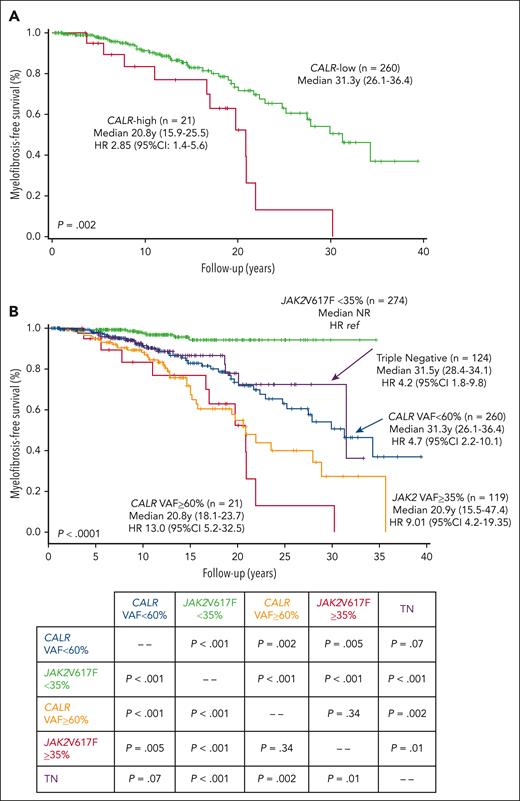
Among 281 patients with essential thrombocythemia and calreticulin (CALR) mutation, we found a variant allele frequency of ≥60% to be associated with significantly shortened myelofibrosis-free survival, mostly apparent with CALR type-1 and CALR type-indeterminate mutations.
TO THE EDITOR:
A mutation in calreticulin (CALRm) is found in 30% of essential thrombocythemia (ET) patients.1,2 There are 2 CALRm types, a 52-bp deletion (type 1/like; CALR-1) and a 5-bp insertion (type 2/like; CALR-2), plus other rare variants (CALR-indeterminate). In patients with myelofibrosis (MF), a high CALR variant allele frequency (VAF [set at ≥55% by receiver operating characteristic (ROC) analysis])3 was associated with features of more advanced disease (anemia, circulating CD34+ cell counts, ASXL1 mutation, ≥2 mutated myeloid genes, and shorter leukocytosis-free survival) compared with lower VAF.3 A VAF >50% may originate from uniparental disomy or copy-neutral loss of heterozygosity.4
The aim of this study was to analyze the impact of CALRm VAF in a cohort of 281 patients with CALRm, unbiasedly retrieved from databases of the Center Research and Innovation of Myeloproliferative Neoplasms (Florence), centers of the Quebec Myeloproliferative Neoplasms (MPN) Research Group (Canada) and Mayo Clinic (Rochester, MN). For comparison, data for 393 patients with JAK2V617F mutated and 124 patients with triple-negative (TN) ET, and 55 patients with CALRm post-ET-MF (PET-MF), were retrieved from Center Research and Innovation of Myeloproliferative Neoplasms database. Diagnosis of ET was confirmed according to 2022 World Health Organization and International Consensus Classification criteria to avoid inadvertent inclusion of prefibrotic-MF5-7; PET-MF diagnosis was according to International Working Group-Myeloproliferative Neoplasms Research and Treatment criteria.8 Using DNA from purified granulocytes collected at diagnosis or <1 year in the absence of active treatment, CALRm VAF, determined by capillary gel electrophoresis of polymerase chain reaction (PCR)-amplified fragments, was expressed as the ratio (percentage) of interpolated areas under-the-curve of CALRm/CALRm + CALRwt3; quantification of JAK2V617F VAF was by real-time quantitative-PCR and digital PCR amplification. A panel of 45 myeloid neoplasm-associated genes was sequenced by next generation sequencing.9 The nonparametric Wilcoxon rank-sum test, the Kaplan-Meier estimate of survival and the log-rank test were used as appropriate. Continuous variable analysis (ROC curve) was used to identify the threshold VAF level eventually resulting in correlation to anyone of the following outcomes: progression to PET-MF, transformation to blast phase (BP), thrombosis, hemorrhage, and overall survival (OS). Reported P values were 2-sided, and P < .05 was considered statistically significant.
Characteristics of study population are reported in Table 1. A total of 152 (54%) patients were CALR-1, 101 (36%) CALR-2, and 28 (10%) CALR-indeterminate. The mean (± standard deviation) VAF was 40.8% (±15.3%) for the entire population, 40.4% (±15.7%) for CALR-1, 40.1% (±13.7%) CALR-2, and 47.2% (±19.0%) CARL-indeterminate (supplemental Table 1, available on the Blood website). During a median follow-up of 8.6 years, 25 patients (8.9%) died; OS at 10, 20, and 30 years was 92%, 85%, and 75%. Fifty patients (17.8%) progressed to PET-MF after a median of 14.6 year, and 2 patients (0.7%) transformed to BP. Thrombosis (14 arterial, 3 venous) occurred in 17 (6.0%) patients coincident with or immediately preceding the diagnosis of ET and 35 new events (54.3% arterial) were noted in 28 (10.0%) patients during the follow-up. Major hemorrhage occurred in 4 patients (2.0%). Analysis of clinical and hematologic features of patients with CALRm according to CALRm type discovered significant differences regarding older age in CALR-indeterminate (P = .014), male sex predilection in CALR-1 (P = .04) and higher platelet count in CALR-2 (P = .001), in line with previous reports10-12 (supplemental Table 1).
Interrogation of the impact of CALRm VAF on major clinical outcomes (PET-MF, BP, thrombosis, bleeding, and OS) highlighted significant correlation of CALRm VAF ≥60% (determined by ROC analysis) with MF-free survival (MFS), with high risk (HR) of 2.85 (95% confidence interval [CI], 1.4-5.6; P = .002; Figure 1A). Conversely, CALRm VAF as continuous variable did not correlate with thrombosis, bleeding, BP, or OS. We therefore used a threshold of ≥60% to categorize patients as CALR-high and CALR-low and compare their phenotype at diagnosis and the outcomes during follow-up.
Myelofibrosis-free survival curves. (A) Kaplan-Meier curves for MFS of patients categorized as CALR-low and CALR-high VAF (CALRm VAF <60% and ≥60%, respectively) are presented. (B) Kaplan-Meier curves for MFS are presented for patients with CALR-low and CALR-high, as well as patients with ET with JAK2V617F mutation, stratified by their VAF <35% or ≥35%, as in Guglielmelli et al9, and patients with TN. The significance levels for comparison of different patient cohorts are reported in the lower table.
Myelofibrosis-free survival curves. (A) Kaplan-Meier curves for MFS of patients categorized as CALR-low and CALR-high VAF (CALRm VAF <60% and ≥60%, respectively) are presented. (B) Kaplan-Meier curves for MFS are presented for patients with CALR-low and CALR-high, as well as patients with ET with JAK2V617F mutation, stratified by their VAF <35% or ≥35%, as in Guglielmelli et al9, and patients with TN. The significance levels for comparison of different patient cohorts are reported in the lower table.
A total of 21 patients (7.5%) were CALR-high and were enriched in CALR-indeterminate variants: 28.6% of CALR-high vs 8.5% of CALR-low (P = .008). At baseline, the 2 cohorts did not differ as regarded age, sex, symptoms, splenomegaly, thrombosis, International Prognostic Score for Essential Thrombocythemia (IPSET) revised-thrombosis score,13 IPSET-survival score,14 and bleeding. Leukocyte and platelet counts did not differ, whereas hemoglobin levels were lower (12.8 g/dL; range, 8.4-14.0) in CALR-high compared to CALR-low (13.6 g/dL; range, 10.1-16.4; P = .02) (Table 1). Finally, we noticed that mutations in myeloid genes were enriched in patients with CALR-high (66.7% vs 30.2%; P = .013).
No difference was noticed in thrombosis, bleeding, BP, and death during follow-up. Transformation to PET-MF occurred in 17.8% (n = 50) of the whole population, 52.4% of CALR-high (n = 11), and 15.0% (n = 39) of CALR-low (P < .001); MFS was significantly shorter in patients with CALR-1 (HR, 2.0; 95% CI, 1.0-3.9; P = .04) and CALR-indeterminate (HR, 2.7; 95% CI, 1.1-6.5; P = .03), using CALR-2 as the reference. There was an impact of a CALRm VAF ≥60% on MFS regardless of the type of CALRm (P = .017). Anemia developed in 69.2% of patients with CALR-high vs 18.3% of patients with CALR-low, leukoerythroblastosis in 46.2% vs 7.8%, and palpable splenomegaly in 50.0% vs 12.1% (P < .001 for all). The median anemia-FS in CALR-high was 17.8 years (14.9-20.6) vs 32.1 years (24.3-39.8; HR, 2.9; 95% CI, 1.3-6.3) in CALR-low, leukoerythroblastosis-FS 20.0 years (16.6-23.4) vs not-reached (HR, 3.71; 95% CI, 1.3-10.5) and splenomegaly-FS 21.0 years (13.4-28.6) vs not-reached (HR, 3.40; 95% CI, 1.3-9.3) (all, P < .01) (supplemental Figure 1). In a multivariate analysis for MFS in which CALR VAF (≥60% and <60%) was added to variables (age, sex, splenomegaly, symptoms, and CALR-type) previously reported as being independently associated with PET-MF in a multicenter study of 1607 patients,15 only male sex and CALR VAF ≥60% remained significant (P = .04 and P = .005, respectively). Similarly, inclusion of the IPSET revised-thrombosis score or IPSET-survival score did not affect the association of CALR VAF with PET-MF (not shown in detail). We also analyzed the role of additional mutations in 34 informative patients who transformed to PET-MF; 7 of 8 patients with CALR-high with additional mutations transformed to PET-MF (87.5%) compared to 18 of 26 CALR-low (69.2%; P = .7).
Finally, we compared MFS of patients with CALR-high and CALR-low with JAK2V617F mutated (stratified by VAF <35% and ≥35%, as in15) and patients with TN (Figure 1B). The resulting Kaplan-Meier curves resulted significantly different (P < .0001), with median survival ranging from not-reached for JAK2V617F <35% to 31.5, 31.3, 20.9, and 20.8 years for TN and JAK2V617F ≥35% for CALR-low and CALR-high, respectively (see Figure 1B for respective HRs using JAK2V617F <35% as reference category). Therefore, CALR-high and JAK2V617F ≥35% constituted a “high-risk” mutation category for MFS (median, 20.8 years; 15.5-47.4) that differed significantly from a “low-risk” category comprising CALR-low and JAK2V617F <35% (34.3 years; 15.9-25.7) (P < .0001). Of note, patients with TN had MFS similar to the “low-risk” category (31.5 years; 28.4-34.1) (Figure 1B).
In conclusion, a CALRm VAF ≥60% is associated with greater risk of and shorter time to MF progression in patients with ET, similar to patients having higher JAK2V617F VAF (set at ≥35%). A role for JAK2V617F VAF on MF progression was reported also in polycythemia vera.16,17 Therefore, accumulation of CALR mutated alleles might contribute to PET-MF progression, as indirectly supported by the higher CALR VAF (56.0% ± 11.8%) found in 55 patients with PET-MF vs 281 patients with ET (40.8% ± 15.3%; P < .001) (supplemental Figure 2). In a small series of 38 patients with ET, those in the fourth quartile (VAF >48.2%) had a 6.7 greater HR of PET-MF.18 An association between MF progression and CALR VAF increase, independent of additional mutations, was demonstrated in 45 patients with ET studied longitudinally.19 Evidence regarding allele accumulation in relation to MF progression in ET might eventually support a rationale for CALR VAF as surrogate end point in clinical trials. Finally, whether achieving meaningful reduction of the burden of CALR mutated cells with target therapy, such as a novel monoclonal antimutant CALR antibody,20 will translate in improved MFS, remains to be assessed.
Acknowledgments
This study received financial support from the Associazione Italiana per la Ricerca sul Cancro (AIRC) (grant type 5×1000) “Metastatic disease: the key unmet need in oncology” to MYNERVA (MYeloid NEoplasms Research Venture AIRC), project #21267. P.G., M.B., and E.N. were also supported by the Ministero della Salute, Rome, Italy (Finalizzata 2018, NET-2018–12365935, Personalized medicine program on myeloid neoplasms: characterization of the patient’s genome for clinical decision making and systematic collection of real world data to improve quality of health care).
Authorship
Contribution: P.G., N.S., N.G., L.B., A.T., and A.M.V. designed research, analyzed and interpreted data, and wrote the manuscript; C.M., M.B., E.N., I.S., and G.R., performed molecular characterization; L.B., O.K., M.A., A.D., G.C., A.A., G.G.L., and M.H. collected clinical data; and all authors checked and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alessandro M. Vannucchi, Department of Experimental and Clinical Medicine, University of Florence, Center Research and Innovation of Myeloproliferative Neoplasms, Azienda Ospedaliero-Universitaria Careggi, Largo Brambilla, 3 pad 27B, 50134 Florence, Italy; email: a.vannucchi@unifi.it.
References
Author notes
P.G., N.S., N.G., A.T. and A.M.V. contributed equally to this study.
Data are available on request from author Paola Guglielmelli (paola.guglielmelli@unifi.it).
The online version of this article contains a data supplement.