Visual Abstract
Acute respiratory failure (ARF) is common in patients with hematological malignancies notably those with acute leukemia, myelodysplastic syndrome, or allogeneic stem cell transplantation. ARF is the leading reason for intensive care unit (ICU) admission, with a 35% case fatality rate. Failure to identify the ARF cause is associated with mortality. A prompt, well-designed diagnostic workup is crucial. The investigations are chosen according to pretest diagnostic probabilities, estimated by the DIRECT approach: D stands for delay, or time since diagnosis; I for pattern of immune deficiency; R and T for radiological evaluation; E refers to clinical experience, and C to the clinical picture. Thorough familiarity with rapid diagnostic tests helps to decrease the use of bronchoscopy with bronchoalveolar lavage, which can cause respiratory status deterioration in those patients with hypoxemia. A prompt etiological diagnosis shortens the time on unnecessary empirical treatments, decreasing iatrogenic harm and costs. High-quality collaboration between intensivists and hematologists and all crossdisciplinary health care workers is paramount. All oxygen delivery systems should be considered to minimize invasive mechanical ventilation. Treatment of the malignancy is started or continued in the ICU under the guidance of the hematologists. The goal is to use the ICU as a bridge to recovery, with the patient returning to the hematology ward in sufficiently good clinical condition to receive optimal anticancer treatment.
Introduction
In patients with hematological malignancies, acute respiratory failure (ARF) is a frequent and life-threatening complication.1 In this population, when intensive care unit (ICU) admission is required, the reason is ARF in 60% to 80% of cases.2 ARF is typically characterized by tachypnea >30/min or labored breathing, signs of respiratory distress, low PaO2 (<60 mm Hg) or peripheral oxygen saturation (SpO2) (<90% on room air), or oxygen therapy ≥6 L/min, and recent pulmonary infiltrates (<7 days).
In patients with acute myeloid leukemia (AML) or those receiving allogeneic hematopoietic cell transplantation, ARF may indicate intricate immunological impairments leading to severe lung infections, frequently accompanied by acute respiratory distress syndrome3 and multiorgan dysfunction.4,5 Moreover, the use of multiple immunosuppressive agents, targeted immunotherapies, and chimeric antigen receptor T-cell therapy is expected to increase the incidence of respiratory complications in the future.
Managing ARF in patients with hematological malignancy involves 3 key components.1,6,7 First, urgently provide supportive treatment to restore oxygenation (targeting SpO2 88%-95%) and address associated organ dysfunctions. Second, promptly initiate investigations tailored to the patient’s malignancy, clinical presentation, and risk-to-benefit ratio of each diagnostic approach. Third, administer optimal malignancy treatment through daily interactions with hematologists. Close collaboration among hematologists, intensivists, chest physicians, infectious disease specialists, clinical microbiologists, and interdisciplinary health care workers is essential, ensuring that ICU management serves as a bridge to curative anticancer treatment.
The epidemiology of ARF in patients with hematological malignancy has significantly evolved. Substantial progress in both cancer and supportive treatments has emerged, shedding light on distinct immune impairments linked to each malignancy. Given these changes, a personalized approach to each patient is now paramount.8
This review explores ARF in patients with hematological malignancies and its current diagnostic and therapeutic challenges.
Epidemiology and causes of ARF
The frequency of ARF varies significantly among malignancy types. Five factors directly increase the risk of ARF: myeloid over lymphoid proliferation9; neutropenia, particularly if prolonged10; allogeneic hematopoietic cell transplantation,11,12 notably, in elderly patients13; underlying structural lung disease14; and absence of appropriate prophylaxis.15,16 ARF incidence is on the rise among patients with hematological malignancy because of improved survival from advances in cancer therapy.17 Aggressive chemotherapy regimens, allogeneic hematopoietic cell transplantation, and the emergence of multidrug-resistant pathogens combine to increase the risk of respiratory infections.18 Prolonged neutropenia and lung infiltration by malignant cells are also associated with ARF.19,20
In a population-based cohort study from Ontario, the 1-year incidence of ICU admission of adults with a newly diagnosed hematological malignancy was 14%. Patients with AML, aggressive non-Hodgkin lymphoma, or acute lymphoblastic leukemia (ALL) faced the highest risk.21 Endotracheal mechanical ventilation was required in 37% of patients, and in-hospital mortality was 31%.21 In a Danish nationwide cohort study, the 1-year cumulative risk of ICU admission was 23% in patients with AML and ALL. ICU admission was most common within the first months after diagnosis.22 Early ICU admission should be reserved for patients with AML with hyperleukocytosis,23-25 bleeding, severe coagulopathies, or ARF.26 Routine preemptive admission fails to provide benefits.27
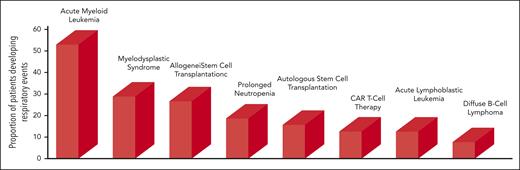
Figure 1 shows the proportion of patients with a respiratory event across different hematological malignancy. Bacterial pneumonia and invasive aspergillosis are associated with prolonged neutropenia.10 Patients with lymphoproliferative disorders28 or myeloma are less affected because neutropenia is uncommon and immunoglobulin replacement therapy effective.29
Proportion of patients developing respiratory events across different types of hematological malignancies. Patients with prolonged neutropenia were those with AML and recipients of allogeneic stem cell transplants. Data for bars 1 and 2 from Rabbat et al,31 Chaoui et al,9 and Azoulay et al.98 Data for bar 3 from Azoulay et al98 and Pichereau et al.122 Data for bar 4 from Orasch et al.10 Data for bar 5 from Kerhuel et al.127 Data for bar 6 from Azoulay et al.5 Data for bar 7 from Rabbat et al,31 Chaoui et al,9 and Azoulay et al.98 Data for bar 8 from Algrin et al128 and Wohlfarth et al.28 CAR, chimeric antigen receptor.
Proportion of patients developing respiratory events across different types of hematological malignancies. Patients with prolonged neutropenia were those with AML and recipients of allogeneic stem cell transplants. Data for bars 1 and 2 from Rabbat et al,31 Chaoui et al,9 and Azoulay et al.98 Data for bar 3 from Azoulay et al98 and Pichereau et al.122 Data for bar 4 from Orasch et al.10 Data for bar 5 from Kerhuel et al.127 Data for bar 6 from Azoulay et al.5 Data for bar 7 from Rabbat et al,31 Chaoui et al,9 and Azoulay et al.98 Data for bar 8 from Algrin et al128 and Wohlfarth et al.28 CAR, chimeric antigen receptor.
Among hematological malignancies, AML is most often associated with respiratory events, which affected 50% and 82% of patients in 2 retrospective studies from Austria30 and France,31 respectively. Older age increased the risk.32 In Canada, 62% of patients with AML required mechanical ventilation.33 The respiratory events were independently associated with higher 1-year mortality.9
Hematological malignancies all lead to profound immunological impairments that promote pneumonia development.6 Early antibacterial treatment is indicated for all patients with ARF and hematological malignancies.34 Decisions to add antifungal and/or antiviral agents and start chemotherapy or steroids are made on a case-by-case basis after discussion among hematologists, intensivists, and, when needed, external consultants. Many mechanisms can affect the lungs in patients with hematological malignancies, and >1 is present in 20% of cases.35 The causes of ARF can be roughly categorized as infectious (opportunistic or nonopportunistic) or noninfectious. Categorizations may be misleading for several reasons. Patients might have multiple ARF causes. It is crucial to take a comprehensive approach, ruling out coinfections. Second, categories overlook the unique immune impairments related to each hematological malignancy not just the treatment. For example, opportunistic infections can occur early in T-cell malignancies.36 Third, categories do not account for epidemiological shifts, the introduction of new diagnostic tests, and potential clinical changes brought about by new anticancer drugs.37-39 Finally, categorizations might suggest equal probabilities for all causes, resulting in confusion and overtreatment.
The diagnostic approach should be personalized for each patient. The initial bedside evaluation comprises 3 steps. First, clinical and radiological findings determine the pretest probability of each diagnosis (Figure 2). This guides the start of empirical treatment and the design of a diagnostic workup with the best risk-benefit balance. Then, clinicians conduct the diagnostic workup. Finally, a conclusion is drawn about the cause according to specific rules, aiming to minimize cases with unknown etiology. Additionally, decisions are made to discontinue anti-infectious agents if found ineffective, to prevent toxicity, exposure, and to reduce costs.
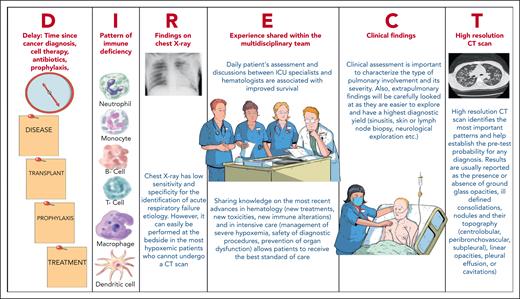
Initial clinical assessment: the DIRECT approach
Case 1 (patient 2)
A 61-year-old man with newly diagnosed acute monocytic leukemia was admitted to the ICU with dyspnea, desaturation, and respiratory distress (Figure 3). Diffuse pulmonary infiltrates with consolidations were found. With 12 L oxygen per minute, the respiratory rate decreased, and the patient felt more comfortable. The most likely causes were identified and the probability of each assessed at the bedside using the DIRECT approach (Figures 2 and 3). Corresponding treatment and diagnostic strategy were applied.
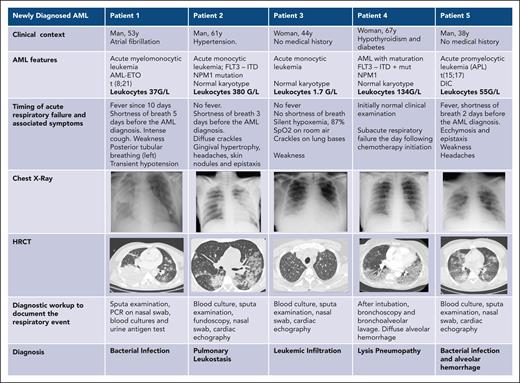
Different types of respiratory events in patients with newly diagnosed AML. DIC, disseminated intravascular coagulation; HRCT, high-resolution CT of the chest; ITD, internal tandem duplication.
Different types of respiratory events in patients with newly diagnosed AML. DIC, disseminated intravascular coagulation; HRCT, high-resolution CT of the chest; ITD, internal tandem duplication.
The initial assessment is conducted at the bedside. It involves the patient’s medical history, a comprehensive physical examination, standard laboratory tests, and imaging studies to gauge the pretest clinical probability for potential causes of ARF. In our experience, an effective means of obtaining these estimates is the DIRECT approach (Figure 2).7,40 The DIRECT is a clinical approach that allows to establish pretest probability of ARF etiologies and to guide the diagnostic workup. Table 1 and Figures 2 and 3 illustrate the application of DIRECT in real-life patients (infection, leukostasis, leukemic infiltration, pulmonary tumor lysis syndrome, or diffuse alveolar hemorrhage).41 Distinguishing among types of leukemic infiltrate holds major therapeutic implications.42 Leukostasis characterizes hyperleukocytic AML, with endothelial injury from microvascular invasion and cellular hyperviscosity, leukocytic microthrombi, oxygen steal, and hypoxia. In leukemic infiltration, chiefly in monoblastic AML, blasts aggregate in vascular lumens43 and can induce ARF in the absence of hyperleukocystosis.44 There is no relationship between the leukocyte count and the incidence of parenchymal leukemic infiltration.45 The infiltrates typically follow the lymphatic routes along the bronchovascular bundles, interlobular septa, and pleural interstitial tissue. Conversely to the 2 previous leukemic infiltrates, lysis pneumopathy occurs immediately or early after chemotherapy. It refers to a diffuse alveolar damage with degenerating blast cells in the interstitium and in organizing alveolar exudates.46
Ten clinical vignettes illustrating the personalized approach to identifying the cause of ARF in patients with hematological malignancies
 |
 |
ARDS, acute respiratory distress syndrome; BAL, bronchoalveolar lavage; B-ALL, B-cell ALL; CAR, chimeric antigen receptor; CLL, chronic lymphocytic leukemia; CMV, cytomegalovirus; CRS, cytokine release syndrome; HRCT, high-resolution CT of the chest; ICANS, immune effector-cell-associated neurotoxicity syndrome; NK/T-EBV, natural killer cell and Epstein-Barr virus–associated T-cell lymphoproliferation; SARS-CoV-1, severe acute respiratory syndrome coronavirus 1; SCT, stem cell transplantation; WBCs, white blood cells.
Studies with methodological limitations suggest that steroid therapy may improve short-41,47,48 and long-term outcomes in patients with AML with hyperleukocytosis.49-51 Treatments targeting cytokines and adhesion molecules involved in myeloblast-endothelium interactions may also deserve consideration. However, randomized trials on potential benefits from these treatments is challenging, because of the very low incidence of hyperleukocytic AML. To date, only group comparisons in retrospective studies are available. Similarly, pulmonary leukostasis may benefit from slow cytoreduction using hydroxyurea to shrink the tumor burden, thereby reducing the risk of tumor lysis in the kidneys and lungs.52,53 However, this strategy is based on local experience and has not been proven beneficial. Leukapheresis remains controversial,54 because most of the leukemic burden is in the bone marrow and promptly shifts to the peripheral blood leading to postprocedure rebound. In a systematic review on patients with AML and an initial white blood count ≥100 × 109/L, Oberoi et al demonstrated that neither leukapheresis nor hydroxyurea significantly influenced mortality.55 However, more evidence is needed.56 ARF development with falling leukocyte counts strongly suggests pulmonary lysis syndrome, although treatment for pneumonia should also be given and cytarabine-induced pulmonary damage considered.56
In the DIRECT approach, D stands for delay, or time since the hematological malignancy diagnosis, stem cell transplantation, or drug initiation.
I stands for pattern of immune deficiency, from both the underlying malignancy and the treatments received. This governs the likelihood of each ARF cause. For instance, neutropenia is associated with sepsis and fatal infection.57 Lymphoproliferative B-cell disorders can result in bacterial infections because of both the B-cell deficiency and the hypogammaglobulinemia induced by anti-CD20 monoclonal antibody treatment.6 Patients with T-cell deficiencies may experience opportunistic infections without having received any treatment.36 Bruton tyrosine kinase inhibitors may alter the clinical or radiological features of pulmonary infections.38 Overall, considering the type of immune deficiency helps to determine whether the treatment must target opportunistic infections (T-cell deficiency or higher prolonged steroid exposure) or specific pathogens (encapsulated; slow growing, such as tuberculosis and histoplasmosis; or intracellular pathogens). This crucial treatment guidance is obtained at ICU admission. Protein electrophoresis and a flow-cytometry analysis of the lymphocyte population should be obtained.
The R and T stand for radiological evaluation of the lungs by chest radiography and computed tomography (CT). High-resolution CT is now performed routinely in patients with hematological malignancies and ARF, although proof of clinical benefits is lacking.58 It is crucial to assess the risk of respiratory deterioration before transporting a spontaneously breathing patient with high fraction of inspired oxygen (FiO2) requirements to the radiology department. Chest imaging helps determine the best diagnostic test. A prospective study of 112 patients with febrile neutropenia and normal chest radiographs found pulmonary infiltrates on CT in 60% of the cases.58 The yield can be increased by considering the pretest probability of each ARF cause.59 Alveolar consolidation suggests a bacterial or fungal infection. Septal thickening may indicate fluid overload, lung infiltration by the malignancy, or intracellular pathogens. Ground-glass opacities are seen in Pneumocystis jirovecii pneumonia, viral pneumonia, and infections by intracellular pathogens. Micronodules may point to a bacterial, viral, or mycobacterial lung infection, whereas large, solid, or excavated nodules may be produced by invasive fungal, mycobacterial, or bacterial infections due to, for instance, Nocardia, Geotrichum, or Actinomyces. Staphylococcus aureus and Pseudomonas aeruginosa should also be considered. Lastly, pleural effusions are rare in P jirovecii pneumonia but can develop in all types of lung involvement.6 The increasing use of lung ultrasound can be expected to produce new guideposts to the etiological diagnosis.
The E in clinical experience pertains to insights shared during rounds by hematologists and intensivists. Recent evidence reveals immunological deficiencies, drug toxicities, optimal oxygenation methods, procedure safety, and infection patterns in patient subgroups. In-depth familiarity with this evidence aids in selecting optimal diagnostic and treatment strategies. For instance, septic shock suggests bacterial infection, hemoptysis necrotizing pneumonia, and chest pain necessitates urgent investigations for classical diagnoses but may also result from invasive aspergillosis invading lung vasculature. C refers to the clinical picture. After this bedside approach, treatment begins, and a choice is made between invasive or noninvasive diagnostic strategies. Table 1 illustrates how DIRECT is applied in 10 real-life situations, determining whether to perform bronchoscopic-bronchoalveolar lavage and progression to the definite diagnosis.
Case 1 (patient 2, continued)
The patient required escalation to a high-flow nasal cannula (HFNC) (50 L/min; 50% FiO2) and then felt substantially better despite a high breathing rate of 30/min (Figure 2). Cefotaxime and spiramycin were started. Echocardiography was normal. Pulmonary leukostasis was the most likely diagnosis. The skin and gingiva were infiltrated. Hyperhydration and cytoreduction using hydroxyurea were started. The combination of monoblastic AML with thoracic CT findings of thickening of interlobular septa suggested extravascular leukemic infiltration. Although based on studies with considerable methodological limitations, dexamethasone (10 mg 4 times per day) was administered until neutropenia was achieved. The patient improved substantially as the leukocyte count declined. Oxygen and dexamethasone were stopped on day 11.
Diagnostic strategy
Case 2 (patient 5)
This 67-year-old man had been receiving rituximab and venetoclax for the last 9 months to treat chronic lymphocytic leukemia (Table 1). Profound hypogammaglobulinemia had prompted IV immunoglobulin replacement therapy 3 weeks earlier. He had started experiencing respiratory symptoms 2 weeks earlier. He was admitted directly to the ICU with labored breathing, a 44/min breathing rate, and a 77% SpO2 level. HFNC (60 L/min; 85% FiO2) raised the SpO2 to 94% and slowed the breathing rate to 32/min, but he remained uncomfortable. Anti-Pneumocystis prophylaxis had been stopped, indicating a very high pretest probability of P jirovecii pneumonia, and treatment was started at admission.
Because patients with an undetermined ARF etiology are at higher risk of dying,35,60 every patient should undergo a diagnostic workup. Three different diagnostic approaches have been evaluated: lung biopsy, bronchoscopic-bronchoalveolar lavage, and noninvasive rapid diagnostic tests. The reference standard, lung biopsy,61,62 induces high morbidity and mortality rates, limiting its use.63 Post mortem lung examinations in patients with no identified ARF cause show invasive fungal infection; malignant lung infiltration; and potentially steroid-sensitive conditions, such as organizing pneumonia, acute interstitial pneumonia, acute eosinophilic pneumonia, and diffuse alveolar hemorrhage.64 CT-guided lung biopsy has been increasingly performed over the past 10 years, chiefly when fungal infection is suspected.65,66 The advent of bronchoscopic-bronchoalveolar lavage in the early 1990s reshaped the management of lung infiltrates in patients who are immunocompromised. However, it has a low diagnostic yield for patients with hematology, notably those with neutropenia.7 Moreover, in patients with ARF, bronchoscopic-bronchoalveolar lavage was associated with respiratory status deterioration.7 We recently reported an association between the use of bronchoscopic-bronchoalveolar lavage and higher mortality.67 However, despite our propensity score–matching efforts, we might not have considered all confounding variables in our analysis. A major change introduced over the past 2 decades is the development of rapid diagnostic tests performed on samples that are collected using noninvasive or minimally invasive procedures. Over time, their diagnostic performance has improved6 to the extent that they are now sound alternatives to bronchoscopic-bronchoalveolar lavage.68 Some of these tests identify biomarkers (eg, C-reactive protein, procalcitonin, B-natriuretic peptide, or β-D-glucan), serum antigens (eg, galactomannan or Cryptococcus), or urine antigens (eg, Pneumococcus or Legionella). Others use polymerase chain reaction (PCR) amplification to detect circulating DNA (eg, herpes viruses and cytomegalovirus) or respiratory-pathogen RNA in nasal swabs, nasopharyngeal aspirates, or respiratory secretions (eg, rhinoviruses, respiratory syncytial virus, parainfluenza viruses, influenza viruses, human metapneumovirus, adenovirus, bocavirus, and coronaviruses). PCR also effectively detects bacteria, fungi, and parasites in serum or other fluids (eg, Nocardia, mycobacteria, Mucorales, Aspergillus, Toxoplasma, and Histoplasma). PCRs are very helpful for diagnostic strategies, but they do not inform on sensitivity to treatments (eg, antifungals). Some rapid diagnostic tests require minimally invasive but well-tolerated sample collection, such as peripheral node biopsy, skin biopsy (patient 4; Table 1), or marrow aspiration. In a diagnostic strategy trial comparing combined early bronchoscopic-bronchoalveolar lavage (day 1) and rapid diagnostic tests vs rapid diagnostic tests only (with delayed bronchoscopic-bronchoalveolar lavage if needed by day 3), we found no significant differences in the proportion of unidentified ARF causes, mechanical ventilation needs, or mortality between the 2 groups.69 Interestingly, among the 106 patients in the rapid diagnostic only group, 38 (36%) underwent bronchoscopic-bronchoalveolar lavage, providing a diagnosis not revealed by noninvasive tests in only 2 patients (5%). Table 2 shows the basic principles that underlie the choice, optimization, and interpretation of rapid diagnostic tests. Furthermore, the routine incorporation of metagenomic and transcriptomic data is being increasingly considered.70,71 An algorithmic approach incorporating the genomic portrait of pathogens determined by metagenomic next-generation sequencing, the airway microbiome, and the host transcriptome was very effective in distinguishing pathogens from commensals in the lower respiratory tract.72
Ten basic principles for optimizing the diagnostic yield of noninvasive diagnostic tests for patients with hematological malignancies and ARF
| Do not perform all tests in every patient. Instead, rely on the pretest probability (DIRECT approach) to select tests with either high sensitivity for confirming a strongly suspected diagnosis or high negative predictive value for ruling out a diagnosis that has a low pretest probability. |
| Identify the diagnosis. Every patient must have a diagnostic workup, which may involve a single diagnostic test when the diagnosis is obvious (echocardiography, pleural drainage, and sputum test). |
| Avoid cognitive bias. Do not be satisfied with a high pretest probability. Document the diagnosis using rapid diagnostic tests or invasive tests. Consider every possible differential diagnosis. |
| In patients with hypoxemic ARF, give priority to noninvasive diagnostic tests. When a diagnosis can be made noninvasively, do not perform bronchoscopic-bronchoalveolar lavage |
| Do not confuse a positive test with a diagnostic test. PCR can detect tiny amounts of viral DNA, whose presence does not confirm pneumonia due to the corresponding virus. Identified pathogens may be commensals or colonizing pathogens. |
| A negative test can contribute substantially to the diagnosis. For a diagnosis with a low pretest probability, a test with a high negative predictive value can allow withdrawal of a treatment, thereby decreasing toxicity and cost. |
| The best diagnostic test assesses the lung compartment predominantly affected by the disease. The diagnostic yield of bronchoscopic-bronchoalveolar lavage is high in patients with ground-glass opacities. These opacities indicate bronchoscopic-bronchoalveolar lavage, unless the patient is considered too unstable or too hypoxemic or a noninvasive diagnostic test can achieve the same diagnostic yield. |
| When bronchoscopic-bronchoalveolar lavage is deemed mandatory for a patient with severe hypoxemia, early intubation that allows for the investigation is acceptable. Early intubation is an option when the patient is responding poorly, to avoid delaying necessary bronchoscopic-bronchoalveolar lavage. In these patients who are severely ill, the adverse effect on outcomes of failure to identify the cause of ARF deserves close consideration. |
| The risk-to-benefit ratio of each procedure must be established by consensus. All alternatives and innovative options should be considered. |
| Diagnostic strategies can be more effective than diagnostic tests. Integrating clinical data with a series of efficient tests can substantially increase diagnostic yields. |
| Do not perform all tests in every patient. Instead, rely on the pretest probability (DIRECT approach) to select tests with either high sensitivity for confirming a strongly suspected diagnosis or high negative predictive value for ruling out a diagnosis that has a low pretest probability. |
| Identify the diagnosis. Every patient must have a diagnostic workup, which may involve a single diagnostic test when the diagnosis is obvious (echocardiography, pleural drainage, and sputum test). |
| Avoid cognitive bias. Do not be satisfied with a high pretest probability. Document the diagnosis using rapid diagnostic tests or invasive tests. Consider every possible differential diagnosis. |
| In patients with hypoxemic ARF, give priority to noninvasive diagnostic tests. When a diagnosis can be made noninvasively, do not perform bronchoscopic-bronchoalveolar lavage |
| Do not confuse a positive test with a diagnostic test. PCR can detect tiny amounts of viral DNA, whose presence does not confirm pneumonia due to the corresponding virus. Identified pathogens may be commensals or colonizing pathogens. |
| A negative test can contribute substantially to the diagnosis. For a diagnosis with a low pretest probability, a test with a high negative predictive value can allow withdrawal of a treatment, thereby decreasing toxicity and cost. |
| The best diagnostic test assesses the lung compartment predominantly affected by the disease. The diagnostic yield of bronchoscopic-bronchoalveolar lavage is high in patients with ground-glass opacities. These opacities indicate bronchoscopic-bronchoalveolar lavage, unless the patient is considered too unstable or too hypoxemic or a noninvasive diagnostic test can achieve the same diagnostic yield. |
| When bronchoscopic-bronchoalveolar lavage is deemed mandatory for a patient with severe hypoxemia, early intubation that allows for the investigation is acceptable. Early intubation is an option when the patient is responding poorly, to avoid delaying necessary bronchoscopic-bronchoalveolar lavage. In these patients who are severely ill, the adverse effect on outcomes of failure to identify the cause of ARF deserves close consideration. |
| The risk-to-benefit ratio of each procedure must be established by consensus. All alternatives and innovative options should be considered. |
| Diagnostic strategies can be more effective than diagnostic tests. Integrating clinical data with a series of efficient tests can substantially increase diagnostic yields. |
Diagnostic strategies are not conflicting for various reasons. Bronchoscopic-bronchoalveolar lavage might not be indicated; echocardiography is the diagnostic tool for cardiogenic pulmonary edema (patient 1; Table 1). Additionally, bronchoscopic-bronchoalveolar lavage might be contraindicated (severe hypoxemia, coagulopathy, and shock). In case 2, a 5-day anti–P jirovecii treatment could have prompted to rapid improvements, averting the need for mechanical ventilation. In centers where mycological staining and immunofluorescence are available, bronchoscopic-bronchoalveolar lavage would hasten the definite diagnosis of P jirovecii pneumonia but would also carry a risk of respiratory status deterioration. PCR testing for P jirovecii performs equally well on bronchoalveolar lavage fluid and on induced sputum.73 Moreover, β-D-glucan testing is a valuable tool for diagnosing P jirovecii, given its high negative predictive value.74 Whether bronchoalveolar lavage fluid analysis is more effective in enabling anti-infectious de-escalation compared with rapid diagnostic tests has not been properly investigated. Third, in patients with ARF with neutropenia, the diagnostic and therapeutic yields of bronchoscopic-bronchoalveolar lavage is low.68 Fourth, in some situations (eg, case 2), PCR tests perform as well on serum or sputum as they do on bronchoalveolar lavage fluid.37,73,75 In some situations, however, bronchoscopic-bronchoalveolar lavage is the only investigation that can confirm the diagnosis, help manage treatment toxicities, and provide guidance for the future chemotherapy courses. Examples include drug-related pulmonary toxicity, eosinophilic lung diseases, diffuse alveolar hemorrhage, or alveolar proteinosis. Table 1 depicts following 2 scenarios: patient 9 with promptly detected invasive aspergillosis of the airways through bronchoscopy, and patient 10 with a diagnosis requiring crucial alveolar-cell analysis.
Importantly, the 3 diagnostic approaches can be combined to maximize procedural safety and diagnostic yield. For instance, first-line rapid diagnostic tests can be performed while the patient is being stabilized. If uncertainty remains, a safer performance of bronchoscopic-bronchoalveolar lavage can follow. CT-guided lung biopsy can be discussed as a back-up strategy for certain lung lesions (eg, nodules and consolidations). However, in a clinical trial, bronchoscopic-bronchoalveolar lavage after first-line rapid diagnostic tests produced the diagnosis in limited number of cases.69 Consequently, second-line bronchoscopic-bronchoalveolar lavage should be selectively used and its results optimized by a careful evaluation of the bronchial tree and routine assessment of the alveolar cells.
Case 2 (patient 5, continued)
Induced sputum sampling failed because of desaturation during the aerosols (Table 1). He was intubated 9 hours after ICU admission, when SpO2 was 81% despite HFNC (60 L/min; 100% FiO2). He rapidly developed refractory hypoxemia but was ineligible for extracorporeal membrane oxygenation because of his age, uncontrolled disease, and thrombocytopenia. Fiberoptic bronchoscopy was not feasible. Mini–bronchoalveolar lavage performed through the endotracheal tube without bronchoscopy produced a positive PCR result for P jirovecii and negative culture results for bacteria and fungi. β-D-Glucan was >500 pg/mL. He died in a setting of refractory acute respiratory distress syndrome.
Oxygenation, ventilation, and ICU admission
Cases 3 and 4 (patients 7 and 9)
Case 3
Patient 7 was a 44-year-old woman with newly diagnosed acute myelomonocytic leukemia and a 3-week history of respiratory symptoms (Table 1). She required only 6 L O2 per minute to maintain her SpO2 at 95% with several episodes of desaturation. CT showed a crazy paving pattern suggestive of alveolar proteinosis. Despite safety concerns, bronchoscopic-bronchoalveolar lavage was performed.
Case 4
This 39-year-old man with relapsing T-cell ALL presented with fever, cough, and shortness of breath (patient 9 in Table 1). Imaging showed ill-defined consolidations suggestive of fungal infection. The patient was stable with 6 L O2 and considered fit to undergo bronchoscopic-bronchoalveolar lavage.
Noninvasive ventilation (NIV) is indicated or patients with ARF because of cardiogenic pulmonary edema or with hypercapnia. However, the evidence does not support the use of NIV for hypoxemic ARF.76,77 Historically, NIV was considered effective based on observations from a single-center, open-label trial in 52 patients with compromised immune system with early hypoxemic ARF.78 The situation has changed considerably. Now, survival is 60% to 70% when no additional organ dysfunction occurs and 40% otherwise.2,79 Evidence against the use of NIV for patients with compromised immune system has accumulated over time. In a randomized controlled trial comparing NIV with O2 supply for 374 patients who are immunocompromised with ARF, NIV did not significantly decrease the proportion of patients who required mechanical ventilation and had no impact on mortality.76 Similar results were reported when NIV was compared with HFNC.77,80
In unselected patients, HFNC therapy compared with standard O2 supply or NIV improves physiological,81 clinical,82 and patient-reported outcomes.83 In a randomized controlled trial of 776 patients who were immunocompromised with ARF, HFNC produced greater oxygenation improvements than conventional oxygen supply but did not significantly reduce intubation or mortality rates.84
We apply a personalized approach to hypoxemic ARF in patients with hematological malignancies. We aim to restore oxygenation following the Acute Respiratory Distress Syndrome Network guidelines, to maintain SpO2 between 88% and 95%. We also aim to alleviate dyspnea, reduce tachypnea, and enhance patient comfort. If standard oxygen supply achieves these goals, it is continued. Otherwise, HFNC therapy is initiated to elevate the SpO2 to 95%, decrease the breathing rate, and enhance patient comfort.85 Clinicians should avoid hyperoxia, because trials assessing the impact of setting SpO2 > 95% have shown no benefit86,87 but possible harm.88 When these objectives are not achieved, the optimal timing of mechanical ventilation initiation is unknown. However, evidence exists that patients at high risk of failure must be recognized early, because delayed intubation is associated with higher mortality.89,90 In an individual participant data meta-analysis that included 11 087 patients, the crude mortality was 53%, with adjusted survival improving over time. However, time between ICU admission and intubation was a strong predictor of mortality, suggesting a detrimental effect of late oxygenation failure.89
For ward patients with hematological malignancies and hypoxemic ARF, ICU admission criteria must be individualized. In cases of ARF, waiting for an ICU bed for over an hour is independently associated with increased mortality and ICU stay.91,92 Moreover, persistent hypoxemia is associated with long-term cognitive impairment.93 Delayed ICU admission is also associated with lower survival in patients with hematological malignancies.26 Factors to consider when assessing each patient include the response to standard oxygen supply, required oxygen flow rate, associated organ dysfunctions, and possible need for bronchoscopic-bronchoalveolar lavage. We advocate early ICU admission for patients with hematological malignancy with ARF for several reasons. First, those requiring >6 L of standard oxygen supply face a 40% intubation and 30% mortality rates, necessitating the unique monitoring provided in ICUs.76,77,84 Second, although HFNC therapy in wards may be considered when ICU beds are scarce, it deprives patients of care by a team experienced in optimal intubation timing tests.69 Fourth, in patients receiving >6 L of oxygen, delayed hospital-to-ICU admission independently predicted mortality in a post hoc analysis of data from a randomized controlled trial.26 Last, the added workload on ward health care staff caring for patients with ARF might compromise the quality of care for other ward patients.
Cases 3 and 4 (continued)
Case 3
The diagnostic was confirmed. HFNC therapy was well tolerated. On day 12, the patient returned to room air. Induction chemotherapy rapidly improved the respiratory status, although the infiltrates persisted for 3 months.
Case 4
During bronchoscopic-bronchoalveolar lavage on standard oxygen supply (12 L/min via a Venturi mask), desaturation and tachycardia developed rapidly. The bronchoscopy was stopped, and the patient was intubated. The diagnosis of invasive aspergillosis was strongly suspected based on extensive tracheobronchial-tree inflammation with a membrane overlaying the mucosa (Table 1). A sample of the mucosa contained filaments further identified as Aspergillus fumigatus.
Short-term mortality and longer-term outcomes
The survival of patients with hematological malignancies who are critically ill has significantly increased over the past 2 decades, including subgroups once deemed unfit for ICU care.94 Several mortality predictors have become less relevant.95 Neutropenia, once crucial for diagnostics and treatment decisions, is now unrelated to mortality and does not argue against ICU admission.96,97 The characteristics of the malignancy are no longer associated with short-term mortality.98 Severity scores, although useful for patient assessment, should not guide individual decision-making.96 Substantial survival improvements have been reported in patients who were provided mechanical ventilation.35 If not delayed, intubation is no longer futile,89 as far as a comprehensive diagnostic workup is made. If late intubation is necessary, involving patients and relatives in the decision-making process is crucial, acknowledging the significantly poor prognosis and loss of autonomy at this stage.
ICU survivors with mechanical ventilation may experience lasting physical issues (pulmonary complications, muscle atrophy, weakness, dental problems, and sensory impairments) and mental challenges (diminished quality of life, increased risk of posttraumatic stress disorder, and various psychological and neurocognitive impairments, including memory deficits, attention problems, concentration difficulties, and reduced mental processing speed).99-101
ARF in hematological malignancies disrupts treatment schedules,9,98 but collaborative efforts among intensivists, hematologists, and all crossdisciplinary specialists help patients stay fit for chemotherapy. Importantly, ICU survivors achieve long-term survival and complete remission rates comparable with those never admitted to the ICU.30
As per Table 3, mortality-associated factors fall into 4 groups: general patient characteristics, history of the underlying hematological malignancy (eg, progression or uncontrolled graft-versus-host disease [GVHD]), characteristics of ICU admission (ICU case volume, time to ICU admission, and severity scores) and, finally, in-ICU–acquired events (eg, response to intensive care, late intubation, invasive aspergillosis, or multiorgan dysfunction).
Factors associated with mortality in patients with hematological malignancy with ARF
| General characteristics |
| Age102-104 |
| Performance status98 |
| Frailty∗35 |
| Structural lung disease (COPD and chronic interstitial pneumonia)31 |
| Charlson comorbidity index2 |
| History of the hematological malignancy |
| Uncontrolled or refractory disease2,98,105 |
| Allogeneic stem cell transplantation with uncontrolled GVHD35 |
| At the time of ICU admission |
| Time between hospital and ICU admission26,104,106 |
| Admission to a low volume ICU107-110 |
| Severity scores2,102,104,111-114 |
| Hypoxemia (PaO2/FiO2 < 100)35 |
| Throughout the ICU stay |
| Development of nonrespiratory organ dysfunction102,113,115 |
| NIV failure105,114,116 |
| Delayed intubation89 |
| Driving pressure (in patients with ARDS)†117 |
| Low platelet count (in patients receiving ECMO)118 |
| Diagnosis of invasive fungal infection2,35 |
| No use of voriconazole in patients with invasive pulmonary aspergillosis119,120 |
| Undetermined ARF etiology60 |
| Prolonged ICU length of stay113 |
| General characteristics |
| Age102-104 |
| Performance status98 |
| Frailty∗35 |
| Structural lung disease (COPD and chronic interstitial pneumonia)31 |
| Charlson comorbidity index2 |
| History of the hematological malignancy |
| Uncontrolled or refractory disease2,98,105 |
| Allogeneic stem cell transplantation with uncontrolled GVHD35 |
| At the time of ICU admission |
| Time between hospital and ICU admission26,104,106 |
| Admission to a low volume ICU107-110 |
| Severity scores2,102,104,111-114 |
| Hypoxemia (PaO2/FiO2 < 100)35 |
| Throughout the ICU stay |
| Development of nonrespiratory organ dysfunction102,113,115 |
| NIV failure105,114,116 |
| Delayed intubation89 |
| Driving pressure (in patients with ARDS)†117 |
| Low platelet count (in patients receiving ECMO)118 |
| Diagnosis of invasive fungal infection2,35 |
| No use of voriconazole in patients with invasive pulmonary aspergillosis119,120 |
| Undetermined ARF etiology60 |
| Prolonged ICU length of stay113 |
ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation.
Frailty is state of decreased physiologic reserve that heightens vulnerability to acute stressors.129
Driving pressure (plateau pressure minus positive end expiratory pressure) is used to optimize mechanical ventilation by providing lung-protective ventilatory adapted to the size of the aerated lung.
Five points deserve special attention. First, admission to ICUs that manage larger numbers of patients with hematological malignancy with ARF is associated with better survival,107-109 emphasizing the significance of collaborative approaches involving hematologists and intensivists. For patients with hematological malignancy admitted to nonspecialized ICUs, daily high-quality communication between hematologists and intensivists is crucial, even when the acute disease is identified and no hematological treatment is required.2,110,121 Second, in allogeneic stem cell transplantation recipients, uncontrolled GVHD remains a risk for poor mechanical ventilation outcomes. When GVHD is controlled or in early stages, substantial survival rates persist.122,123 Third, patients with hyperleukocytic AML are at higher risk for both ICU admission and mortality.53,124 However, biological signatures125 and therapeutic targets126 have shown promise in improving early survival.53,108,109 Large-scale, multicenter studies are needed to evaluate interventions, including the individualized use of ICU resources. Fourth, in patients with hematological malignancy, older age is a mortality risk factor, but it should be assessed with other frailty markers. Fit elderly patients often have better outcomes than younger but frail patients.
Lastly, not all patients receive full-code status, because individual preferences and care goals guide decisions. Some patients undergo a 7- to 10-day ICU trial for treatment assessment, necessitating multidisciplinary discussions and family-centered care to avoid premature or delayed decisions affecting survival or prolonging nonbeneficial care and suffering. Through collaboration, intensivists, hematologists, and crossdisciplinary specialists recognize situations leading to a fatal outcome (eg, persistent multiorgan dysfunction, refractory hypoxemia from invasive fungal infection, or relapsing malignancy) and those in which ICU survivors are healthy enough to pursue an anticancer treatment resembling the initial plan.
Conclusions
Patients with hematological malignancies and hypoxemic ARF, notably common in acute leukemia and postallogeneic stem cell transplantation, necessitate a personalized diagnostic and treatment approach. The paramount importance of high-quality collaboration between hematologists, intensivists, and crossdisciplinary health care workers cannot be overstated. A meticulous bedside assessment ensures optimal initial treatment and diagnostic selection. Crucially, maintaining SpO2 levels between 88 and 95 through effective oxygen therapy is vital for a safe and efficient diagnostic strategy. Research is needed to determine the optimal timing for endotracheal intubation. Efforts should be directed toward identifying the cause of ARF. ICU admission, seen as a bridge to curative treatment, aims to discharge patients who are fit for optimal anticancer treatments, enhancing survival in those with hematological malignancies and ARF.
Authorship
Contribution: E.A. led the manuscript preparation; and J.M. and V.L. contributed to each different step of the manuscript preparation and to the cases and images that are shown.
Conflict-of-interest disclosure: E.A. has obtained research funding for his institution from Alexion and MSD Avenir and has received lecturing fees (2020-2023) from Sanofi, Alexion, Gilead, and Pfizer. J.M. reports personal fees and nonfinancial support from MSD, F2G, and Cidara; and grants, personal fees and nonfinancial support from Pfizer Inc and Gilead Sciences. V.L. declares no competing financial interests.
Correspondence: Elie Azoulay, Médecine Intensive et Réanimation, Assistance Publique–Hôpitaux de Paris, Hôpital Saint-Louis, Paris-Cité University, 1 ave Claude Vellefaux, 75010 Paris, France; email: elie.azoulay@aphp.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal