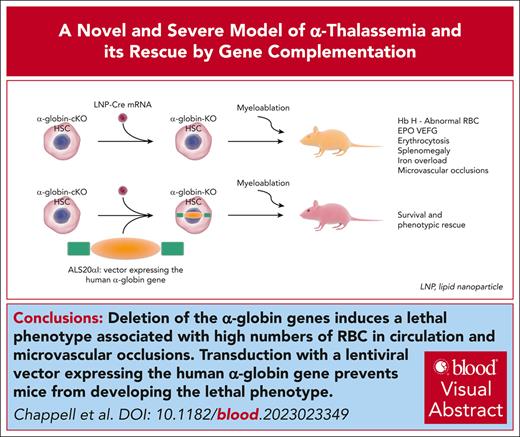
Key Points
Deletion of the α-globin genes induces a lethal phenotype associated with high levels of red blood cells in circulation.
Transduction with a lentiviral vector expressing the human α-globin gene prevents mice from developing the lethal phenotype.
Visual Abstract
α-Thalassemia (AT) is one of the most commonly occurring inherited hematological diseases. However, few treatments are available, and allogeneic bone marrow transplantation is the only available therapeutic option for patients with severe AT. Research into AT has remained limited because of a lack of adult mouse models, with severe AT typically resulting in in utero lethality. By using a lipid nanoparticle (LNP) targeting the receptor CD117 and delivering a Cre messenger RNA (mRNACreLNPCD117), we were able to delete floxed α-globin genes at high efficiency in hematopoietic stem cells (HSC) ex vivo. These cells were then engrafted in the absence or presence of a novel α-globin–expressing lentiviral vector (ALS20αI). Myeloablated mice infused with mRNACreLNPCD117–treated HSC showed a complete knock out (KO) of α-globin genes. They showed a phenotype characterized by the synthesis of hemoglobin H (HbH; also known as β-tetramers or β4), aberrant erythropoiesis, and abnormal organ morphology, culminating in lethality ∼8 weeks after engraftment. Mice infused with mRNACreLNPCD117–treated HSC with at least 1 copy of ALS20αI survived long term with normalization of erythropoiesis, decreased production of HbH, and amelioration of the abnormal organ morphology. Furthermore, we tested ALS20αI in erythroid progenitors derived from α-globin–KO CD34+ cells and cells isolated from patients with both deletional and nondeletional HbH disease, demonstrating improvement in α-globin/β-globin mRNA ratio and reduction in the formation of HbH by high-performance liquid chromatography. Our results demonstrate the broad applicability of LNP for disease modeling, characterization of a novel mouse model of severe AT, and the efficacy of ALS20αI for treating AT.
Introduction
The human α-globin gene cluster is on chromosome 16 (16p13.3).1,2 Per the colinearity rule, α-globin gene loci are arranged according to the order they are expressed during development (5′-ζ-α2-α1–3′), and expression of the HBA2 gene in adults is higher than that of the HBA1 gene.3 α-Globin expression is coordinated by 4 transcriptional enhancers, located between 10 and 48 kilobases upstream of the genes.4-9 In humans, α-globin genes are expressed consistently at high levels beginning at fetal development. Hence, the effects of α-globin gene mutations manifest throughout fetal and adult life.
α-Thalassemia (AT) is caused by insufficient function of the 4 α-globin alleles found in healthy individuals and occurs because of deleted or mutated alleles.10-12 Disease severity in deletional AT is usually proportional to the number of α-globin alleles affected.1,6-11,13-15
Generally, when 3 alleles are affected, patients develop hemoglobin H (HbH) disease that is, in part, characterized by the formation of β-globin homotetramers leading to chronic hemolysis. Patients with HbH survive but may require periodic or chronic blood transfusions. Even when not transfused, many patients with HbH develop iron overload and increased oxidative stress that results in organ injury.16,17
Patients with 4 mutated alleles are affected by Barts hydrops fetalis (BartHF). BartHF is characterized by the formation of γ-chain tetramers, known as Hb Barts, and is generally lethal in utero.1,6-8,10,11,13,14 However, the number of surviving patients with BartHF is increasing because of perinatal and neonatal care advancements. As adults, patients with BartHF predominantly make HbH-producing red blood cells (RBC), requiring chronic blood transfusion for survival.18 Severe forms of AT (rescued BartHF, as well as some forms of HbH) can lead to cirrhosis, hypertension, hypothyroidism, diabetes, and infertility.2,19,20 In many respects, the prognosis for AT resembles that for β-thalassemia.21-23 However, the relationships between HbH production, abnormal erythropoiesis, increased hemolysis, and pathological manifestations have not been fully elucidated.
Over 400 forms of deletional or nondeletional alleles of AT have been identified and documented thus far.24 The considerable diversity of mutations identified is explained by AT carriers being partially protected from Plasmodium falciparum malaria, although in a poorly understood manner.25-27 Consequently, in tropical and subtropical regions of the world (in which malaria is, or was, endemic), the incidence of AT is very high, making it 1 of the most common human genetic diseases.26,27 Historically, AT has been geographically limited to tropical regions and the Mediterranean Basin (eg, Greece), with many cases occurring in Southeast Asia and the Middle East, where AT represents the most prevalent thalassemic condition.28-30 In some geographical areas, the frequencies of patients with AT are similar to, or higher than, those with β-thalassemia.28,31,32 Additionally, geographical areas that received high numbers of immigrants from Southeast Asia now show a higher incidence of AT.33 Thus, AT, which currently has minimal treatment options, represents an increasing public health burden on the medical system, making it an important area of interest in academic and therapeutic research.
Although epidemiological studies are scarce, the number of patients who could benefit from gene therapy applications can be estimated based on available publications. For instance, in the study by Lal et al analyzing 86 patients with HbH, the likelihood of patients with deletional mutations receiving a transfusion by the age of 20 years was 2.8%.34 In contrast, for patients with nondeletional mutations, this likelihood was 13% by age 1 year, 39% by 5 years, 75% by 10 years, and 80% by age 20 years.34 The youngest patient in this study to require a transfusion was aged 3 months and had a Hb level of 6.0 g/dL.31,34,35 Therefore, considering the large number of individuals with mutations or deletions in the α-globin gene cluster, the number of patients with severe HbH that may benefit from allogeneic bone marrow (BM) transplant or gene therapy is expected to be substantial.
Considering that AT is an increasingly widespread condition with the potential to become even more widespread because of immigration, the lack of research regarding its pathogenesis and treatment is surprising and unfortunate. This paucity of information is primarily because of limited mouse models of severe AT, with the only available models that produce viable adults lacking only 2 copies of the α-globin gene (of 4 total alleles) and show a mild phenotype.
Here, we characterize for the first time the complexity of AT in 2 new severe adult mouse models of AT, and generate an effective treatment for this disease.
Materials and methods
BM transplantation
After treatment with lipid nanoparticles (LNP) containing nucleoside-modified messenger RNA (mRNA) coding for Cre recombinase, conjugated to antibodies targeting mCD117 (mRNACreLNPCD117) or lentiviral vector, a minimum of 300 000 viable lineage-negative (lin−) donor cells were resuspended in phosphate-buffered saline (Gibco, Thermo Fisher Scientific) and IV injected into anesthetized lethally irradiated Pepcb/BoyJ CD45.1 recipient mice (8-12 weeks old; The Jackson Laboratory, Bar Harbor, ME) or green fluorescent protein (GFP) recipient mice.36,37 To generate mixed chimeras, CD45.1 GFP− recipient mice received different proportions of mRNACre-LNPCD117–treated lin−Hba-a1-conditional knockout (cKO) hematopoietic stem cells (HSC; AG-KO CD45.2 GFP−) and wild-type (WT) lin− HSC (CD45.2 GFP+). Primary recipient mice were lethally irradiated with 2 administrations of 500 cGy separated by 4 hours, for a total of 1000 cGy, using a X-Rad320 Irradiator (Precision; Madison, CT). Secondary and tertiary transplants were performed using a dose of 10E+06 whole BM cells, intravenously injected into lethally irradiated recipient mice following the same guidelines as for the aforementioned primary transplants.38
Patient samples were obtained after the approval by the Children’s Hospital of Philadelphia institutional review board (protocol 23-020843). All participants gave their informed consent before inclusion in the study. All experiments performed in mice were approved by the institutional animal care and use committee (protocol 1173) at the Children’s Hospital of Philadelphia.
Results
Generation of adult mice affected by severe AT via engraftment of AG-KO cells modified by LNP
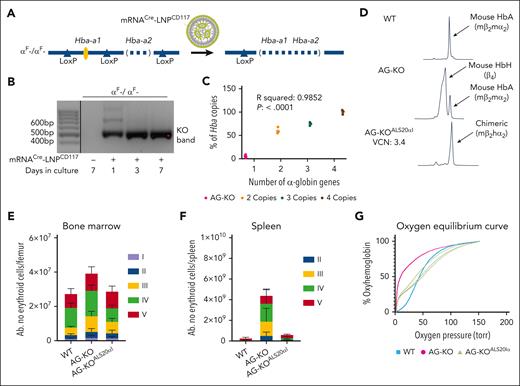
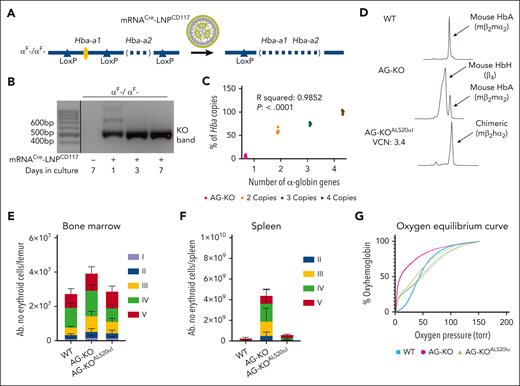
To generate AT mice, we created cKO mice that have been edited to remove the second α-globin gene (Hba-a2) while inserting loxP sites flanking the first α-globin gene (Hba-a1), generating the Hba-a1Flox/Hba-a2KO haplotype (αF- in Figure 1A; supplemental Figure 1A-B, available on the Blood website). Hba-a1Flox/Flox/Hba-a2KO/KO animals show a mild phenotype (Table 1). To achieve complete deletion of the α-globin genes in HSC, we treated lin− cells isolated from Hba-a1Flox/Flox/Hba-a2KO/KO mice (CD45.2) ex vivo with LNP containing nucleoside-modified mRNA coding for Cre recombinase. Furthermore, these LNP were conjugated to antibodies targeting mCD117 (mRNACreLNPCD117; Figure 1A). CD117 is a receptor expressed at high levels on HSC, thus the antibody enhanced targeting of the LNP.42Hba-a1cKO/cKO/Hba-a2KO/KO cells showed the expected deletion of the floxed Hba-a1 genes (Figure 1B). These cells were injected into myeloablated GFP+ or CD45.1 recipient mice to generate animals with BM deletion of the mouse α-globin genes (AG-KO; Figure 1C; supplemental Figure 2).
Characterization of WT, Hba-a1Fl/Fl/Hba-a2KO/KO + mRNACre-LNPCD117 (AG-KO), and HSC Hba-a1Fl/Fl/Hba-a2KO/KO + mRNACre-LNPCD117 + ALS20αI (AG-KOALS20αI) transplant recipients. (A) Scheme showing the Cre-mediated excision of Hba-a1 after administration of mRNACre-LNPCD117. Although administration of mRNACreLNPCD117 offers impressive in vivo recombination, we opted for in vitro treatment and engraftment of isolated lin− cells to ensure the near total gene deletion. (B) Agarose gels showing the deletion of the Hba-a1 (αF) genes in cells collected and kept in vitro after lin− cell collection. Amplification after recombination results in a 496-base pair (bp) product, whereas the absence of recombination (no cells exposed to LNP) does not produce an amplification because of size constraints. The deletion was observed after treatment with mRNACre-LNPCD117 and keeping the cells in culture, as indicated. Only relevant lanes are shown; the full gel is shown in supplemental Figure 37. The black line separates 2 different sections of the gel. (C) Levels of endogenous genomic mouse Hba (Hba-a1 + Hba-a2), after treatment with mRNACre-LNPCD117 measured by droplet digital polymerase chain reaction (ddPCR). The ddPCR assay amplifies any remaining intact genomic α-globin gene, either Hba-a1 or Hba-a2. The residual genomic α-globin amplification in AG-KO level mice is compared with nonrecombined WT control (4 copies of the α-globin genes) and mice carrying 3 or 2 copies of the α-globin genes, shown as a percentage. The WT is set to 100% by default. (D) Cation-exchange HPLC chromatograms of WT, AG-KO, and AG-KOALS20αI mice that received transplantation were analyzed. Absolute counts of erythroblasts in the BM (E) and the spleen (F) include proerythroblasts (I, in purple), basophilic erythroblasts (II, in blue), polychromatic erythroblasts (III, in yellow), orthochromatic erythroblasts and reticulocytes (IV, in green), and RBC (V, in red). Significance was assessed by ordinary 2-way analysis of variance (ANOVA) using Tukey multiple comparisons test (n = 4-6; test described in supplemental Table 1). (G) Oxygen equilibrium curves of WT and AG-KO mice, as well as AG-KOALS20αI with VCN in the range of 1.1 to 2.9, values were extrapolated using the method described in supplementary Materials, in “Oxygen binding affinity.”
Characterization of WT, Hba-a1Fl/Fl/Hba-a2KO/KO + mRNACre-LNPCD117 (AG-KO), and HSC Hba-a1Fl/Fl/Hba-a2KO/KO + mRNACre-LNPCD117 + ALS20αI (AG-KOALS20αI) transplant recipients. (A) Scheme showing the Cre-mediated excision of Hba-a1 after administration of mRNACre-LNPCD117. Although administration of mRNACreLNPCD117 offers impressive in vivo recombination, we opted for in vitro treatment and engraftment of isolated lin− cells to ensure the near total gene deletion. (B) Agarose gels showing the deletion of the Hba-a1 (αF) genes in cells collected and kept in vitro after lin− cell collection. Amplification after recombination results in a 496-base pair (bp) product, whereas the absence of recombination (no cells exposed to LNP) does not produce an amplification because of size constraints. The deletion was observed after treatment with mRNACre-LNPCD117 and keeping the cells in culture, as indicated. Only relevant lanes are shown; the full gel is shown in supplemental Figure 37. The black line separates 2 different sections of the gel. (C) Levels of endogenous genomic mouse Hba (Hba-a1 + Hba-a2), after treatment with mRNACre-LNPCD117 measured by droplet digital polymerase chain reaction (ddPCR). The ddPCR assay amplifies any remaining intact genomic α-globin gene, either Hba-a1 or Hba-a2. The residual genomic α-globin amplification in AG-KO level mice is compared with nonrecombined WT control (4 copies of the α-globin genes) and mice carrying 3 or 2 copies of the α-globin genes, shown as a percentage. The WT is set to 100% by default. (D) Cation-exchange HPLC chromatograms of WT, AG-KO, and AG-KOALS20αI mice that received transplantation were analyzed. Absolute counts of erythroblasts in the BM (E) and the spleen (F) include proerythroblasts (I, in purple), basophilic erythroblasts (II, in blue), polychromatic erythroblasts (III, in yellow), orthochromatic erythroblasts and reticulocytes (IV, in green), and RBC (V, in red). Significance was assessed by ordinary 2-way analysis of variance (ANOVA) using Tukey multiple comparisons test (n = 4-6; test described in supplemental Table 1). (G) Oxygen equilibrium curves of WT and AG-KO mice, as well as AG-KOALS20αI with VCN in the range of 1.1 to 2.9, values were extrapolated using the method described in supplementary Materials, in “Oxygen binding affinity.”
AG-KO animals show high production of RBC that exclusively express HbH
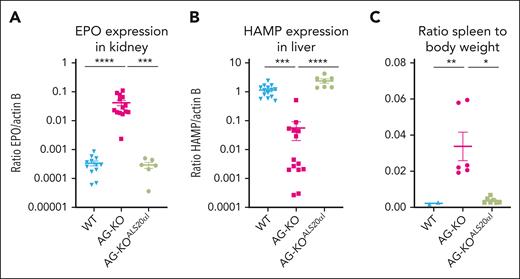
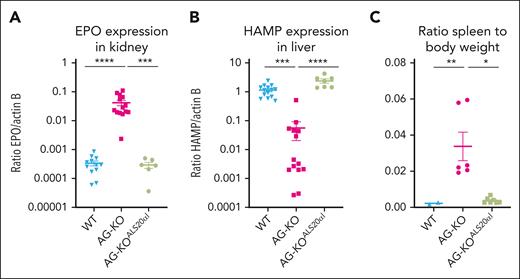
We hypothesized that Hba-a1cKO/cKO/Hba-a2KO/KO or AG-KO derived RBC would almost completely replace WT RBC within 2 months of engraftment.43 Approximately 6 to 8 weeks after transplant, AG-KO mice exhibited high chimerism (>90% donor hematopoietic cells; supplemental Figure 3) with a severe phenotype including low levels of α-globin chains, high levels of β-globin tetramers (HbH) as measured by high-performance liquid chromatography (HPLC; Figure 1D), and the presence of β-globin precipitates on RBC membranes (supplemental Figure 4A). Unexpectedly, and in contrast with the features of animals lacking the β-globin gene, these animals showed significantly elevated hematocrit (68.2% ± 1.2% vs 43.2% ± 1.0% in controls) and RBC (13.0 × 106 ± 0.4 × 106 vs 9.1 × 106 ± 0.2 × 106 cells per μL in controls; Table 1).38 The BM and spleen of AG-KO mice showed a clear expansion in total cellularity (1.5- and ∼10-times more in the BM and spleen, respectively, compared with controls; supplemental Figure 5A), and in the number of progenitor erythroid cells (2.0- and ∼10-times more in the BM and spleen, respectively). In particular, we observed an increase in the number of orthochromatic and reticulocyte cells (Figure 1E-F; supplemental Figures 5B and 6) and abnormal RBC morphology (supplemental Figure 7A). These animals also showed additional abnormal erythroid parameters, including shorter RBC life span (supplemental Figure 7B), high levels of erythropoietin (EPO) mRNA in the kidney (Figure 2A), reduced mean corpuscular Hb (11.4 ± 0.3 pg vs 14.4 ± 0.2 pg in controls) and mean corpuscular Hb concentration levels (21.6 ± 0.2 g/dL vs 30.2 ± 0.4 g/dL in controls), and a 460% increase in reticulocyte count (Table 1).
Evaluation of gene expression and spleen-to-body weight ratios in WT, AG-KO, and AG-KOALS20αIrecipients. (A) EPO gene expression was measured in kidney tissue of WT (n = 12), AG-KO (n = 14), and AG-KOALS20αI (n = 7) animals. (B) Hepcidin (HAMP) gene expression was measured in liver tissue of WT (n = 12), AG-KO (n = 14), and AG-KOALS20αI (n = 6) animals. (C) Ratio of the spleen-to-body weight measured in WT (n = 2), AG-KO (n = 6), and AG-KOALS20αI (n = 7). The mean and standard error of the mean are indicated. Significant differences between groups were measured using a Kruskal-Wallis test with Dunns multiple comparisons test: ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001.
Evaluation of gene expression and spleen-to-body weight ratios in WT, AG-KO, and AG-KOALS20αIrecipients. (A) EPO gene expression was measured in kidney tissue of WT (n = 12), AG-KO (n = 14), and AG-KOALS20αI (n = 7) animals. (B) Hepcidin (HAMP) gene expression was measured in liver tissue of WT (n = 12), AG-KO (n = 14), and AG-KOALS20αI (n = 6) animals. (C) Ratio of the spleen-to-body weight measured in WT (n = 2), AG-KO (n = 6), and AG-KOALS20αI (n = 7). The mean and standard error of the mean are indicated. Significant differences between groups were measured using a Kruskal-Wallis test with Dunns multiple comparisons test: ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P ≤ .0001.
RBC carrying HbH induce systemic hypoxia and high levels of EPO
To further investigate the effect of the RBC expressing HbH on hypoxia, EPO synthesis, and additional parameters, we generated animals with different proportions of AG-KO and WT HSC (supplemental Figure 8). Myeloablated CD45.1 GFP− mice were engrafted mixing AG-KO-CD45.2-GFP− and WT-CD45.2-GFP+ HSC. Donor chimerism in the BM was evaluated by quantifying the level of CD45.1 vs CD45.2 cells (supplemental Figure 9). The proportion of WT vs AG-KO RBC was evaluated in the periphery (AG-KO-GFP− vs WT-GFP+; supplemental Figure 10). Six to 8 weeks after BM transplant, animals that received 100% and 80% AG-KO HSC showed similar nonphysiological complete blood count values, characterized by erythrocytosis, high HCT, and reticulocytosis (supplemental Figure 11). Mice engrafted with 80% AG-KO HSC showed a predominant proportion of GFP− RBC in circulation (supplemental Figure 10). In contrast, animals engrafted with 60%, 40%, and 20% AG-KO HSC showed a large proportion of GFP+ RBC in circulation (supplemental Figure 10) and normalization of the complete blood count parameters (supplemental Figure 11). Accordingly, serum levels of vascular endothelial growth factor (a proxy for hypoxia) were significantly elevated in 100% and 80% AG-KO animals (supplemental Figure 12), and similarly, serum EPO levels were elevated (supplemental Figure 13).44 However, EPO levels in the 80% AG-KO cohort were ∼10-times lower than in the 100% AG-KO cohort (supplemental Figures 13 and 14), suggesting that even a low proportion of normal RBC in circulation can mitigate severe hypoxia and EPO synthesis.
RBC carrying HbH show high oxygen-binding affinity
Using mice expressing GFP− and GFP+ RBC in different proportions, we correlated oxygen equilibrium curves with α:β levels and the Hill coefficient, a parameter to quantify oxygen molecules' positive or negative cooperativity to Hb (supplemental Figure 15A-B). The oxygen-binding affinity of RBC from AG-KO animals was significantly stronger than that of WT animals that received transplants. WT mice that underwent transplantation had a midpoint value of affinity (or p50) of 32.6, whereas AG-KO mice that received transplantation had a p50 of 10.7, indicating that AG-KO RBC have an abnormally elevated oxygen-binding affinity (Figure 1G; supplemental Figure 15A-B). These RBC showed a low Hill coefficient (0.86 in mice engrafted with 100% of AG-KO HSC vs 2.30 in WT; supplemental Figure 15), indicating a strong reduction in cooperativity.45-47
AG-KO animals display iron overload
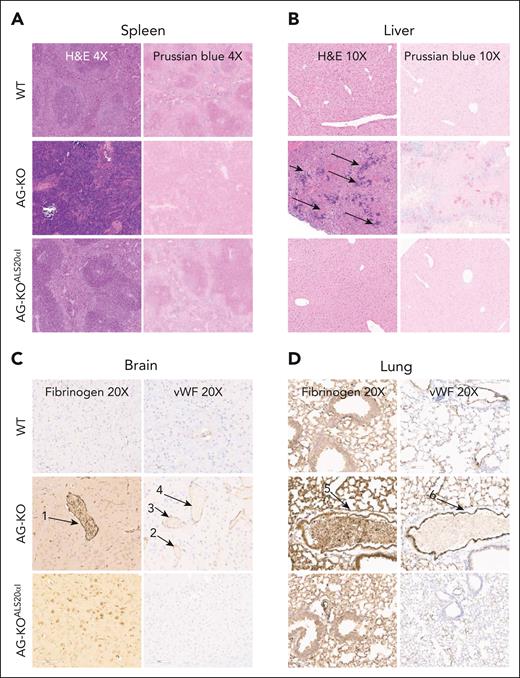
β-Thalassemia patients and mice show iron overload even without blood transfusion, due to the high level of active erythropoiesis, which suppresses hepcidin, a hormone secreted by the liver that controls and limits dietary iron absorption from the duodenum.48-52 Because AG-KO mice showed high erythropoiesis, we hypothesized that they might display dysregulated iron metabolism. In fact, AG-KO mice showed very low levels of hepcidin expression in the liver (<5% of control WT mice; Figure 2B). Organs collected from these animals also showed significant morphological abnormalities. Mice showed splenomegaly (Figure 2C) with a complete breakdown in the division of red and white pulp (Figure 3A; supplemental Figure 16A), with no iron retained in macrophages, consistent with suppression of hepcidin synthesis. Conversely, the liver showed clear signs of iron overload, consistent with low hepcidin levels and increased iron absorption (Figure 3B; supplemental Figure 16A).
Histology of the spleen and liver, and immunohistochemistry of lung and brain tissue from WT, AG-KO, and AG-KOALS20αIrecipient animals. (A) Representative spleens from WT, AG-KO, and AG-KOALS20αI mouse chimeras stained with hematoxylin and eosin (H&E) and Perls Prussian blue, shown at ×4 original magnification. The spleens of WT animals exhibit sites of high iron staining corresponding to the retention of iron in the macrophages. (B) Representative livers from WT, AG-KO, and AG-KOALS20αI recipient animals stained with H&E and Perls Prussian blue, shown at ×10 original magnification. Arrows indicate sites of extramedullary hematopoiesis. (C) Brain tissues from WT, AG-KO, and AG-KOALS20αI mouse chimeras were stained using antifibrinogen and anti–von Willebrand factor (VWF) immunohistochemistry. Numbered arrows indicate sites of possible vessel occlusion. (D) Lung tissue from WT, AG-KO, and AG-KOALS20αI mouse chimeras was stained using antifibrinogen and anti-VWF immunohistochemistry. Numbered arrows indicate sites of possible vessel occlusion. Arrows 1 and 5 indicate sites of fibrinogen staining, whereas arrows 2-4 and 6 indicate sites of high VWF staining.
Histology of the spleen and liver, and immunohistochemistry of lung and brain tissue from WT, AG-KO, and AG-KOALS20αIrecipient animals. (A) Representative spleens from WT, AG-KO, and AG-KOALS20αI mouse chimeras stained with hematoxylin and eosin (H&E) and Perls Prussian blue, shown at ×4 original magnification. The spleens of WT animals exhibit sites of high iron staining corresponding to the retention of iron in the macrophages. (B) Representative livers from WT, AG-KO, and AG-KOALS20αI recipient animals stained with H&E and Perls Prussian blue, shown at ×10 original magnification. Arrows indicate sites of extramedullary hematopoiesis. (C) Brain tissues from WT, AG-KO, and AG-KOALS20αI mouse chimeras were stained using antifibrinogen and anti–von Willebrand factor (VWF) immunohistochemistry. Numbered arrows indicate sites of possible vessel occlusion. (D) Lung tissue from WT, AG-KO, and AG-KOALS20αI mouse chimeras was stained using antifibrinogen and anti-VWF immunohistochemistry. Numbered arrows indicate sites of possible vessel occlusion. Arrows 1 and 5 indicate sites of fibrinogen staining, whereas arrows 2-4 and 6 indicate sites of high VWF staining.
AG-KO animals exhibit microvascular occlusions
As production of abnormal RBC increased after engraftment, AG-KO mice showed features indicative of microvascular occlusion, including increased respiratory rates and stroke-related symptoms, such as spinning or loss of limb use. Upon analysis of AT animals' brains (Figure 3C; supplemental Figure 16B) and lungs (Figure 3D; supplemental Figure 16B), we observed diffuse microvascular occlusions, and high numbers of erythrocytes. Fibrinogen staining was diffusely positive (Figure 3C-D; supplemental Figure 16B, supplemental Figure 17A), indicating activation of coagulation, and we observed von Willebrand factor colocalization with vessel-associated endothelial cells (Figure 3C-D; supplemental Figures 16B and 17A). In line with these observations, plasma of AG-KO mice with a high proportion of GFP− RBC in circulation also showed high levels of thrombin–antithrombin complex concentration (supplemental Figure 17B).
Generation of adult animals affected by severe AT by engraftment of α-KO fetal liver cells
In parallel with, and to confirm the phenotype of, AG-KO mice, we bred animals with a cis deletion of Hba-a1 and Hba-a2.53 Heterozygous mice of this strain are known to have a mild phenotype associated with compensatory reticulocytosis (Table 1), whereas homozygous mice are embryonic lethal.39-41 Fetal liver cells (FLCs) were collected from embryos at day 14.5 gestation and genotyped to identify homozygotes. FLCs from homozygous KO embryos were transplanted into myeloablated recipient animals (indicated as AG-FKO mice; supplemental Figure 18). The AG-FKO chimeras recapitulated the phenotype observed in AG-KO mice, including β-globin precipitates (supplemental Figure 4B), reduction in the synthesis of α-globin chains (supplemental Figure 19) and production of HbH as assessed by HPLC (supplemental Figure 20), abnormal erythroid parameters (supplemental Figure 21), splenomegaly (supplemental Figure 22A), abnormal spleen and liver morphologies, and iron overload, (supplemental Figure 22A-B), culminating in lethality at ∼7 weeks after transplant. However, with this approach, we observed that we needed 10 times more AG-FLC (compared with WT-FLCs) to engraft the recipient mice consistently. This limitation made using this model for downstream applications excessively challenging from a logistical and therapeutic standpoint. Therefore, we focused on the KO model generated by using mRNACreLNPCD117, which provided a consistent source of HSC with high levels of Hba-a1 and Hba-a2 gene deletion.
Generation of lentiviral vectors expressing the human α-globin gene
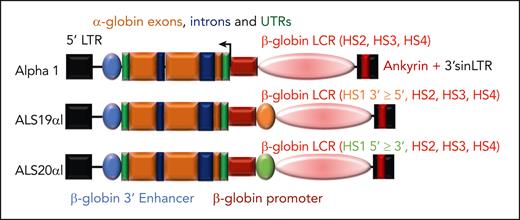
Previous attempts to express the human α-globin gene by lentiviral vectors in embryos of α-globin–KO mice were not successful because of the complexity of the model or the inefficiency of transgene expression.54 Because the regulatory sequences necessary for high levels of α-globin expression are large and thus less able to be contained within a lentiviral vector, we chose to develop vectors making use of regulatory sequences used for β-globin expression, which are well characterized and have been successfully used to achieve high levels of erythroid-specific expression in vitro and in vivo.38,55 This strategy is sound because both α-globin and β-globin have overlapping expression patterns and together form Hb. We postulated that using β-globin gene regulatory elements would result in higher levels of expression given that α-globin chain synthesis involves 4 gene copies whereas β-globin involves only 2. Based on a recent publication from our group, we cloned the α-globin gene into vectors that contain the β-globin promoter and 3′ enhancer, as well as the hypersensitive sites 2, 3, and 4 of the locus control region and the Ankyrin insulator embedded in the 3′ self-inactivating long terminal repeat to generate the α1, ALS19αI, and ALS20αI vectors (Figure 4).56-58 Each vector carried a different-sized locus control region based on our previous studies, with each variant capable of expressing the α-globin gene at a high level. Previous studies (including some from our laboratory) have suggested that including insulator elements reduces integration position effects on expression and genome toxicity, resulting in a safer vector overall.38,56-63
Schematic diagrams of lentiviral vectors expressing the human α-globin gene. Vectors differ in the inclusion and orientation of core sequences of the DNase I hypersensitive sites (HS) of the β-globin locus control region (LCR) but maintain the same short β-globin promoter, 3′ enhancer, and untranslated regions (UTRs). These vectors also use a 3′ self-inactivating LTR (sinLTR) that includes an Ankyrin insulator element and HBA2 genomic sequence. All vectors include HS2, HS3, and HS4 of the β-globin LCR. In addition, ALS19αI includes HS1 (in 3′→5′ orientation), whereas ALS20αI includes HS1 (in 3′→5′ orientation).
Schematic diagrams of lentiviral vectors expressing the human α-globin gene. Vectors differ in the inclusion and orientation of core sequences of the DNase I hypersensitive sites (HS) of the β-globin locus control region (LCR) but maintain the same short β-globin promoter, 3′ enhancer, and untranslated regions (UTRs). These vectors also use a 3′ self-inactivating LTR (sinLTR) that includes an Ankyrin insulator element and HBA2 genomic sequence. All vectors include HS2, HS3, and HS4 of the β-globin LCR. In addition, ALS19αI includes HS1 (in 3′→5′ orientation), whereas ALS20αI includes HS1 (in 3′→5′ orientation).
ALS20αI expresses high levels of the human α-globin gene in mouse and human erythroid cell lines
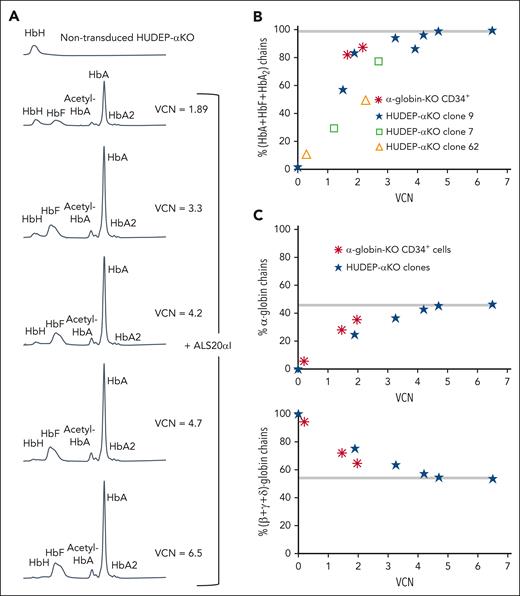
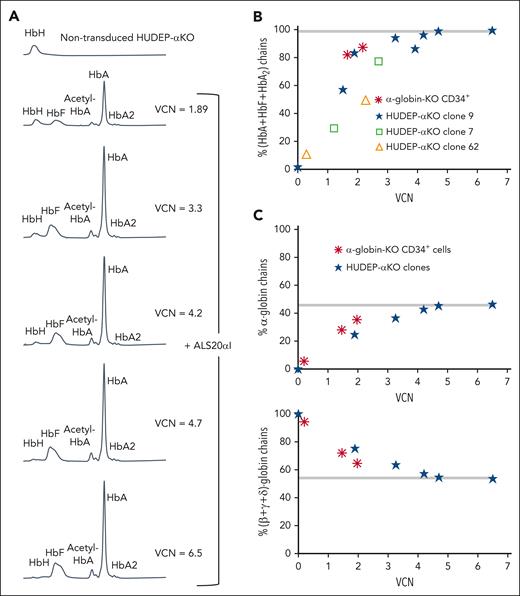
We tested the ability of α1, ALS19αI, and ALS20αI to express the α-globin gene using murine erythroleukemia cells (supplemental Figure 23), and found that ALS20αI expressed the highest level of human α-globin chains and comparable with that of an endogenous murine α-globin gene (supplemental Figure 24). We also used human umbilical cord–derived erythroid progenitor (HUDEP) cells edited using CRISPR-CRISPR–associated protein 9 to remove all endogenous α-globin genes (HUDEP-aKO; supplemental Figure 25) and generate several KO clones. Transduction of clone number 9 with increasing multiplicity of infection of ALS20αI showed a proportional increase in functional Hbs (HbA, HbF, and HbA2; Figure 5A). Functional Hbs increase and vector copy number (VCN) directly correlated in 3 independent HUDEP-αKO clones (Figure 5B). Similarly, cells treated with ALS20αI showed a linear relationship between VCN and increasing amounts of α-globin chains (Figure 5C, top), whereas the relative proportion of β-like chains was normalized (Figure 5C, bottom). Hb tetramers and the ratio of α-globin to (β + γ + δ)-globin chains normalized and reached a plateau with 4 copies of ALS20αI. Based on these observations, it appeared that ALS20αI produced, at 1 VCN, human α-globin proteins equivalent to that of a single endogenous α-globin gene.
Evaluation of the effect of ALS20αI transduction on α-globin–KO cells. (A) Chromatograms from cation-exchange HPLC of hemolysates from nontransduced and ALS20αI–transduced HUDEP-αKO cells (clone 9). In nontransduced HUDEP-αKO cells, only a single peak corresponding to HbH (β4) is visible, because of the lack of all α-globin chains. After transduction, HbA (α2β2) and HbF (α2γ2) peaks become visible, whereas HbH decreases. (B) Correlation between VCN and percent HbA + HbF + HbA2 tetramers in HUDEP-αKO clones and α-globin-KO CD34+–derived erythroid cells. (C) Correlation between VCN and percent α-globin chains (top); correlation between VCN and percent single β-globin-like chains (β + γ + δ; bottom) detected by reversed-phase HPLC in hemolysates from terminally differentiated primary α-KO CD34+ cells or immortalized HUDEP-αKO erythroblast untreated or treated with ALS20αI. Gray bars represent values in healthy human erythroblasts. The values normalize at VCN of ∼4 for both α-globin–KO CD34+ and HUDEP-αKO cells, indicating that 1 copy of ALS20αI can generate as many α-globin chains as 1 endogenous α-globin gene.
Evaluation of the effect of ALS20αI transduction on α-globin–KO cells. (A) Chromatograms from cation-exchange HPLC of hemolysates from nontransduced and ALS20αI–transduced HUDEP-αKO cells (clone 9). In nontransduced HUDEP-αKO cells, only a single peak corresponding to HbH (β4) is visible, because of the lack of all α-globin chains. After transduction, HbA (α2β2) and HbF (α2γ2) peaks become visible, whereas HbH decreases. (B) Correlation between VCN and percent HbA + HbF + HbA2 tetramers in HUDEP-αKO clones and α-globin-KO CD34+–derived erythroid cells. (C) Correlation between VCN and percent α-globin chains (top); correlation between VCN and percent single β-globin-like chains (β + γ + δ; bottom) detected by reversed-phase HPLC in hemolysates from terminally differentiated primary α-KO CD34+ cells or immortalized HUDEP-αKO erythroblast untreated or treated with ALS20αI. Gray bars represent values in healthy human erythroblasts. The values normalize at VCN of ∼4 for both α-globin–KO CD34+ and HUDEP-αKO cells, indicating that 1 copy of ALS20αI can generate as many α-globin chains as 1 endogenous α-globin gene.
Successful rescue of AG-KO animals by ALS20αI
To assess whether ALS20αI was able to prevent the lethal phenotype in AG-KO mice, we treated Hba-a1cKO/cKO/Hba-a2KO/KO cells with both mRNACreLNPCD117 and ALS20αI (supplemental Figure 26). Myeloablated recipient mice received only AG-KO HSC were euthanized out of concern for animal welfare ∼7 weeks after transplant because of the expected severe pathological phenotype. In contrast, mice receiving Hba-a1cKO/cKO/Hba-a2KO/KO treated with both mRNACreLNPCD117 and ALS20αI (AG-KOALS20αI) and with VCN as low as 1.1 survived (Figure 6A), showing high chimerism (supplemental Figure 27), high levels of deletion of the endogenous α-globin genes (Figure 1C), and high levels of chimeric Hb (human-α/mouse-β or hα2mβ2; Figure 1D).
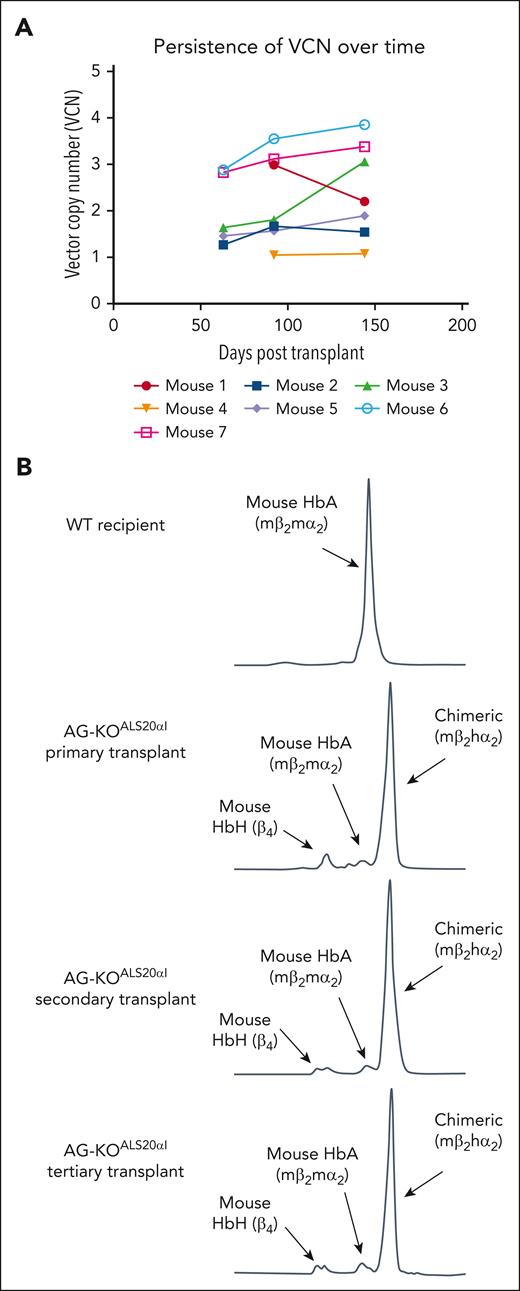
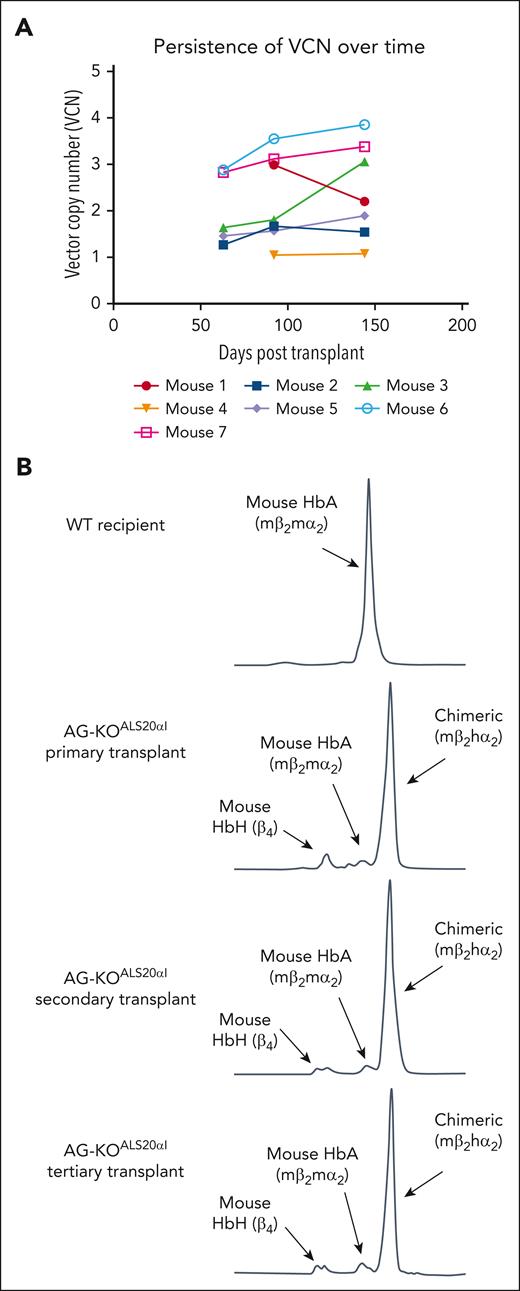
Stable engraftment and expression of human α-globin protein in primary, secondary, and tertiary transplanted mice. (A) The VCN was measured via ddPCR analysis of genomic DNA (gDNA) from AG-KOALS20αI mice. gDNA for the first 2 time points was isolated from the peripheral blood, whereas gDNA from the final time point was isolated from the BM. Each point represents a single mouse. (B) Cation-exchange HPLC analysis of RBC lysate collected from primary, secondary, and tertiary transplanted AG-KOALS20αI mice. This analysis indicated that ALS20αI transduced long-term HSC, and its expression is stable over time.
Stable engraftment and expression of human α-globin protein in primary, secondary, and tertiary transplanted mice. (A) The VCN was measured via ddPCR analysis of genomic DNA (gDNA) from AG-KOALS20αI mice. gDNA for the first 2 time points was isolated from the peripheral blood, whereas gDNA from the final time point was isolated from the BM. Each point represents a single mouse. (B) Cation-exchange HPLC analysis of RBC lysate collected from primary, secondary, and tertiary transplanted AG-KOALS20αI mice. This analysis indicated that ALS20αI transduced long-term HSC, and its expression is stable over time.
ALS20αI improves p50, erythropoiesis, and iron metabolism in AG-KO mice
Erythroid parameters in AG-KOALS20αI mice (Table 1) were in the range of animals carrying 2 copies of the α-globin gene (Hba-a1KO/+/Hba-a2KO/+). The improvement in these animals was remarkable considering that animals showed residual HbH of various degrees (22.2% ± 14.0% of the total Hb tetramers by HPLC) and that mβ2hα2 is an artificial Hb with noncanonical oxygen-binding affinity (Figure 1G).64 We also characterized the p50 and Hill coefficient in animals treated with ALS20αI. Although their p50 was similar in WT and AG-KOALS20αI mice (Figure 1G), their Hill coefficient was different and low in AG-KOALS20αI mice (0.97-0.99, extrapolated using the method described in supplementary Materials in “Oxygen binding affinity”). In AG-KOALS20αI mice, the Hill coefficient did not change with increasing VCN (even with the highest VCN and lowest levels of HbH), suggesting that this chimeric Hb (mβ2hα2) has a low cooperativity.
Despite these trans-species limitations, ALS20αI led to improvement or normalization of BM and spleen cellularity and erythropoiesis (Figure 1E-F; supplemental Figures 5A-B and 6), β-globin precipitates and RBC quality (supplemental Figures 4C and 7A), as well as EPO and hepcidin expression (Figure 2A-B). The mice survived long term (>5 months; supplemental Figure 28) with normal spleen size (Figure 2C), normal spleen and liver anatomy (Figure 3A; supplemental Figure 16A), and absence of coagulation defects (Figure 3C-D; supplemental Figure 16B).
ALS20αI stably expresses the human α-globin gene and corrects the AT phenotype in animals that underwent secondary and tertiary transplantion
Secondary transplants (N = 12 recipient mice, generated from 4 donor mice) showed expression of the transgene and steady correction of the phenotype, confirming that the expression of human α-globin, as a result of ALS20αI treatment, is robust and stable (Figure 6B). Furthermore, tertiary transplants (N = 4 recipient mice, generated from 1 donor mouse) demonstrate the longevity of ALS20αI expression (Figure 6B).
Lentiviral gene transfer improves human α-globin synthesis and morphology in patient samples and α-globin–KO CD34+ cells
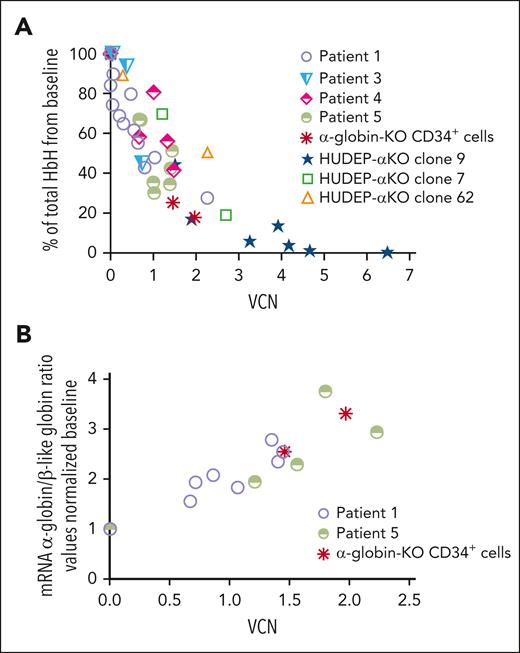
We tested ALS20αI’s ability to improve the phenotype of erythroid progenitor cells derived from peripheral CD34+ cells from patients with HbH disease characterized by various deletional and nondeletional mutations as described in Table 2.65-75 Across all genotypes, the level of HbH decreased as the VCN increased (Figure 7A). In 2 specific examples, patients with −α3.7/− −SEA and αAgrinioα/αAgrinioα mutations, transduction of erythroid progenitor cells with ALS20αI increased the ratio of α-globin to β-like globin mRNA (Figure 7B).
Evaluation of the effect of ALS20αI transduction on HbH and α-globin mRNA levels in mutant cells. (A) The percentage of total HbH relative to VCN measured by cation-exchange HPLC in erythroblasts differentiated from HUDEP-αKO clones, α-globin–KO CD34+ cells, and cells from patients with HbH. Each symbol represents 1 biological replicate: Patients 1 (n = 12), 3 (n = 3), 4 (n = 5), and 5 (n = 5). Genotypes are summarized in Table 2. In HUDEP-αKO clone 9, n = 7, in clone 7, n = 2, and in clone 62, n = 2. In α-globin–KO CD34+, n = 2. (B) The HBA:(HBB + HBG + HBD) mRNA relative to VCN in a patient with the –α3.7/− −SEA deletion (n = 8, patient 1 in Table 2), in a patient with homozygous Agrinio (427 T>A) mutation (n = 5, patient 5 in Table 2), and in α-globin–KO CD34+–derived erythroid cells. These analyses also suggested that 1 copy of ALS20αI produced as many human α-globin chains as those of a single endogenous α-globin gene.
Evaluation of the effect of ALS20αI transduction on HbH and α-globin mRNA levels in mutant cells. (A) The percentage of total HbH relative to VCN measured by cation-exchange HPLC in erythroblasts differentiated from HUDEP-αKO clones, α-globin–KO CD34+ cells, and cells from patients with HbH. Each symbol represents 1 biological replicate: Patients 1 (n = 12), 3 (n = 3), 4 (n = 5), and 5 (n = 5). Genotypes are summarized in Table 2. In HUDEP-αKO clone 9, n = 7, in clone 7, n = 2, and in clone 62, n = 2. In α-globin–KO CD34+, n = 2. (B) The HBA:(HBB + HBG + HBD) mRNA relative to VCN in a patient with the –α3.7/− −SEA deletion (n = 8, patient 1 in Table 2), in a patient with homozygous Agrinio (427 T>A) mutation (n = 5, patient 5 in Table 2), and in α-globin–KO CD34+–derived erythroid cells. These analyses also suggested that 1 copy of ALS20αI produced as many human α-globin chains as those of a single endogenous α-globin gene.
Because of the limitations in obtaining CD34+ HSC from patients with severe α-thalasemia major, we deleted the α-globin genes (HBA1/HBA2) in freshly isolated CD34+ cells by CRISPR-CRISPR–associated protein 9 (supplemental Figure 29), and generated control CD34+ cells, targeting the integration locus for AAVS1 (supplemental Figure 30). Deletion efficiency in these cells was 96.6%, with a residual 5.6% synthesis of α-globin chains upon erythroid differentiation (supplemental Figure 31). Transduction of α-globin–KO CD34+ cells twice with 0.5 or 1.0 multiplicity of infection of ALS20αI showed an increase in VCN (1.46 and 1.97) and in the production of α-globin protein chains (supplemental Figure 31). Similarly, ALS20αI increased functional Hbs (HbA, HbF, and HbA2), whereas HbH was markedly reduced (Figure 5B; supplemental Figure 32). Correlation between the reduction of HbH and VCN in HUDEP-αKO, patient and α-globin–KO CD34+ cells (Figure 7A), as well as the increase in healthy Hbs (HbA + HbF + HbA2; supplemental Table 2), suggested that ALS20αI produced, at 1 VCN, human α-globin protein equivalent to that of a single endogenous α-globin gene.
We also compared the morphology of maturing erythroid cells from patient 5 (αAgrinioα/αAgrinioα; Table 2) and the α-globin–KO CD34+ specimen with that of healthy erythroblasts. On day 7 of differentiation in culture, untreated cells showed a delayed and incomplete differentiation pattern, with a more significant proportion of late-basophilic to polychromatic features (supplemental Figure 33). In contrast, cells treated with ALS20αI showed improved maturation reaching polychromatic/orthochromatic stages at terminal differentiation, more similar to healthy WT cells (supplemental Figure 33).
Safe genomic integration profile of ALS20αI
We performed integration site analysis in genomic DNA isolated from CD34+ cells transduced with ALS20αI (with ∼1 VCN for all the samples), observing that all samples yielded highly polyclonal distributions (supplemental Figure 34). As is commonly seen with lentiviral vectors, integration was enriched in active transcription units and associated features (supplemental Figures 34 and 35). Furthermore, a statistical analysis of the proportion of integration sites near cancer-associated genes from the Catalogue of Somatic Mutations in Cancer (COSMIC) database showed no enrichment during growth (paired Wilcoxon test, day 1 vs day 14 after transduction; P > .05).76-80 Thus, we concluded that the integration site distributions of the samples studied showed no obvious signals leading to safety concerns.
Discussion
Here, we have described 2 methods for creating complete KOs of the α-globin gene in mice, 1 using an FLC transplant from a previously described line of constitutive KO mice, and a second using a novel cKO.51 In both cases, mice suffering from a complete knock out of α-globin demonstrated the same phenotype. These mice suffer from paradoxical hypoxia due to the high oxygen-binding affinity of HbH tetramers. Because of functional hypoxia, levels of EPO increase and hepcidin decrease, producing additional RBC and kickstarting ineffective erythropoiesis. However, because RBC can only be made with HbH, these new RBC continue to perpetuate the cycle, stimulating further erythropoiesis and leading to extramedullary hematopoiesis. As a result, the animals quickly become inundated with RBC incapable of effectively transferring oxygen, leading to an escalating hematocrit. Because both HbBart and HbH tetramers also have high oxygen-binding affinity in humans, it is conceivable that the production of some RBC expressing HbH, despite relatively high Hb levels, can be associated with paradoxical functional anemias, considering the suboptimal oxygen delivery.81 This might trigger hypoxia and EPO production, further increasing the production of RBC nonfunctional in delivering oxygen. This phenomenon may explain why, compared with patients with β-thalassemia, individuals affected by severe AT (those affected by BartHF and rescued by blood infusion in utero) still produce a large proportion of RBC expressing HbH and require larger transfusion volumes to reduce the production of endogenous RBC.18 Despite these high transfusion volumes, patients still show elevated reticulocytosis, marking a significant difference compared with patients with β-thalassemia who are chronically transfused who remain reticulocytopenic.81,82 A potential model for these observations can be found in supplemental Figure 36. Based on this model and our data, we hope to inform clinicians to investigate the relationship between the level of HbH, hypoxia, and coagulation markers in patients with severe AT and, potentially, improve their clinical care.
In AT mice, ineffective erythropoiesis and excess of abnormal RBC lead to many of the organ pathologies observed, including splenomegaly and iron overload in the liver. At the same time, the erythrocytosis produces conditions conducive to the formation of clots and the occurrence of fibrin-rich vaso-occlusive thrombi. These mice represent a model of the most severe form of AT, meaning that developing a successful treatment in this model would be helpful in all forms of AT. Here, we also investigated the development of a novel lentiviral vector expressing human α-globin, ALS20αI. Transduction of AG-KO HSC with ALS20αI resulted in the efficient production of high levels of human α-globin and the formation of chimeric mβ2hα2 heterotetramers. ALS20αI transduction improved the phenotype and prevented lethality in mice at VCNs as low as 1.1. Furthermore, at low VCNs, mice displayed a substantial improvement in hematological parameters such as those with higher VCNs. These results suggest that lethality in AT requires a near complete absence of α-globin expression, and that recovery of a low level of its expression may be sufficient to partially improve life span. Secondary and tertiary BM transplantation resulted in the continued expression of human α-globin, indicating that ALS20αI can successfully transduce long-term HSC and that the correction of the AT phenotype is durable.
Research and development of novel treatments for hemoglobinopathies have had remarkable success in the past several years, most notably the approval by the US Food and Drug Administration of betibeglogene autotemcel (Zynteglo) and lovotibeglogene autotemcel (Lyfgenia), lentivirus-based ex vivo treatments for β-thalassemia and sickle cell disease, respectively.83,84 This outcome results from many studies and the creation of numerous research tools dedicated to investigating β-thalassemia and sickle cell disease. In contrast, AT has received a fraction of the attention despite representing a significant and growing health care challenge. Generating the mouse models of severe AT described here can be a powerful tool for future investigations and provide avenues of investigation for human patients.18,81,82,85 This model also offers a platform for developing novel therapies such as ALS20αI.
Acknowledgments
The authors thank the Pathology Core Laboratory at The Children’s Hospital of Philadelphia Research Institute for providing histological, immunohistochemical, and imaging services.
This research was supported by the Frontier Program (The Children's Hospital of Philadelphia); Comprehensive Center for the Cure of Sickle Cell Disease and Other Red Cell Disorders grant GRT-00001923-05; Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health; Penn Institute for Regenerative Medicine grant GRT-00003991; National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants R01DK095112 and R01DK133475 [S.R.], NIH/National Heart, Lung, and Blood Institute (NHLBI) grants 5T32HL007150 and 5T32HL007622 [M.P.T.], and NIH/National Cancer Institute grant R01 CA241762 [F.D.B]); The Thomas B. and Jeannette E. Laws McCabe Fund at the University of Pennsylvania (H.P.); Naamira Biomedicals (H.P.); the NIH, NHLBI T32 training grant in hemostasis and thrombosis (project number 2T32HL007971-21A [M.C.]); The Children’s Hospital of Philadelphia Research Institute Bridge to Faculty Program (A.G.); the NIH/NHLBI (grants 5K99HL156060-03 [K.L.G.] and 5R61HL156158-02 [O.A.]) and the Special Account for Research Grants–National and Kapodistrain University of Athens (SARG-NKUA) code 12260 (A.K.). The authors acknowledge that CD34 cells were supplied by the Fred Hutchinson Co-Operative Centers of Excellence in Hematology Cell Processing Core, which is funded through NIH, NIDDK (grant U54 DK106829).
Authorship
Contribution: S.R., M.E.C., L.B., L.T., A.G., M.P.T., V.G., A.K., N. Teawtrakul, and O.A. conceptualized the study; M.E.C., S.R., L.B., M.P.T., D.J., A.G., C.C.C., T.E.P., N. Tanaka, V.B., N.H., L.T., A.M.R., J.K.E., and E.J.C. developed the methodology; M.E.C., L.B., D.J., A.G., V.G., M.T.F., S.G., K.L.G., and L.T. performed the investigation; M.E.C., S.R., L.B., L.T., and A.G. were responsible for visualization of data; S.R. acquired funding; S.R., M.E.C., and C.C.C. were responsible for project administration; M.E.C., S.R., L.B., L.T., A.G., and F.D.B. wrote the original draft of the manuscript; and M.E.C., S.R., L.B., L.T., D.J., A.G., M.P.T., V.G., K.L.G., A.K., N. Teawtrakul, B.L.M., Y.K.T., D.W., O.A., and H.P. reviewed and edited the manuscript.
Conflict-of-interest disclosure: H.P. and D.W. are scientific founders and hold equity in Capstan Therapeutics. Y.K.T. and B.L.M. are employees and hold equity in Acuitas Therapeutics. D.W. receives research support from BioNTech. F.D.B. is founder of Biocept; reports intellectual property licensed to Novartis; and serves as a consultant for Sana Biotechnology, Poseida Therapeutics, Encoded Therapeutics, and Johnson & Johnson. In accordance with the University of Pennsylvania policies and procedures and our ethical obligations as researchers, H.P., and D.W. are named on several patents that describe the use of nucleoside-modified mRNA and targeted LNP as platforms to deliver therapeutic proteins and vaccines. The vector ALS20αI is protected in the patent “Generation of a lentiviral vector for the cure of alpha-thalassemia” (The Children's Hospital of Philadelphia, M.E.C., and S.R.). The remaining authors declare no competing financial interests.
Correspondence: Stefano Rivella, Division of Hematology, Department of Pediatrics, The Children's Hospital of Philadelphia, 3615 Civic Center Blvd, Leonard and Madlyn Abramson Pediatric Research Center, Room 316B, Philadelphia, PA 19104; email: rivellas@chop.edu; and Hamideh Parhiz, Department of Systems Pharmacology and Translational Therapeutics, University of Pennsylvania Perelman School of Medicine, Biomedical Research Building II/III Room 1256, 421 Curie Boulevard, Philadelphia, PA 19104; email: hamideh.parhiz@pennmedicine.upenn.edu.
References
Author notes
M.E.C., L.B., L.T., and A.G. are joint first authors.
For original data, please contact the corresponding author, Stefano Rivella (rivellas@chop.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.