Introduction: Salvage autologous stem cell transplantation (ASCT) is an effective and feasible treatment option for patients with relapsed multiple myeloma (MM). It is standard practice to collect stem cells adequate for two transplants for ASCT eligible patients with newly diagnosed multiple myeloma (NDMM). Apheresis is an expensive procedure with the added risk of complications from central venous access such as bleeding, infection and, granulocyte colony stimulating factor administration. However, the rate of utilization of second ASCT is not known.
Methods: We conducted a single institution, retrospective study of 511 consecutive patients with NDMM who underwent ASCT to identify factors predictive of receiving second ASCT. Patients received ASCT as front-line therapy between 1/1/2015-2/13/2019. Demographic and disease characteristics were summarized using median and range for continuous variables, and frequency and percentages were calculated for categorical variables. The cumulative incidence of second ASCT was estimated using the Fine and Gray method, and competing risk regression models were used to estimate the hazard ratios for risk of second ASCT. Analyses were performed using Stata version 18 (StataCorp, College Station, TX), and all statistical tests were two-sided with a type 1 error of <0.05 indicating statistical significance. All estimates were reported with 95% confidence intervals (95% CI).
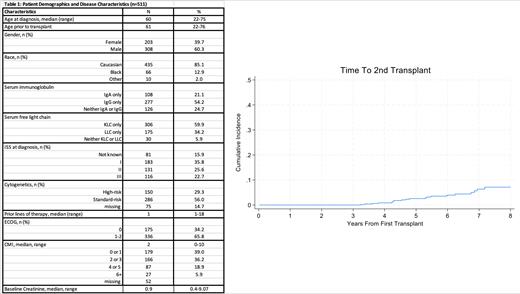
Results: At a median follow-up was 4.7 years, 25 out of 511 patients received a second ASCT. The cumulative incidence rate at 5 years was 2.6% (95% CI 1.4-4.4%) (Figure 1).Age at diagnosis, age at first ASCT and Charlson comorbidity index (CMI) were significantly associated with the risk of second ASCT in univariable analysis (UVA). In multivariable analysis (MVA) age at first ASCT was the only factor that was statistically significantly associated with the likelihood of a second ASCT. We devised a predictive score for utilization of second ASCT using hazard ratios for each of the three significant factors noted in the UVA. Scoring was done as follows: age <65 years - 1 point, >65 years - 7 points; 1 point for CMI 0-1, 2 points for CMI 2 or higher; serum creatinine </=1.2 - 1 point, >1.2 - 3 points. Based on the total score, patients are grouped into low (<=3), medium (4-6) and high (7+) likelihood categories, with cumulative incidence rates of receiving second ASCT at 4.0% (95% CI: 1.3-9.1%), 3.4% (95% CI 1.4-7.0%) and 0.6% (95% CI 0.1-3.1%) respectively. Patients older than 65 years were 87% less likely to receive a second ASCT compared with those younger than 65 years (HR=0.13, 95% CI 0.02-0.98).
Conclusion: Using this predictive score, goal of stem cell collection for patients with MM could be determined more efficiently. This could avoid extra days of apheresis, chemo mobilization, hospitalizations and need for stem cell storage at least for patients with a low likelihood of second ASCT, translating into cost effective utilization of resources. We plan to validate this score prospectively in a larger group of patients with NDMM undergoing ASCT in the future.
Disclosures
Khan:Janssen: Honoraria; Secura Bio: Consultancy, Research Funding; BMS: Research Funding; Amgen, Sanofi: Speakers Bureau. Cottini:The Dedham Group: Consultancy. Efebera:Adaptive: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; Sanofi: Honoraria, Speakers Bureau. Rosko:FDA ODAC Committee: Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding. Vasu:Omeros Inc: Research Funding; Sanofi Inc: Research Funding. de Lima:Miltenyi Biotec: Research Funding; AbbVie: Other: Data Safety Monitoring Board; Novartis: Other: Data Safety Monitoring Board; Bristol Myers Scribb: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees.