In this issue of Blood, te Vrugt et al report recurrent T-cell receptor Β (TRB)::NOTCH1 fusions in a large cohort of patients with T-cell lymphoblastic lymphoma (T-LBL), with a notable absence in patients with T-cell acute lymphoblastic leukemia (T-ALL).1 In contrast to activating NOTCH1 mutations, the authors demonstrate that TRB::NOTCH1 translocations confer a poor prognosis through an increased relapse risk in T-LBL.
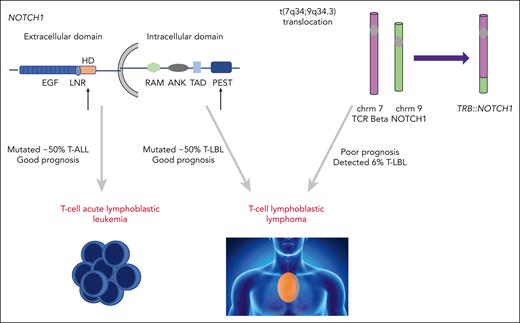
NOTCH1 is a transmembrane protein with a critical role in T-cell development. Upon ligand binding, full-length NOTCH1 is cleaved to release intracellular NOTCH (ICN) from the cell membrane. ICN then translocates to the nucleus and activates a plethora of target genes, with MYC being the most noteworthy. Activating NOTCH1 mutations are highly prevalent in both T-ALL and T-LBL, occurring in just over half of both malignancies (see figure).2-4 The mutations commonly affect the extracellular heterodimerization domain and/or the intracellular PEST domain. Although there have been discrepant reports regarding the prognostic implications of activating NOTCH1 mutations in T-ALL, the majority of studies associate such lesions with an improved outcome in both T-ALL and T-LBL.5-7
Activating NOTCH1 mutations occur in over 50% of T-ALL and T-LBL cases. The majority of these occur in the heterodimerization or PEST domain (black arrows) and are associated with favorable outcomes in T-ALL and T-LBL. The translocation of NOTCH1 gene to TRB locus t(7;9) results in aberrant NOTCH activation. te Vrugt et al show this fusion is identified in T-LBL, but not T-ALL, and its presence correlates with higher relapse risk. chrm, chromosome; TCR, T-cell receptor.
Activating NOTCH1 mutations occur in over 50% of T-ALL and T-LBL cases. The majority of these occur in the heterodimerization or PEST domain (black arrows) and are associated with favorable outcomes in T-ALL and T-LBL. The translocation of NOTCH1 gene to TRB locus t(7;9) results in aberrant NOTCH activation. te Vrugt et al show this fusion is identified in T-LBL, but not T-ALL, and its presence correlates with higher relapse risk. chrm, chromosome; TCR, T-cell receptor.
The TRB::NOTCH1 fusion was first described over 30 years ago in 3 cases of T-ALL, providing early evidence for the role of aberrant NOTCH1 signaling in T-cell leukemogenesis.8 An intronic breakpoint in NOTCH1 (chromosome 9q34.3) places the truncated ICN1 under the control of the TCR-B promoter (chromosome 7q34), leading to constitutive production of ICN1. Notably, material from 2 of the 3 TRB::NOTCH1-positive cases in this initial report, as well as the TRB::NOTCH1-positive SUP-T1 cell line, was collected from malignant pleural effusions, giving the first indication these translocations may be more prevalent in T-LBL than T-ALL.
Given the rarity of both conditions, te Vrugt et al report an impressively large number of patients in their study (192 T-LBL, 167 pediatric T-ALL). The authors developed a multiplexed polymerase chain reaction–based assay that detects the presence of the translocation with high sensitivity and specificity, with clear potential for broad-scale rollout in diagnostic laboratories. They show that 6% of T-LBL cases harbor a TRB::NOTCH1 translocation, whereas it was not detected in any of the pediatric T-ALL cohort. Interestingly, its presence in T-LBL correlated with a markedly lower event-free survival, occurring as a result of a high relapse rate of 67%. Identifying prognostic markers in T-LBL has proven challenging, thus this finding could provide a novel and simple assay for prognostication in T-LBL. Determining robust prognostic markers may not only enable more directed upfront therapies, but also the potential for deintensification of treatment for those at lowest risk.
The different prevalence of TRB::NOTCH1 in T-LBL vs T-ALL contributes to the ongoing discussion relating to whether T-LBL and T-ALL are distinct entities, or a continuum of the same disease. The curation of carefully biobanked cohorts of these rare diseases, together with advances in sequencing capabilities including spatial transcriptomics, will likely shed further light onto the biological drivers of these phenotypes. Furthermore, the authors raise questions about the context-dependent role of NOTCH1. It remains unclear why TRB::NOTCH1 fusions and NOTCH1-activating mutations have such contrasting prognostic implications. It is possible that these lesions differ in their degree of activation potential, and that ICN1 dose, and in turn MYC levels, have implications for responses to chemotherapy. Further mechanistic work is clearly required to determine the reasons for these differences.
te Vrugt et al have potentially defined a new high-risk subtype of T-LBL through the presence of the TRB::NOTCH1 fusion, which warrants validation internationally across different T-LBL cohorts. Historically poor outcomes in T-ALL have been improved by using early risk-directed treatments. The paucity of effective salvage regimens for both T-LBL and T-ALL necessitates the use of effective upfront treatments. One possible option may include the addition of the proteasome inhibitor bortezomib in this high-risk cohort. Bortezomib has been shown to reverse chemotherapy and glucocorticoid resistance in NOTCH1-overexpressing models.9 Interestingly, the Children’s Oncology Group reported that the addition of bortezomib to chemotherapy (modified augmented Berlin-Frankfurt-Munster regimen) improved survival in the T-LBL but not T-ALL cohort.10 Once genomic data are available for this cohort, it will be informative to assess the impact of bortezomib on the outcome of patients with TRB::NOTCH1 positivity.
The LBL 2018 trial (NCT04043494) is currently prospectively examining outcomes of patients according to genomic profile; integrating TRB::NOTCH1 status with other prognostically important lesions, such as those affecting KMT2D, will be important in developing a robust risk score for this disease.5 Determining whether the TRB::NOTCH1 fusion is detectable in cell-free DNA, potentially in quantitative minimal residual disease assays, could also be of clinical utility in such patients. Last, it will be essential to reassess the prognostic relevance of these lesions in the context of novel immunotherapies that are now emerging for T-cell malignancies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.