Smoldering multiple myeloma (MM) is an asymptomatic clonal plasma cell condition considered as a premalignant entity that may evolve over time to symptomatic MM. Based on a “poorly defined” risk of progression, some well-intended investigators proposed prospective interventional trials for these individuals. We believe this may be a harmful intervention and favor a close “wait and watch” approach and rather enroll these patients in dedicated observational biological studies aiming to better identify patients who will evolve to MM, based on their plasma cells' biology, including genomics, epigenetics, and the immune microenvironment.
Introduction
Smoldering multiple myeloma (SMM) is a biological entity defined in the 1970s,1 characterized by the bone marrow expansion of monoclonal plasma cells in otherwise asymptomatic individuals. The definition requires 10% to 60% plasma cells on a bone marrow biopsy or aspirate or the detection of a monoclonal immunoglobulin in the blood >30 g/L, in the absence of disease defining events.2 The iStopMM study, an Icelandic nationwide screening study for myeloma precursor conditions recently reported that the prevalence of SMM in the total population was 0.53% (95% confidence interval [CI], 0.49-0.57) in individuals aged ≥40 years.3 It is considered as a precancerous state, with some of these individuals progressing to symptomatic diseases such as MM or AL amyloidosis. However, the rate of this transformation exhibits nonlinearity over time, being higher in the first 5 years (∼10% per year) but significantly lower thereafter (1%-3% per year).2 As a precancerous entity, the immediate question is whether it is possible to prevent the SMM evolution to symptomatic, life-threating diseases by offering treatments to these asymptomatic individuals. Eradicating the monoclonal plasma cells before they lead to end-organ damages would be the optimal goal of interventional trials dedicated to SMM.
Where are we starting from?
To avoid overtreatment of individuals who may never require any therapy, researchers have endeavored to develop prognostic models capable of identifying those considered high-risk SMM. To date, 2 recognized models are available.4,5 The Spanish PETHEMA group in 2007 proposed a model based on the percentage of phenotypically abnormal plasma cells and immunoparesis.4 More recently, the Mayo Clinic proposed the so-called 20/2/20 model, based on bone marrow plasmacytosis, monoclonal immunoglobulin levels in the blood, and the serum free light chain ratio.5 These 2 models classify patients into low-, intermediate-, and high-risk SMM; however, there is a very poor concordance between the 2 classifiers.3,6 Furthermore, when examining patients classified as “high risk,” it becomes very evident that not all patients genuinely exhibit a high-risk profile, with 20% to 25% not progressing to MM at 5 years. Of note, applying 2 of the high-risk criteria from the 20/2/20 model or the International Myeloma Working Group logistic regression score model7 to the recently proposed more comprehensive PANGEA model8 yields a 2-year risk of progression of only 25%, as opposed to 44% and 51% with the aforementioned 2 models. Hence, it is clear that the current prediction models are largely inaccurate, likely due to their plasma cell–centric nature and their failure to account for the significant roles palyed by both adaptive and innate immunity in controlling smoldering disease.9-13
Notwithstanding these significant limitations of the currently utilized risk prediction models, well-intentioned investigators have advocated for treatment in the case of these patients with “high-risk” SMM. The Spanish PETHEMA group has been a pioneer in this field with the QUIREDEX trial.14 They randomized 119 patients with high-risk SMM (based on the Spanish model) to lenalidomide-dexamethasone for 2 years (57 patients) vs observation (62 patients), with time to progression to MM as the study primary end point. Indeed, this objective was achieved, and the study findings served as the foundation for the development of numerous trials in this population. However, it is essential to consider some of the limitations of this trial, including: (1) the relatively small sample size, (2) the inclusion of patients who are now classified as symptomatic MM according to the new disease criteria,15 and (3) the lack of statistical power to analyze the effect on overall survival (OS).
What are the treatment objectives?
Proponents of treating patients with SMM often draw an analogy from the treatment of precancerous conditions such as ductal carcinoma in situ. However, such analogy is arguably flawed because the treatment of ductal carcinoma in situ is curative and limited to surgery with a defined course of radiation therapy in some cases. The same concept applies for a dysplastic colonic polyp or precancerous skin lesions that are eradicated with a minor surgical intervention. This is clearly not the case with SMM. On the ClinicalTrials.gov website, 54 interventional trials involving individuals with SMM are currently listed. However, only a few have already been published. All these trials have response rates, minimal residual disease (MRD), and/or progression free survival (PFS; which includes progression to MM) as primary end points, aiming to delay the occurrence of organ damage such as renal failure or vertebral fractures. Most of them are randomized trials, comparing the experimental approach with observation or lenalidomide-dexamethasone. Of note, 2 academic trials (GEM-CESAR NCT02415413 and ASCENT NCT03289299) are single-arm, phase 2 studies, with a fairly aggressive intervention, including autologous transplant in the Spanish CESAR trial. In the published trials to date, the primary end points such as response rates and PFS were successfully achieved. However, this outcome was entirely anticipated, particularly when comparing treatment with observation, as seen in the ECOG trial, despite a low response rate of only 50%.16 Thus, we contend that the tested primary end points of these trials may be misleading, particularly when no OS benefit is demonstrated. Importantly, through these experimental interventions and using current classifiers, patients with truly high-risk SMM will inevitably progress to overt MM, with some developing refractoriness to effective anti-MM drugs, whereas those incorrectly categorized as high risk will have undergone harmful therapies devoid of any survival benefit.
What have we learned from this therapeutic approach?
First, defining the primary objective of these trials is a crucial aspect. In a healthy population afflicted by a premalignant condition such as SMM, trials investigating early treatment should prioritize the extension of survival (or even achieving cure), despite the formidable challenge this presents, potentially necessitating decades to accomplish. Choosing overall response rates, MRD, or PFS as primary objectives is misleading. Firstly, these objectives are very easy to achieve especially when comparing treatment vs observation. Secondly, interventions not aimed at curing patients with SMM, such as doublets involving lenalidomide-based treatments or anti-CD38 antibodies, may merely postpone the onset of MM symptoms, with little to no effect on OS. Conversely, some patients, particularly those with aggressive SMM, may acquire drug resistance during these trials, rendering the subsequent use of these drugs in the treatment of overt MM ineffective and reducing the likelihood of achieving durable remissions. Furthermore, exposure of patients with SMM to established anti-MM backbone therapies may exclude them from participating in prospective clinical trials upon progression to MM. Moreover, these interventions will impede the analyses of biological samples collected at the time of overt progression to MM, because these treatments have the potential to alter both the tumoral clone itself as well as its microenvironment.
The second major limitation of these SMM trials is the definition of “high risk SMM.” The current models include 20% to 25% of patients who will never evolve to MM. This point is highly problematic for trials using very aggressive approaches such as in the CESAR (intensive induction, transplant, consolidation, and maintenance) or ASCENT (long intensive quadruplet treatment) trials. The postulated benefit with early intervention in patients with SMM stems from the belief that monoclonal plasma cells would be genomically less complex and likely more susceptible to anti-MM drugs. Although not experimentally demonstrated, this hypothesis is understandable. However, it is problematic to propose such aggressive therapies to patients who would never evolve to symptomatic MM, exposing to potentially harmful and life-threatening toxicities. Furthermore, preliminary communications of these 2 trials showed that the MRD negativity rates were not superior to that obtained in overt MM treated with similar treatments such as in the FORTE trial.17 These data put into question the validity of the primary hypothesis, that SMM would be more sensitive to therapy than overt MM and thus more accessible to cure. Finally, trials based on adaptive immunotherapeutic approaches (bispecific antibodies or chimeric antigen receptor T cells) are currently proposed or actively enrolling patients with “high-risk” SMM, whereas the efficacy of such approaches is yet to be established in newly diagnosed MM. The same concern regarding toxicity can be raised as well, especially potentially fatal infections with bispecific antibodies or secondary leukemias/lymphomas with chimeric antigen receptor T cells.
What are the remaining questions?
Probably the most crucial one is whether SMM is a true precancerous condition, more sensitive to drugs used in MM, and whether treating before occurrence of overt MM may eradicate the plasma cell clone. Parallel to this question is whether this concept of SMM is relevant. It has been wisely proposed that this concept is a misunderstanding of the biology of plasma cell malignancies. Some of the SMM could be already true plasma cell malignancies and thus be classified as MM requiring treatment, whereas others are still monoclonal gammopathies of undetermined significance (MGUS), which should be monitored.
To answer this fundamental question, more in-depth biological studies are needed, including phenotypic, genomic, and immune environment analyses. Such studies include the MGUS-like phenotypic classification of monoclonal gammopathies in which 19% of SMM may be reclassified as MGUS-like with low risk of progression to MM.18 Similarly, several studies identified genomic features, beyond fluorescence in situ hybridization (FISH)-based analyses, associated with progression of SMM to MM. Among these genomic features are single nucleotide variants in genes such as TP53, ATM, NRAS, KRAS, MAF, BRAF, NFKBIA, and DIS3 as well as copy number variations with del 14q, del 16q (CYLD), t(4;14), t(14;16), MYC translocations or copy number gains, as well as APOBEC (SBS2+SBS13) mutational signatures.10-13,19-22 Importantly, these genomic studies show that in contrast to their clinical risk score, patients with “high-risk” SMM with genomically indolent disease have low risk of progression.19 Even more pertinent are the emerging immunological studies and immune fitness assessments that are clearly associated with SMM progression to MM but yet to be incorporated into the progression risk evaluations.10-13
From a practical point of view, and until we have validated a highly accurate prediction tool of progression in SMM, the “primum non nocere” approach is a close “watch and see” recommendation. Patients with SMM should receive adequate radiological evaluations using imaging techniques such as low dose whole body tomodensitometry, magnetic resonance imaging, and positron emission tomography. These patients should also be closely followed, at least during the first 5 years, to detect clinical evolution, such as renal deterioration or bone focal lesion appearance.
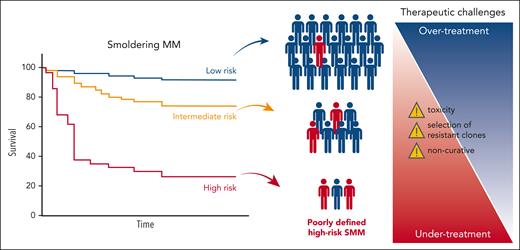
Finally, we believe that most of the currently ongoing or proposed therapeutic trials in SMM are not the appropriate answer to an unresolved question of what defines high-risk SMM (Figure 1). This recommendation against treating SMM gains further significance as some investigators now advocate for treating non–high-risk SMM or even MGUS (NCT06140524).
Schematic depicting the current limitations in treating SMM represented by the poorly defined risk in SMM (blue representing true low risk vs red representing true high risk) as well as some of therapeutic challenges.
Schematic depicting the current limitations in treating SMM represented by the poorly defined risk in SMM (blue representing true low risk vs red representing true high risk) as well as some of therapeutic challenges.
Acknowledgments
H.A.-L. is supported by grants from the ARC Foundation and the Riney Foundation. N.J.B. is supported by grants from the Terry Fox Foundation, International Myeloma Society, Multiple Myeloma Research Foundation, Myeloma Canada, and Leukemia and Lymphoma Society.
Authorship
Contribution: H.A.-L. and N.J.B. designed, wrote, and approved the manuscript.
Conflict-of-interest disclosure: N.J.B. has received research funding from Pfizer, has received speaker’s bureau honoraria from AbbVie, Amgen, Bristol Myers Squibb (BMS), Genentech, Janssen, Pfizer, and Sanofi, and is a consultant/advisory board member for BMS, Janssen, and Pfizer. H.A.-L. declares no competing financial interests.
Correspondence: Herve Avet-Loiseau, IUC-T Oncopole, Unité de Génomique du Myélome, 1, Ave Irene Joliot-Curie, 31059 Toulouse, France; email: avetloiseau.herve@iuct-oncopole.fr.