In this issue of Blood, Ghesquières et al1 conclude that the prednisone, vinblastine, doxorubicin, and bendamustine (PVAB) regimen, which lacks any novel drugs, could be a valuable nonbleomycin regimen for older patients with classical Hodgkin lymphoma (cHL). They also note that the outcome of older patients with chemotherapy-treated cHL remains dismal, regardless of the chemotherapy regimen used, and needs improvement.
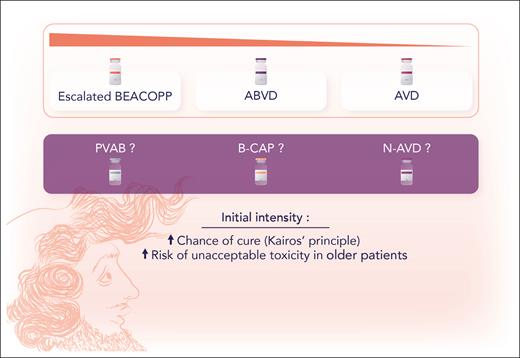
Older patients with cHL do not share the favorable prognosis of their younger counterparts. They often suffer from more comorbidities and have a lower tolerance to toxic chemotherapy. There is no chemotherapy gold standard demonstrated by large randomized trials to guide the treatment decisions in this group. The Kairos principle (coined by Volker Diehl; Kairos had hair in the front but was bald at the back of his head), to hit hard early to grab the chance of cure, seems valid for young patients, given the evidence for dose-dense therapies like escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP). Unfortunately, older patients do not tolerate that approach. Hence, less intensive regimens, like the regimen studied here, PVAB, are used (see figure).
According to the Kairos principle, coined by Volker Diehl, the chance of cure increases with initial intensity of chemotherapy, but intensive combinations, like BEACOPP, may result in unacceptable toxicity in older patients with cHL. Professional illustration by Somersault18:24.
According to the Kairos principle, coined by Volker Diehl, the chance of cure increases with initial intensity of chemotherapy, but intensive combinations, like BEACOPP, may result in unacceptable toxicity in older patients with cHL. Professional illustration by Somersault18:24.
The inferior prognosis for older patients with cHL is partly explained by comorbidities and frailness.2 The biological characteristics of the disease are also different in older compared with younger patients. Older patients more commonly have mixed cellularity histology, advanced disease stage, and more frequent Epstein-Barr virus positivity. Anthracycline-based therapy remains important in curative treatment, but more intensive regimens like BEACOPP often result in intolerable toxicity and even death.3 Doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) is commonly used in clinical practice, but bleomycin-induced lung toxicity is a major problem for older patients.4-6 Also, granulocyte colony-stimulating factor is not recommended after ABVD, due to the risk of bleomycin toxicity. Omission of bleomycin (AVD) has been tried and seems to be a feasible strategy, but cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) seems inferior.7 PD-1 inhibitors and the antibody drug conjugate brentuximab vedotin are promising additions to standard therapy but both the optimal timing and combination of agents are still being explored.8,9 The latest contribution is combining brentuximab vedotin with dacarbazine or nivolumab for the slightly different group of patients not eligible for chemotherapy.10
The patients in the Ghesquières study were all aged >60 years and diagnosed with advanced stage disease. The 89 included patients had a median age of 68 years. They received treatment with 6 cycles of the chemotherapy combination PVAB. Complete metabolic remission after treatment was 77.5%. Four-year progression-free survival (PFS) and overall survival (OS) were 50% and 69%, respectively. Patients aged >69 years were evaluated according to the geriatric cumulative illness rating scale (CIRS-G) and, interestingly, a mini nutritional assessment. Unfortunately, the relatively small sample size limits the power of the study. However, compared with other prospective studies of this age group, 89 patients is a respectable number. Performing a study of older patients with cHL is, in itself, quite an achievement. Despite this limitation, as older patients with cHL have worse PFS and OS than young patients, the statistics in the study are convincing.
The PVAB regimen contains no novel agents such as PD-1 inhibitors or antibody drug conjugates. Combining the standard anthracycline doxorubicin, with the proven (yet not too toxic) agent vinblastine and bendamustine, which has known efficacy both as a single agent and in combination in the relapse setting, seems reasonable. The absence of bleomycin is positive considering the risk of lung toxicity, and all patients received granulocyte colony-stimulating factor.
This study, like other cHL studies in this age group, is a nonrandomized phase 2 study. Ideally, a randomized trial should be performed in the older age group but that seems all but impossible, even for very large international groups. The results of the study, in terms of PFS and OS, seem to be relatively in line with other combinations. Unfortunately, the lack of randomization, different inclusion criteria, and different time points for evaluation make it impossible to draw any firm conclusions.
In conclusion, the PVAB regimen seems tolerable, resulting in PFS and OS in line with other combinations. If possible, a large, randomized study would be performed, which would require a vast international collaboration. The introduction of a tolerable and relatively effective new combination is valuable and moves the field forward. Standard PVAB will probably fall short compared with PD-1 inhibitor and brentuximab vedotin combinations but might still prove very helpful since the optimal chemotherapy backbone for combinations with novel drugs is not yet established. Very good results have been seen using pretreatment with brentuximab vedotin or PD-1 inhibitors, a kind of Kairos principle where the opportunity is taken but in a more careful way, not to tear the gray hair away from the aging Kairos’ head.11
Conflict-of-interest disclosure: D.M. has received honoraria from Roche, Merck, Bristol Myers Squibb, and Takeda (not related to this article).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal