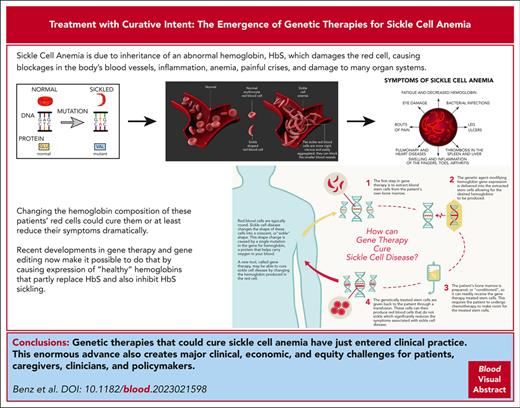
Visual Abstract
The root cause of sickle cell anemia has been known for 7 decades, yet no curative therapies have been available other than allogeneic bone marrow transplantation, for which applicability is limited. Two potentially curative therapies based on gene therapy and gene editing strategies have recently received US Food and Drug Administration approval. This review surveys the nature of these therapies and the opportunities and issues raised by the prospect of definitive genetically based therapies being available in clinical practice.
Linus Pauling’s demonstration in 1949 that sickle cell anemia (SCA) was characterized by an abnormal hemoglobin (sickle cell hemoglobin [HbS]) possessing an altered electrostatic charge,1 coupled with Vernon Ingram’s 1957 discovery that HbS contains β chains bearing valine instead of glutamic acid at position 6,2 marked SCA as medicine’s first molecular disease. HbS polymerizes when it unloads its oxygen to the tissues, producing gelatinous fibers that pathologically increase erythrocyte viscosity and stiffness. They also inflict metabolic, cytoskeletal, and membrane damage, creating the eponymous sickle cell shape and causing the erythrocyte surfaces to become excessively adherent to the endothelium.3 The activated endothelium and enhanced adherence of other blood cells provoke inflammatory and procoagulant diatheses. Taken together, these changes render red cells unable to traverse the microcirculation, causing microvascular occlusion and ischemia, thereby generating the classic syndrome of hemolytic anemia, recurrent painful vaso-occlusive crises, and protean end-organ damage.3,4 Median life expectancy in the United States is only 50 to 55 years.4 The clinical phenotype emerges only after birth because fetal hemoglobin (HbF) contains γ subunits instead of β. Patients coinheriting SCA and hereditary persistence of HbF produce more HbF in adult life and have milder clinical courses.3
No curative treatments have been available for patients with sickle cell disease other than allogeneic bone marrow transplantation. Its applicability is limited by significant morbidity and mortality risks, including graft-versus-host disease, a paucity of compatible donors, and expense.4 Only 4 drugs have been US Food and Drug Administration approved specifically for treatment of sickle cell anemia. Only 1, hydroxyurea, even partially improves survival. Hydroxyurea reduces morbidity in responsive patients primarily by inducing variable levels of HbF, which partially replaces HbS and is also a potent biochemical inhibitor of HbS sickling.4 Indeed, HbF levels as low as 20% to 30% can reduce symptoms. Hydroxyurea’s efficacy is variable and requires lifelong daily treatment, but it does demonstrate that pharmacologically modifying the hemoglobin composition of HbS erythrocytes is possible and beneficial.
Finally, 7 decades after the delineation of SCA’s root cause, potentially curative therapies are in hand. Two promising modalities based on distinct gene manipulation strategies have entered clinical practice, having transited the final stages of the US Food and Drug Administration approval process. Each achieves clinical impact by partially replacing HbS with a nonsickling hemoglobin that also inhibits polymerization by any remaining HbS.
Lovotibeglogene autotemcel, developed by bluebird bio, employs a gene addition strategy, delivering a gene encoding an engineered hemoglobin containing a β(87T>Q) mutation into hematopoietic stem cells (HSCs).5 This variant possesses anti-sickling activity roughly equipotent to HbF.6 Exagamglogene autotemcel (generally referred to as exa-cel, formerly named CTX-001), developed by CRISPRTx and Vertex, applied gene editing to the discovery by researchers7,8 that the cessation of HbF synthesis in adult erythrocytes requires the action of a bcl11a repressor protein. In exagamglogene autotemcel–edited HSCs, the erythroid-specific bcl11a enhancer is crippled, reducing bcl11a expression and derepressing γ globin synthesis.9 Most participants receiving either agent during clinical trials experienced significant hematologic and clinical benefits with few adverse events.5,9 Some patients became nearly symptom free. Most others experienced significant relief, welcome results compatible with movement into clinical practice.
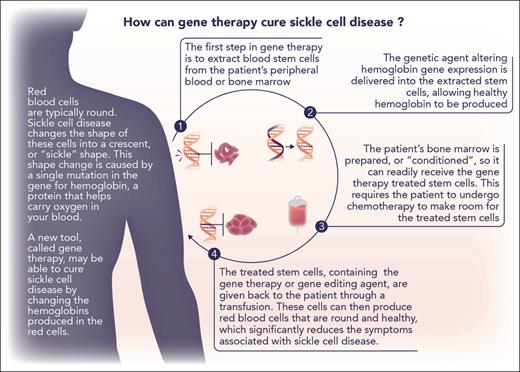
It is best to regard these landmark therapies as treatment with curative intent rather than as curative treatments until the completeness and durability of clinical benefit are better known. They are also encumbered with limitations that will impede access to all who might benefit, especially in geographic regions where SCA is most prevalent. Both use ex vivo protocols to modify HSCs, necessitating mobilization and harvesting of circulating HSCs, expansion of the transfected clones, ablation of endogenous HSCs with a genotoxic agent (busulfan), and rescue with the modified HSCs (Figure 1). Most facilities capable of safely executing these autologous stem cell transplant regimens are not available in the equatorial regions where the disease is most frequently encountered. Moreover, comparable gene therapy products now on the market, including the similar bluebird bio T87Q gene replacement for thalassemia, are priced at $1 000 000 to $3 000 000.10 Ensuring equitable access will be a considerable challenge to providers, payers, and policy makers.
Explanation of the process of ex vivo gene therapy. Modified (with permission) from a figure provided by the Cure Sickle Cell Initiative (https://curesickle.org/genetic-therapies) for patients, caregivers, and the general public.
Explanation of the process of ex vivo gene therapy. Modified (with permission) from a figure provided by the Cure Sickle Cell Initiative (https://curesickle.org/genetic-therapies) for patients, caregivers, and the general public.
Short-term adverse events were in keeping with those associated with autologous transplantation and were manageable. However, genomic manipulation and ex vivo expansion of HSCs coupled with genotoxic marrow ablation increase the risk of developing myelodysplastic syndrome or leukemia. Cases have been reported in SCA gene therapy trials.11 There is also suggestive evidence for and against the idea that patients with SCA may experience accelerated clonal hematopoiesis of indeterminant potential (CHIP),12 potentially enhancing these risks. This raises the possibility that treatment might be most safely administered at the earliest age at which the need for this therapy is apparent, before CHIP or irreversible organ damage is extensive.
Despite these challenges, both therapies demonstrate that gene therapy/editing technologies can reduce or eliminate sickling in patients with SCA. Gene-based treatments with curative intent are thus going to be among the clinical options available to patients and their clinicians. Given that reality, what are the implications for patients and their loved ones, clinicians, payers, and policy makers? Who should get this therapy and at what age? Who should provide them? How will access be ensured, given the complexity and expense of the therapy? What short- and longer-term monitoring and support will these patients need? Although these are general concerns for all genetic therapies, they are especially complex for SCA because this disease is most prevalent in underresourced regions globally and among an underserved demographic in the Western hemisphere.
Patients, their caregivers, and their loved ones have been paying keen attention to the emergence of genetic therapies. They express both hope and trepidation about their implications personally and for the SCA community. The National Heart, Lung, and Blood Institute’s Cure Sickle Cell Initiative, of which the authors are members, has been interacting extensively with stakeholders to learn their concerns and questions. These commentaries represent issues raised repeatedly by patients, caregivers, and advocates ranging in age from adolescence to 60-70 years at webinars, in person and during online retreats, symposia and forums, and in consultations with the patient representatives and Community Input Panel of the Cure Sickle Cell Initiative. These questions were expressed with about equal frequency. Among them are the following:
“What does a “cure” mean?” The evidence indicates that these therapies are effective at diminishing the sickling process, improving anemia, reducing hemolysis, and reducing or eliminating vaso-occlusive crises. It is not yet clear whether there will be similar beneficial impacts on preexisting end organ damage, or the chronic pain, encephalopathy, and profound malaise and fatigue experienced by many of these patients. Many question if the possibility of a cure justifies consequences of the procedure (e.g., infertility or malignancy risk).
“Will this treatment give me cancer?” The specter of treatment-related malignancy is fully grasped by the community. The possibility that one might “trade” SCA for leukemia is a major source of concern. These risks need to be discussed realistically in a balanced risk/benefit context if patients are to make an informed decision. Long-term monitoring for the appearance of pathogenetic mutations and progression of CHIP will be required. Expert subspecialty support must be readily accessible should this infrequent complication develop. Centers offering these therapies should do so only if able to deliver these resources.
“I’ve lived my whole life identified as a patient with SCA.How will a ‘cure’ change my sense of self?” For individuals whose SCA is sufficiently severe to merit gene therapy/editing, illness is a major definer of their existence, shaping their ability to thrive and approximate normal living. Removal of the somatic burdens of SCA, although clearly beneficial, can be a source of stress and anxiety for individuals who then must recalibrate their sense of self, aspirations, and capacities. Some patients worry that they would no longer “belong” in their support or advocacy groups or to a sickle cell clinic, where they had been receiving more expert care than in the general medical milieu.
“How long will this therapy work? What happens if itstops working? Will I continue to be supported?” The long-term durability of genetic therapies remains a source of anxiety for patients contemplating this open question. What is also not clear is whether these or subsequent technologies will be compatible with repetition of the therapy or the use of subsequent modalities. Some worry that, once treated, they will be regarded as cured and no longer in need of support in dealing with their long-term issues.
“What if I am not eligible to get the therapy?” Gene therapy/editing will initially be appropriate and accessible for only a minority of patients with SCA. Being unable to receive an available definitive treatment is even more stressful than the lack of one. Proper attention and support must be provided to those individuals who are either not eligible or unable to access these treatments.
The issues articulated by the SCA community are both insightful and practically important. They must be addressed if these promising therapies are to achieve their goal of enabling a normal lifespan with a high quality of life. The community repeatedly stresses the importance of mental health issues in dealing with the disease and in contemplating the possibilities that a cure might entail. There should be relevant mental health and well-being resources available to patients with SCA, whether receiving these novel therapies or not. Cancer survivorship programs are now widely available at most institutions capable of delivering genetic therapies. They are equipped to deal with similar issues faced by patients for whom cancer is increasingly becoming a chronic illness. These centers should extend those services and resources to patients with SCA undergoing these new treatments. Indeed, one could hope that these new therapies might catalyze survivorship care for all patients with SCA in the same way that effective treatments for childhood leukemia spurred the creation of the field of cancer survivorship.
The emergence of treatments with curative intent for SCA is exciting and should, at last, offer patients an intervention that eliminates or significantly slows its progression. One hopes that this advance will intensify the search for safer, simpler, and more affordable methods for conditioning the marrow and delivering therapeutic genetic material to HSCs. In vivo gene delivery that achieves adequate and precisely targeted dosing of HSCs would avoid the need for autologous stem cell transplantation. Efforts to use alternative viral vectors,13 endosomes, liposomes, or nanoparticles14 targeted to HSCs are showing promise but encountering issues of inadequate potency and HSC targeting specificity. These are significant but potentially solvable hurdles. Alternatively, now that several key regulators of fetal hemoglobin repression in adult life are known, small molecules or inhibitory RNAs targeting them might be feasible.
The development of 2 potentially curative therapies for SCA highlights once again the essentiality of the rigorous application of basic and translational science to the study of blood diseases. These therapies exist only because of the concerted effort of many scientists and clinicians who elucidated the regulatory mechanisms by which globin genes are expressed at such extraordinarily high levels, but only in developing erythroblasts, and only in a strictly regulated ontological sequence of embryonic-to-fetal-to-adult hemoglobin synthesis. The design of anti-sickling hemoglobins, like T87Q, required the description of the biochemical mechanisms mediating HbS sickling, and the identification of the structural basis for the anti-sickling properties of HbF. Without these and many other contributions, there would have been no rationale or molecular strategy on which to base successful efforts to alter globin gene expression and impede sickling in a clinically meaningful way.
Lovotibeglogene autotemcel and exagamglogene autotemcel prove the principle that one can use gene therapy or gene editing to alter the hemoglobin phenotype in a manner that warrants clinical application. Their success should broaden and intensify the quest for enhanced next-generation options. Fortunately, in addition to the efforts focused on improved delivery, investigators are pursuing novel approaches, such as targeting the mutation directly, editing γ globin gene promoters, RNA therapeutics, and blocking other suppressors of HbF production.15-17 To say that these new therapies should not be regarded as the final answer does not diminish their critical importance as breakthroughs. Seven decades after the delineation of SCA’s root cause, medicine’s first molecular disease is finally yielding to the relentlessness of medical research.
Acknowledgment
The views expressed are those of the authors and do not necessarily represent the views of the National Institutes of Health or the US government.
Authorship
Contribution: All authors developed the basic framework and scope of content for the article. E.J.B. prepared the first drafts. L.E.S. and J.P. contributed editing, and content and tone modifications. J.P. contributed Figure 1. All authors developed the references. E.J.B. edited and refined the final draft and prepared the manuscript for submission.
Conflict-of-interest disclosure: E.J.B. is a member of the boards of directors of Deciphera Pharmaceuticals, CoRegen, Inc, and Candel Therapeutics, 3 oncology biotech companies, and a member of the advisory boards of Riverside Partners (back-office health services), Autoimmunity Biological Solutions (autoimmune diseases), and Kernal Therapeutics (oncology). None of these companies is engaged in hemoglobinopathy or gene therapy activities. All authors are members of the Cure Sickle Cell Initiative, a National Institutes of Health (NIH)–sponsored initiative focused on curative therapies for sickle cell disease. It is an NIH-sponsored and -directed activity that is nonprofit.
Correspondence: Edward J. Benz Jr, Department of Medical Oncology, Dana-Farber Cancer Institute, Room D1644a, 450 Brookline Ave, Boston, MA 02215; email: edward_benz@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal