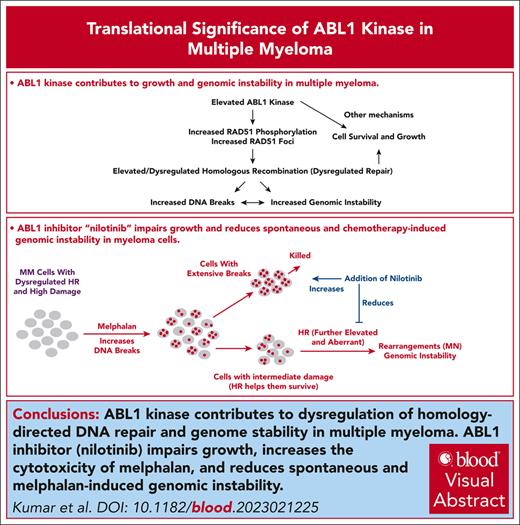
Elevated ABL1 kinase contributes to growth and dysregulation of HR and genome stability in myeloma.
Our data suggest that nilotinib, clinically approved ABL1 inhibitor, can potentially reduce or delay progression in patients with MM.
Visual Abstract
Genomic instability contributes to cancer progression and is at least partly due to dysregulated homologous recombination (HR). Here, we show that an elevated level of ABL1 kinase overactivates the HR pathway and causes genomic instability in multiple myeloma (MM) cells. Inhibiting ABL1 with either short hairpin RNA or a pharmacological inhibitor (nilotinib) inhibits HR activity, reduces genomic instability, and slows MM cell growth. Moreover, inhibiting ABL1 reduces the HR activity and genomic instability caused by melphalan, a chemotherapeutic agent used in MM treatment, and increases melphalan’s efficacy and cytotoxicity in vivo in a subcutaneous tumor model. In these tumors, nilotinib inhibits endogenous as well as melphalan-induced HR activity. These data demonstrate that inhibiting ABL1 using the clinically approved drug nilotinib reduces MM cell growth, reduces genomic instability in live cell fraction, increases the cytotoxicity of melphalan (and similar chemotherapeutic agents), and can potentially prevent or delay progression in patients with MM.
Introduction
Genomic instability or adaptability is a common feature of cancer cells1-6 that enables them to acquire mechanisms that confer greater growth and resistance to treatment.7-11 In multiple myeloma (MM), a cancer of the plasma cells, tumor cells continue to acquire genomic changes over time, and this genomic instability is linked to disease progression.6,11-13 A fundamental problem is that most chemotherapeutic agents are DNA damaging agents. Although such treatment can kill a large fraction of cancer cells, it is also likely to increase chromosomal instability in surviving cancer cells, possibly resulting in the emergence of drug-resistant clones and subsequent relapse. In fact, having an increased rate of DNA repair allows a cancer cell to survive treatment and appears to be associated with chemotherapy resistance in several malignancies.14-17 Particularly in patients with relapsed MM, melphalan resistance is associated with increased rates of DNA repair.18
Homologous recombination (HR), which accurately repairs DNA by copying the missing information from the homologous sequence, maintains genomic integrity and stability.19-22 Dysregulation or hyperactivation of HR can lead to aberrant forms of HR such as nonallelic HR, homeologous recombination, and microhomology-based recombination and is, therefore, associated with a variety of genomic aberrations23-26 and instability.27,28 Microhomology-mediated break–induced replication can cause chromothripsis, which is characterized by massive genomic rearrangements in a localized area of the genome.29 Chromosomal fragments and whole chromosomes produced by defective DNA repair and genomic instability can get encapsulated in a lipid bilayer to form micronuclei,30 which can induce further DNA damage through aberrant DNA repair within the micronucleus.30-35 In fact, spontaneously elevated HR in MM contributes to the acquisition of genomic changes over time as well as the emergence of drug resistance.11 Thus, dysregulated HR in a cancer cell not only repairs DNA breaks but can also produce new breaks (to initiate unscheduled or unnecessary repair), mediate genomic rearrangements (through its aberrant forms), and increase overall genomic instability and chemotherapy resistance.
ABL1 tyrosine kinase is associated with various aspects of DNA damage repair.36 The protein c-ABL phosphorylates RAD51, which is the central component of HR repair.37 Similarly, BCR-ABL1 kinase–mediated phosphorylation of RAD51 can promote aberrant HR with a possible role in the progression of chronic myeloid leukemia (CML).38 In fact, tyrosine kinase inhibitors (such as imatinib and nilotinb) targeting ABL1 are currently used in the treatment of patients with CML, with nilotinib being the drug of choice for CML in the chronic phase.38,39 Although the BCL-ABL1 fusion gene has not been reported in MM, a recent study suggests that ABL1 signaling plays a role in plasma cell survival.40 We therefore evaluated the role of ABL1 kinase in endogenous and chemotherapy-induced genomic instability and growth of MM cells. We show that elevated expression of ABL1 contributes to the overactivation of HR and genomic instability in MM, and its inhibitor nilotinib inhibits chemotherapy-induced genomic damage and instability, while increasing cytotoxicity.
Materials and Methods
Cell lines and patient samples
Normal human fibroblasts, noncancerous human bone marrow stromal cell line (HS-5), and human MM cell lines (MM.1S and RPMI8226) were purchased from American Type Tissue Culture Collection (Rockville, MD). KMS-12-BM, AMO1, U266, NCI-H929, and JJN3 and were purchased from DSMZ (Braunschweig, Germany). All cell lines used were confirmed to be free of mycoplasma and cultured in RPMI1640 growth medium, which contained 10% fetal bovine serum (HyClone, South Logan, UT), as reported previously.11,41-43
Blood samples from healthy donors were collected, and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque gradient (GE Healthcare, Boston, MA). Informed consent was obtained in accordance with the Declaration of Helsinki and the review board of the Dana-Farber Cancer Institute.
Reagents
Nilotinib and melphalan (Sigma Aldrich, Saint Louis, MO) were dissolved in dimethyl sulfoxide and diluted in cell culture medium to the desired concentrations before use. Anti-RAD51 antibody (catalog no. SC-8349) was purchased from Santa Cruz, Dallas, TX. Antibodies against GAPDH (catalog no. mAb2118), HP1 (catalog no. 261), and γH2A.X (Ser139; catalog no. 2577) were from Cell Signaling Technology, Inc (Danvers, MA).
Western blotting
Total cell lysates were prepared using the Thermo Fisher Scientific Cell Lysis Buffer (Thermo Fisher Scientific) supplemented with a protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific). Equal amounts of proteins were resolved by SDS-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Immunoblotting was performed using the following antibodies: β-actin (Santa Cruz), γ-H2AX, and RAD51 (Cell Signaling).
Lentiviral particles and infections
Suppression of ABL1 was achieved using lentivirus-based short hairpin RNAs (shRNAs; Sigma Aldrich). Briefly, cells were transduced with lentiviral particles in the presence of 8-μg/mL polybrene and selected in 2-μg/mL puromycin. Before the experiments, dead cells were eliminated using a Ficoll-Pague gradient (GE Healthcare, Boston, MA), and live cells were allowed to recover for 24 hours.
Immunofluorescence staining and microscopy
For immunofluorescence staining, 20 000 cells were suspended in 100 μL of 1.5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), cytospun onto glass slides at 1000 rpm for 5 minutes, fixed in 4% paraformaldehyde for 10 minutes, washed with PBS, and permeabilized with 0.1% Triton X-100 containing 1.5% bovine serum albumin in PBS for 10 minutes. Cells were then incubated overnight at 4°C with primary antibodies against γ-H2AX (mouse monoclonal anti-γH2AX antibody) and RAD51 (rabbit polyclonal anti-RAD51 antibody, Santa Cruz). Cells were washed and incubated with the appropriate fluorescein-conjugated secondary antibodies (Alexa Fluor 488–labeled goat anti-mouse immunoglobulin G [IgG] from Abcam for γ-H2AX; and Alexa Fluor 594–labeled goat anti-rabbit IgG from Abcam for RAD51). After washing, cells were mounted under coverslips with Prolong Antifade reagent containing DAPI (Invitrogen). Images were acquired with Yokogawa Spinning Disk Confocal/TIRF System with a 63× oil objective lens.
Cell viability assays
Cells were seeded at 2000 to 5000 cells per well in 96-well plates, treated as indicated for 72 hours, and assessed for cell viability using the Cell Titer-Glo Luminescent Cell Viability Assay (Promega) or Cell Counting Kit-8 (CCK-8) assay (Sigma Aldrich) according to the manufacturer’s protocol. Viability was expressed as percentage (± standard error of the mean) of ≥3 independent biological replicates relative to control-treated cells.
Recombination assays
Detection of DNA breaks
Evaluating impact on genomic instability
To evaluate the impact on genomic instability, the cells were cultured in the presence or absence of nilotinib, melphalan, or a combination of both and were evaluated for micronuclei, which is a marker of an unstable genome,30,32,33 as reported by us previously.42-47 Genomic instability was also assessed using single nucleotide polymorphism (SNP) arrays (Affymetrix). Briefly, cells were cultured in the presence or absence of nilotinib. An aliquot of cells was harvested and frozen at the beginning of the experiment (Day 0) to be used as a reference or baseline genome. The acquisition of new copy number alterations in culture relative to the reference cells was monitored using SNP arrays as described by us previously.45
Impact of nilotinib/melphalan in a subcutaneous tumor model
Five-week-old male CB-17 SCID (severe combined immunodeficiency) mice were purchased from Charles River Laboratories (Wilmington, MA) and maintained following the guidelines of the Institutional Animal Care and Use Committee. All experimental procedures were approved by the Institutional Animal Care and Use Committee and the Occupational Health and Safety Department of the Dana-Farber Cancer Institute (Boston, MA). MM.1S cells (3.0 × 106 in 100 μL saline) were injected subcutaneously into the interscapular area of each mouse. After the appearance of tumors (∼7-10 days), the mice were treated with normal saline containing a working dilution of solvent dimethyl sulfoxide, nilotinib (50 mg/kg; 5 days per week), and/or melphalan (2 mg/kg; twice per week), injected intraperitoneally. Tumor sizes were measured regularly, and animals were euthanized when tumors reached 2 cm3 in volume or when paralysis or major compromise in their quality of life occurred. Tumors were removed and evaluated for HR activity.
Results
ABL1 contributes to elevated HR and more DNA breaks in MM cells
Compared with normal plasma cells, ABL1 was upregulated across all stages of plasma cell dyscrasias (P = .004; data set: GSE2568) (Figure 1A). Concordantly, ABL1 protein expression was also upregulated in MM cell lines compared with undetectable or low levels in normal PBMCs (Figure 1B) and CD138+ normal plasma cells (supplemental Figure 1A, available on the Blood website). Because ABL1 regulates RAD51 recombinase through phosphorylation, we investigated the impact of its inhibition on HR as well as other parameters of genome stability (DNA breaks, micronuclei, and acquisition of copy number changes) in MM cell lines. Here, we highlight that genomic impact of ABL1 inhibition was always evaluated in live cell fraction. ABL1 was inhibited using nilotinib or shRNA-mediated knockdown, and the impact on HR was evaluated using a functional HR activity assay. We observed significant inhibition of HR activity in MM cell lines (MM.1S and RPMI8226) in a dose-dependent manner with nilotinib (Figure 1Ci). ABL1 knockdown in these cell lines also caused a significant inhibition of HR activity as assessed by a functional assay (Figure 1Cii). Consistent with these data, ABL1 expression in a myeloma (Multiple Myeloma Research Foundation [MMRF]) data set significantly correlated with the expression of HR gene signature (supplemental Figure 1B). Moreover, nilotinib also inhibited RAD51 phosphorylation and reduced the number of DNA breaks, which are the first stages of the HR process, as evidenced from reduced γ-H2AX expression (Figure 1Di; quantitation of blot shown in supplemental Figure 2A). After normalization with GAPDH, nilotinib inhibited the expression of γ-H2AX by 2.7-fold in RPMI and by 3.4-fold in MM.1S cells (Figure 1D; supplemental Figure 2A). Consistent with these observations, the nilotinib treatment also reduced both the RAD51 and γ-H2AX foci (Figure 1Dii,Diii). Moreover, the ABL1-knockdown led to inhibition of γ-H2AX expression by 11-fold in MM.1S and by sevenfold in RPMI cells (Figure 1E; quantitation of blot shown in supplemental Figure 2B). These results demonstrate a strong impact of ABL1 on HR activity and DNA breaks in MM cells.
ABL1 kinase is overexpressed and contributes to dysregulation of HR activity and genomic integrity in MM. (A) Relative expression (Log2) of ABL1 in normal and MM samples in GSE5900-GSE2658 (NPC = 22; MGUS = 44; SMM = 12; MM = 559) data set. (B) Western blot showing ABL1 expression in normal PBMC samples and MM cell lines. (C) HR activity, assessed by functional assay described in “Methods,” in MM cell lines treated with DMSO (D; control), the ABL1 inhibitor nilotinib for 48 hours (Ci) or shRNAs (C, control; sh-ABL1, ABL1-shRNA) (Cii). Error bars indicate SDs of triplicate experiment and 2-tailed P values derived by t test (∗P < .05-.001; ∗∗P < .001). (D) MM cell lines treated with DMSO or nilotinib (Nil; 5 μM) for 48 hours were evaluated for phosphorylation of RAD51 (Y315) and DNA breaks (by investigating levels of γ-H2AX) using western blotting (Di) and for RAD51 and γ-H2AX foci using fluorescence microscopy (Dii-Diii). Approximately 100 cells representing 3 different microscopic fields were investigated and cells with foci counted. Error bars indicate SDs; 2-tailed P values derived by t test (∗∗P < .001). (E) ABL1 was suppressed using shRNAs (C, control; sh-ABL1, ABL1-shRNA), and the cells were evaluated for DNA breaks (by investigating levels of γ-H2AX) using western blotting. DMSO, dimethyl sulfoxide; NPC, normal plasma cells; MGUS, monoclonal gammopathy of unknown significance; SMM, smoldering multiple myeloma; MM, multiple myeloma; NPC, normal plasma cells; SD, standard deviation; SMM, smoldering multiple myeloma.
ABL1 kinase is overexpressed and contributes to dysregulation of HR activity and genomic integrity in MM. (A) Relative expression (Log2) of ABL1 in normal and MM samples in GSE5900-GSE2658 (NPC = 22; MGUS = 44; SMM = 12; MM = 559) data set. (B) Western blot showing ABL1 expression in normal PBMC samples and MM cell lines. (C) HR activity, assessed by functional assay described in “Methods,” in MM cell lines treated with DMSO (D; control), the ABL1 inhibitor nilotinib for 48 hours (Ci) or shRNAs (C, control; sh-ABL1, ABL1-shRNA) (Cii). Error bars indicate SDs of triplicate experiment and 2-tailed P values derived by t test (∗P < .05-.001; ∗∗P < .001). (D) MM cell lines treated with DMSO or nilotinib (Nil; 5 μM) for 48 hours were evaluated for phosphorylation of RAD51 (Y315) and DNA breaks (by investigating levels of γ-H2AX) using western blotting (Di) and for RAD51 and γ-H2AX foci using fluorescence microscopy (Dii-Diii). Approximately 100 cells representing 3 different microscopic fields were investigated and cells with foci counted. Error bars indicate SDs; 2-tailed P values derived by t test (∗∗P < .001). (E) ABL1 was suppressed using shRNAs (C, control; sh-ABL1, ABL1-shRNA), and the cells were evaluated for DNA breaks (by investigating levels of γ-H2AX) using western blotting. DMSO, dimethyl sulfoxide; NPC, normal plasma cells; MGUS, monoclonal gammopathy of unknown significance; SMM, smoldering multiple myeloma; MM, multiple myeloma; NPC, normal plasma cells; SD, standard deviation; SMM, smoldering multiple myeloma.
Inhibition of ABL1 reduces acquisition of new genomic changes over time in MM
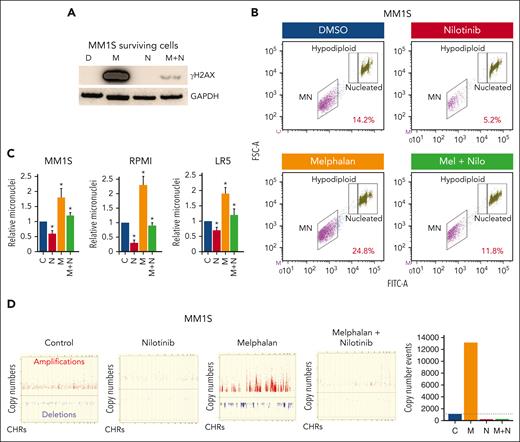
Given that inhibiting ABL1 reduced HR activity, we next investigated whether it could reduce genomic instability. We inhibited ABL1 in MM cells by gene knockdown or nilotinib and investigated the impact of these modulations on the number of micronuclei, a marker of ongoing genomic instability and rearrangements.30,32,33 ABL1 inhibition in both MM cell lines significantly reduced (∼50%; P < .05) the number of micronuclei in 3 independent experiments, indicating reduced genomic instability (Figure 2A-B). To further investigate genomic instability on a finer scale, we cultured MM cells in the presence or absence of nilotinib for 3 weeks and investigated the acquisition of new copy number events relative to “day 0” cells (serving as the baseline genome), using SNP arrays (Affymetrix). Images of copy number events acquired on chromosomes 4 and 9 are shown as example (Figure 2Ci). Over the whole genome, the acquisition of copy number change events in nilotinib-treated cells was reduced to ∼55% of control cells (Figure 2C). These data confirm that ABL1 inhibition reduces genomic instability in MM cells and suggest that it could reduce the rate of genomic evolution in patients with MM.
Inhibition of ABL1 by shRNA or nilotinib reduces genomic instability in MM cells. (A-B) ABL1 was inhibited in MM cell lines (MM.1S and RPMI8226) either using shRNA (C, control; sh-ABL1, ABL1-shRNA) (A) or by culturing in the presence of DMSO or 5 μM ABL1 inhibitor (nilotinib) for 48 hours (B), and cells were evaluated for genomic instability using the micronucleus assay. Flow cytometry images of micronuclei (Ai,Bi) and bar graphs showing relative fold change in micronuclei (Aii,Bii) are shown. Error bars indicate SDs of 3 independent experiments; 2-tailed P values are derived by t test (∗P < .05-.001; ∗∗P < .001). (C) RPMI8226 cells were treated with DMSO only (control) or nilotinib for 21 days, and acquisition of new copy number events relative to the baseline genome of “day 0” cells was evaluated using SNP6.0 arrays (Affymetrix). Representative image of copy number events (amplifications, red dots; deletions, blue dots) on chromosomes 4 and 9 and bar graph showing copy events over whole genome are shown. DMSO, dimethyl sulfoxide; SD, standard deviation.
Inhibition of ABL1 by shRNA or nilotinib reduces genomic instability in MM cells. (A-B) ABL1 was inhibited in MM cell lines (MM.1S and RPMI8226) either using shRNA (C, control; sh-ABL1, ABL1-shRNA) (A) or by culturing in the presence of DMSO or 5 μM ABL1 inhibitor (nilotinib) for 48 hours (B), and cells were evaluated for genomic instability using the micronucleus assay. Flow cytometry images of micronuclei (Ai,Bi) and bar graphs showing relative fold change in micronuclei (Aii,Bii) are shown. Error bars indicate SDs of 3 independent experiments; 2-tailed P values are derived by t test (∗P < .05-.001; ∗∗P < .001). (C) RPMI8226 cells were treated with DMSO only (control) or nilotinib for 21 days, and acquisition of new copy number events relative to the baseline genome of “day 0” cells was evaluated using SNP6.0 arrays (Affymetrix). Representative image of copy number events (amplifications, red dots; deletions, blue dots) on chromosomes 4 and 9 and bar graph showing copy events over whole genome are shown. DMSO, dimethyl sulfoxide; SD, standard deviation.
Inhibition of ABL1 reduces melphalan-induced HR activity in MM cells
Melphalan is a chemotherapeutic agent that causes extensive DNA damage. In fact, treatment with melphalan increases RAD51 expression (Figure 3A, top panel; quantitation of blot shown in supplemental Figure 2C) and HR activity (Figure 3A, bottom panel) in MM cells in a dose-dependent manner, likely due to the cells attempting to repair their genome. To investigate whether ABL1 inhibition can reverse melphalan-induced HR activity, thus increasing melphalan cytotoxicity, we treated MM cells with nilotinib and melphalan, alone as well as in combination, and evaluated the impact on HR activity. Although melphalan significantly increased HR activity, nilotinib not only inhibited endogenous HR activity but also reversed melphalan-induced HR activity in MM cells (Figure 3B).
Melphalan-induced HR activity in MM cells is reversed by nilotinib. (A) MM.1S cells were treated with different concentrations of melphalan and evaluated for RAD51 expression by western blotting (top panel) and HR activity (bottom panel). (B) HR activity assessed in MM.1S cells treated with nilotinib (2.5 μM), melphalan (5 μM), and a combination of both drugs for 48 hours. Error bars indicate SDs of experiments conducted in triplicate and 2-tailed P values are derived by t test (∗P < .05-.001; ∗∗P < .001). SD, standard deviation. D, DMSO; M, melphalan; M+N, melphalan and nilotinib; N, nilotinib.
Melphalan-induced HR activity in MM cells is reversed by nilotinib. (A) MM.1S cells were treated with different concentrations of melphalan and evaluated for RAD51 expression by western blotting (top panel) and HR activity (bottom panel). (B) HR activity assessed in MM.1S cells treated with nilotinib (2.5 μM), melphalan (5 μM), and a combination of both drugs for 48 hours. Error bars indicate SDs of experiments conducted in triplicate and 2-tailed P values are derived by t test (∗P < .05-.001; ∗∗P < .001). SD, standard deviation. D, DMSO; M, melphalan; M+N, melphalan and nilotinib; N, nilotinib.
Inhibition of ABL1 reduces melphalan-induced DNA breaks and genomic instability in MM cells
Given that ABL1 inhibition reduces melphalan-induced HR activity, we next investigated whether melphalan also induces genomic instability and whether that could be reversed by ABL1 inhibition. Briefly, we treated MM cells with nilotinib (2.5 μM), melphalan (5 μM), and a combination of both drugs for 48 hours and evaluated the live cell fractions for DNA breaks (by measuring γ-H2AX level) and genomic instability (using the micronucleus assay). Melphalan was associated with a massive increase in the number of DNA breaks in the live MM cell fraction, and nilotinib almost completely reversed this (Figure 4A). We also evaluated the impact of these treatments on micronuclei, a marker of genomic instability. Representative image of micronuclei in MM1S cells is shown in Figure 4B, whereas bar graphs of relative micronuclei in nilotinib- and/or melphalan-treated 3 MM cell lines are presented in Figure 4C. Consistent with DNA break data, the melphalan increased the number of micronuclei by ∼1.8 to twofold in all 3 cell lines, whereas nilotinib significantly reduced the number of both the basal and melphalan-induced micronuclei in all MM cell lines tested. To investigate the impact of these treatments on copy number, we cultured MM.1S cells in the presence or absence of nilotinib and/or melphalan for 3 weeks and evaluated live cell fractions for copy number changes (relative to day 0 cells) using SNP arrays. We found that, over the whole genome, melphalan led to a >12-fold increase in the number of copy number events, whereas nilotinib not only inhibited the spontaneous but also the melphalan-induced acquisition of copy number events in MM cells (Figure 4D). Altogether, these data show that ABL1 inhibition reduces spontaneous as well as melphalan-induced genomic instability in MM cells.
Nilotinib reduces genomic damage and instability caused by melphalan. (A-B) MM cells were treated with DMSO only (D), melphalan (M), nilotinib (N), or a combination of both drugs (M+N) for 48 hours and evaluated for DNA breaks (by measuring γ-H2AX level) (A) and genomic instability (using micronucleus assay) (B-C). A representative flow cytometry image of micronuclei in MM.1S cells (B) and bar graphs showing percent micronuclei in 3 MM cell lines (C) are shown. Error bars indicate SDs of 3 independent experiments, and 2-tailed P values are derived by t test (∗P < .05). (D) MM.1S cells treated with nilotinib (N; 2.5 μM), low dose melphalan (M; 1 μM), or combination were cultured for 3 weeks. DNA from the cultured and “day 0” (baseline control) cells was analyzed using SNP arrays (Affymetrix). Copy number events in cultured cells were identified using the genome of “day 0” cells as a baseline. A copy number event was defined as a change in ≥3 consecutive probes by 1 copy. Images showing amplifications (red dots) and deletions (blue dots) on all chromosomes and bar graph showing total copy number change events throughout genome. DMSO, dimethyl sulfoxide; SD, standard deviation.
Nilotinib reduces genomic damage and instability caused by melphalan. (A-B) MM cells were treated with DMSO only (D), melphalan (M), nilotinib (N), or a combination of both drugs (M+N) for 48 hours and evaluated for DNA breaks (by measuring γ-H2AX level) (A) and genomic instability (using micronucleus assay) (B-C). A representative flow cytometry image of micronuclei in MM.1S cells (B) and bar graphs showing percent micronuclei in 3 MM cell lines (C) are shown. Error bars indicate SDs of 3 independent experiments, and 2-tailed P values are derived by t test (∗P < .05). (D) MM.1S cells treated with nilotinib (N; 2.5 μM), low dose melphalan (M; 1 μM), or combination were cultured for 3 weeks. DNA from the cultured and “day 0” (baseline control) cells was analyzed using SNP arrays (Affymetrix). Copy number events in cultured cells were identified using the genome of “day 0” cells as a baseline. A copy number event was defined as a change in ≥3 consecutive probes by 1 copy. Images showing amplifications (red dots) and deletions (blue dots) on all chromosomes and bar graph showing total copy number change events throughout genome. DMSO, dimethyl sulfoxide; SD, standard deviation.
Inhibition of ABL1 reduces MM cell viability and increases cytotoxicity of chemotherapeutic agents in MM cells
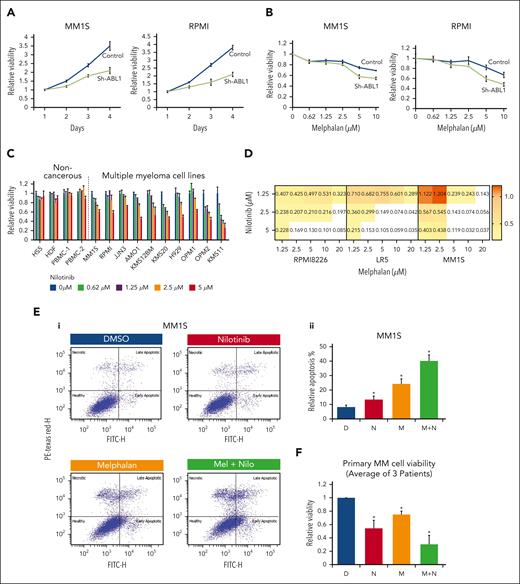
Because ABL1 inhibition prevents a cancer cell from attempting to repair its DNA through HR, we next asked whether ABL1 inhibition reduces cell viability. We inhibited ABL1 in MM cell lines (MM.1S and RPMI8226) using shRNA and found significantly reduced viability of both MM cell lines (Figure 5A). Furthermore, ABL1 knockdown significantly increased the cytotoxicity of melphalan in both MM cell lines tested (Figure 5B). Consistently, nilotinib also reduced MM cell growth in a dose-dependent manner, with minimal or no impact on 3 different normal/noncancerous cell types (HS5, human bone marrow stromal cells; HDF, human normal diploid fibroblasts; and PBMCs from 2 different donors) (Figure 5C). Nilotinib also sensitized 3 MM cell lines, including a melphalan-resistant cell line (LR5), to melphalan treatment with a combination index <1 (Figure 5D) and mean HAS synergy score >16 (supplemental Figure 3A-C), indicating a synergistic impact.
ABL1 inhibition reduces MM cell viability and increases cytotoxicity of melphalan in MM cells. (A) ABL1 in MM cells was inhibited using shRNAs (control, C; ABL1-targeting, sh-ABL1), and after selection, cell viability was assessed for 4 days. (B) Control and ABL1-knockdown (sh-ABL1) MM cells were treated with different concentrations of melphalan for 48 hours, and cell viability was measured. (C) Normal/noncancerous cell types (HS5, human bone marrow stromal cells; HDF, human normal diploid fibroblasts; and PBMC, peripheral blood mononuclear cells from 2 different donors) and MM cell lines were treated with different concentrations of nilotinib, and cell viability was measured. Bar graph showing impact of nilotinib on cell viability; error bars represent SDs of triplicate assays. (D) MM cell lines (MM1.S, RPMI8226, and LR5) were treated with different concentrations of nilotinib and melphalan, and cell viability was measured after 48 hours and combination index (CI) calculated using CalcuSyn software (CI < 1, synergism; CI = 1, additive; CI > 1: antagonism). (E) MM.1S cells were treated with either DMSO (D), 2.5 μM nilotinib (N), 5 μM melphalan (M), or a combination (M + N) and evaluated for apoptosis using annexin/PI staining. Flow cytometry images of a representative experiment (Ei) and bar graphs showing apoptosis in triplicate experiments (Eii) are shown. (F) Bone marrow plasma cells from 3 patients with relapsed MM were treated as indicated for 24 hours and cell viability measured. Error bars indicate SDs of experiments conducted in triplicate, and 2-tailed P values are derived by t test (∗P < .05). DMSO, dimethyl sulfoxide; PI, propidium iodide; SD, standard deviation.
ABL1 inhibition reduces MM cell viability and increases cytotoxicity of melphalan in MM cells. (A) ABL1 in MM cells was inhibited using shRNAs (control, C; ABL1-targeting, sh-ABL1), and after selection, cell viability was assessed for 4 days. (B) Control and ABL1-knockdown (sh-ABL1) MM cells were treated with different concentrations of melphalan for 48 hours, and cell viability was measured. (C) Normal/noncancerous cell types (HS5, human bone marrow stromal cells; HDF, human normal diploid fibroblasts; and PBMC, peripheral blood mononuclear cells from 2 different donors) and MM cell lines were treated with different concentrations of nilotinib, and cell viability was measured. Bar graph showing impact of nilotinib on cell viability; error bars represent SDs of triplicate assays. (D) MM cell lines (MM1.S, RPMI8226, and LR5) were treated with different concentrations of nilotinib and melphalan, and cell viability was measured after 48 hours and combination index (CI) calculated using CalcuSyn software (CI < 1, synergism; CI = 1, additive; CI > 1: antagonism). (E) MM.1S cells were treated with either DMSO (D), 2.5 μM nilotinib (N), 5 μM melphalan (M), or a combination (M + N) and evaluated for apoptosis using annexin/PI staining. Flow cytometry images of a representative experiment (Ei) and bar graphs showing apoptosis in triplicate experiments (Eii) are shown. (F) Bone marrow plasma cells from 3 patients with relapsed MM were treated as indicated for 24 hours and cell viability measured. Error bars indicate SDs of experiments conducted in triplicate, and 2-tailed P values are derived by t test (∗P < .05). DMSO, dimethyl sulfoxide; PI, propidium iodide; SD, standard deviation.
Flow cytometry–based annexin/PI staining demonstrated that treatment of MM.1S cells with nilotinib, melphalan, and a combination of both drugs was associated with apoptosis in ∼12%, 25%, and 40% of cells, respectively, indicating that nilotinib increases melphalan-induced apoptosis in MM cells (Figure 5E). We also evaluated the impact of other DNA damage–related chemotherapy drugs (bendamustine and PARP inhibitor olaprib) in combination with nilotinib in MM cell lines and found nilotinib also significantly sensitized MM cells to these chemotherapeutic drugs (supplemental Figures 4 and 5). Importantly, nilotinib also significantly sensitized bone marrow plasma cells from patients with relapsed MM to melphalan treatment (Figure 5F).
Inhibition of ABL1 significantly increases cytotoxicity of melphalan in a subcutaneous model of MM
We next evaluated the impact of nilotinib, melphalan, and their combination in an in vivo subcutaneous tumor model of MM. Briefly, MM.1S cells were injected subcutaneously into SCID mice, and after the appearance of tumors, mice were treated with nilotinib (50 mg/kg; 5 days per week) and/or melphalan (2 mg/kg; twice per week) and tumor volumes measured regularly. After 2 weeks of treatment, tumor volumes in mice treated with vehicle control, nilotinib, melphalan, and a combination of both drugs increased by sixfold, threefold, 2.8-fold, and 1.2-fold, respectively (P ≤ .02), relative to their initial sizes. The combination treatment resulted in the smallest tumor volumes in mice (Figure 6A), demonstrating the ability of nilotinib to increase the cytotoxicity of melphalan in vivo. Similar results were observed in another independent experiment (supplemental Figure 7). In this experiment also, the tumor sizes in mice treated with both nilotinib and melphalan were significantly (P ≤ .01) shorter than in control mice. Moreover, mice treated with combination of these drugs had the smallest tumors. Mice treated with the combination of nilotinib and melphalan also had a significant (P < .05) increase in survival (supplemental Figure 7B). At the end of 2 weeks, mice were euthanized, and the tumors were removed and evaluated for HR activity in the lysates of the tumor cells. Consistent with in vitro data, melphalan treatment was associated with a significant increase in HR activity, whereas nilotinib significantly inhibited both the endogenous and melphalan–induced HR activities in vivo (Figure 6B). These data suggest that nilotinib increases the cytotoxicity of chemotherapeutic agents, such as melphalan, while reducing genomic instability in MM cells.
Nilotinib increases melphalan sensitivity in MM cells in vivo. (A) MM.1S cells were injected subcutaneously into SCID mice (5 mice per group), and after the appearance of tumors, mice were treated with either DMSO, nilotinib (50 mg/kg, 5 days per week), melphalan (2 mg/kg, twice per week), or in combination. Line plots showing tumor volumes (with error bars indicating SDs) and representative images of tumors in different treatments are shown. (B) Tumors were lysed and evaluated for HR activity by in vitro strand exchange assay; error bars indicate SDs of experiments conducted in triplicate, and 2-tailed P values are derived by t test (∗P < .05-.001). DMSO, dimethyl sulfoxide; SD, standard deviation.
Nilotinib increases melphalan sensitivity in MM cells in vivo. (A) MM.1S cells were injected subcutaneously into SCID mice (5 mice per group), and after the appearance of tumors, mice were treated with either DMSO, nilotinib (50 mg/kg, 5 days per week), melphalan (2 mg/kg, twice per week), or in combination. Line plots showing tumor volumes (with error bars indicating SDs) and representative images of tumors in different treatments are shown. (B) Tumors were lysed and evaluated for HR activity by in vitro strand exchange assay; error bars indicate SDs of experiments conducted in triplicate, and 2-tailed P values are derived by t test (∗P < .05-.001). DMSO, dimethyl sulfoxide; SD, standard deviation.
Discussion
Dysregulation of HR induces imprecise, unscheduled, and unnecessary recombination events leading to genomic rearrangements and instability.21-27 This genomic instability arises early during oncogenesis and contributes to oncogenic transformation and the progression of cancer.1-10 For example, in esophageal cancer, elevated HR not only mediates genomic instability but also contributes to telomere maintenance and tumor growth in vivo.28 Targeting mechanisms that promote genomic instability may be an effective strategy to delay progression, prevent the development of resistance to treatment, minimize the harmful genomic impact of chemotherapy, and increase the cytotoxicity of chemotherapeutic agents. Consequently, drugs (such as nilotinib) that inhibit HR may delay progression in patients with MM and other cancers. In fact, a clinical trial for CML shows that 99% of patients treated with nilotinib did not progress to the accelerated phase.
One major problem in managing patients with cancer is the development of resistance to treatment. The mechanisms underlying this problem could be intrinsic to cancer cells, acquired during treatment, or induced by treatment itself. These mechanisms may include altered cellular drug transport, inadequate cytotoxicity of the therapeutic agent, alterations in the drug targets, and altered DNA repair pathways. Most chemotherapeutic agents induce DNA breaks, thus killing cells. However, surviving cells have more DNA breaks and higher HR activity, predisposing them to genomic instability and eventual genomic evolution toward drug resistance. Here, we found that cells surviving melphalan treatment had increased γ-H2AX expression, and this increase was blocked (to near background level) by the addition of nilotinib. Consistently, the genomic instability was also increased by melphalan and prevented by the addition of nilotinib. Importantly, these analyses were done on live cell fractions only, because apoptosis is associated with DNA breaks. Therefore, in the presence of dead cells, an accurate interpretation of γ-H2AX results is not possible.
Although melphalan-induced DNA breaks and genomic instability in MM cells were reduced by nilotinib, its cytotoxicity was increased. This may seem unexpected; however, myeloma cells have high endogenous DNA damage and overactive HR, which contributes to ongoing genomic rearrangements and instability11,27 as well as the growth and survival of cancer cells.28 In this environment, melphalan treatment further increases DNA damage as well as HR activity, thus prompting heavily damaged cells to undergo apoptosis. However, in surviving cells, further increase in (dysregulated) HR not only helps them survive (by engaging the breaks) but also increases genomic rearrangements and instability. This is the reason that nilotinib increases cytotoxicity of melphalan but reduces spontaneous and melphalan-induced genomic instability.
In summary, our data demonstrate that ABL1 contributes to genomic instability and growth in MM cells. Because ABL1 phosphorylates RAD51 and regulates HR activity (as shown in Figure 1), ABL1-induced HR is attributed to genomic instability in MM cells. However, impact of ABL1 on genome stability and growth of MM cells is not limited to its impact on HR. Evaluation of the impact of nilotinib on expression profile in MM.1S cells using RNA sequencing demonstrated that several DNA repair and recombination related pathways were downregulated. These included P53, ATM, regulation of DNA damage response, DNA damage response, meiotic recombination, and HR. In addition, P53 Hypoxia, E2F, and DNA replication–related pathways were also downregulated by nilotinib treatment in MM.1S cells (supplemental Figure 6). These data suggest that inhibition of ABL1 with nilotinib had a prominent impact on dysregulated DNA repair (including HR) and replication in MM cells.
To our knowledge, our study is the first to demonstrate that a clinically approved ABL1 inhibitor (nilotinib) has the potential to inhibit growth and reduce genomic evolution in MM cells. When used in combination with a chemotherapeutic agent such as melphalan, nilotinib can reduce chemotherapy-induced genomic damage and instability while increasing its cytotoxicity. Such treatments also have the potential to delay or prevent disease progression including development of drug resistance in myeloma.
Acknowledgments
This work was supported by Department of Veterans Affairs Merit Review Award I01BX001584-01 (N.C.M.), National Institutes of Health, National Cancer Institute grants P01-155258 and P50 CA100707 (N.C.M., M.A.S.), and a Paula and Rodger Riney grant (N.C.M.).
Authorship
Contribution: S.K. executed major experiments, analyzed and interpreted the data, and prepared the manuscript; S.T., J.Z., C.L., L.B.P., S.M., J.S., C.C., and Y.-T.T. carried out specific experiments and assisted in data analysis; L.B. and M.K.S. analyzed single nucleotide polymorphism data and helped with statistical and bioinformatic analyses; M.A.S. envisioned the study and participated in designing, data interpretation, and preparation of the manuscript; N.C.M. envisioned the study and participated in its design and coordination, and also helped in drafting the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nikhil C. Munshi, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; email: nikhil_munshi@dfci.harvard.edu; and Masood A. Shammas, Dana Farber Cancer Institute, 44 Binney St, D1B25 Boston, MA 02215; email: Masood_Shammas@DFCI.Harvard.edu.
References
Author notes
Original data and other relevant material are available upon reasonable request from the corresponding author, Masood Shammas (Masood_Shammas@DFCI.Harvard.edu).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.