Issue Archive
Table of Contents
BLOOD COMMENTARIES
PLENARY PAPER
Identification and surveillance of rare relapse-initiating stem cells during complete remission after transplantation
Relapse following complete remission (CR) is the leading cause of death following allogeneic transplant for myeloid malignancy. In this Plenary Paper, Dimitriou et al tracked measurable residual disease (MRD) to predict relapse following transplant for myelodysplasia and related myeloid malignancies. Using flow cytometry to sort hematopoietic stem and progenitor cells (HSPCs) on sequential samples, the authors found that only 60% of unsorted mononuclear cells from patients in CR who later relapsed had detectable MRD, but HSPCs from the same patients were 100% MRD-positive, suggesting a means to identify patients for whom preemptive therapy may prevent overt relapse.
BLOOD SPOTLIGHT
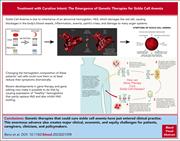
“Treatment with curative intent”: the emergence of genetic therapies for sickle cell anemia
In a Blood Spotlight, Benz and colleagues review the curative potential of recently approved gene therapy approaches to sickle cell disease. The authors discuss the background for their development, the issues of their potential curative intent, and the limitations on their availability in clinical practice.
HOW I TREAT
How I manage acute respiratory failure in patients with hematological malignancies
Clinical Trials & Observations
Using 4 illustrative cases, Azoulay and coauthors describe a structured approach to diagnosis of and therapy for acute respiratory failure in patients with hematologic malignancies.
CLINICAL TRIALS AND OBSERVATIONS
The phase 2 LYSA study of prednisone, vinblastine, doxorubicin, and bendamustine for untreated Hodgkin lymphoma in older patients
Clinical Trials & Observations
Older patients with classical Hodgkin lymphoma (cHL) tolerate intensive bleomycin-containing chemotherapy regimens poorly. Ghesquières and colleagues report on results of a phase 2 study of a new regimen comprised of doxorubicin (Adriamycin), vinblastine, bendamustine, and prednisone in 89 patients aged 61 and over with advanced cHL. Complete metabolic response as determined by positron emission tomography scan was achieved in 78% of patients; progression-free survival and overall survival were 50% and 69%, respectively. Toxicity was deemed acceptable, with a 20% rate of serious adverse events.
LYMPHOID NEOPLASIA
ABL1 kinase plays an important role in spontaneous and chemotherapy-induced genomic instability in multiple myeloma
Kumar et al elucidated a role for ABL1 kinase overactivation in contributing to genomic instability in multiple myeloma (MM) cells. In vitro studies showed that ABL1 inhibition with short hairpin RNA or nilotinib slows MM cell growth and increases sensitivity to melphalan in a subcutaneous tumor model. These studies suggest a novel function for clinically approved ABL1 kinase inhibitors in the treatment of MM.
MYELOID NEOPLASIA
Tumor necrosis factor α promotes clonal dominance of KIT D816V+ cells in mastocytosis: role of survivin and impact on prognosis
Greiner et al investigated the role of tumor necrosis factor α (TNF) in KIT D816V+ systemic mastocytosis (SM). The authors demonstrate that KIT mutation increases expression and secretion of TNF. In turn, TNF suppresses colony formation of normal human bone marrow cells while the KIT-mutant cells are less susceptible to TNF, facilitating clonal dominance of mutant mast cells. Further studies show that resistance of KIT-mutant cells depends on an apoptosis mediator, BIRC5 (survivin). Elevated TNF levels are associated with inferior survival in patients with SM, and these studies suggest that TNF inhibitors may provide a targeted therapy for SM.
RED CELLS, IRON, AND ERYTHROPOIESIS
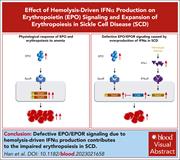
Hemolysis-driven IFNα production impairs erythropoiesis by negatively regulating EPO signaling in sickle cell disease
Han et al demonstrate that while erythroid progenitors are markedly increased in thalassemic mice, they are only modestly increased in mice with sickle cell disease (SCD). Further examination reveals that colony formation and erythropoietin (EPO) signaling are impaired in SCD erythroid cells. Hemolysis and the release of free heme impair erythropoiesis through activation of interferon-α (IFNα), which leads to upregulation of cytokine-inducible SH2-containing protein (CISH), a negative regulator of EPO signaling. Knockout of the IFNα receptor rescues erythropoiesis, confirming a novel interaction between hemolysis and decreased erythropoiesis mediated via a heme-IFNα-CISH axis.
THROMBOSIS AND HEMOSTASIS
Low-frequency inherited complement receptor variants are associated with purpura fulminans
Bendapudi and colleagues investigated the genetic basis for infection-associated purpura fulminans (PF). Using whole exome sequencing, the authors identified rare variants in the complement system in patients with PF. By functional testing, most variants resulted in partial or complete loss of anti-inflammatory CR3 function or gain of proinflammatory CR4 function. Their findings support that inherited defects in complement proteins predispose to hyperinflammation leading to PF.
LETTERS TO BLOOD
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
Immunohistochemistry of a bone marrow biopsy stained for tryptase (red-brown) from a patient with systemic mastocytosis. The tryptase-positive atypical mast cells promote clonal selection of
KIT D816V-positive cells in mastocytosis. See the article by Greiner et al on page 1006. - PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals









A road map of relapse in MDS after allo-HSCT