Abdominal aortic aneurysm (AAA) is a relatively common condition associated with very high mortality if rupture occurs. In this issue of Blood, Benson et al1 show a critical role for platelets in AAA, defining soluble glycoprotein VI (sGPVI) as both a marker for AAA progression and a therapeutic target.
AAA has long been known to cause intraluminal thrombus formation that occurs within the outpouching of the aortic wall (see figure). Historically, thrombus formation in this context was viewed as more of a consequence than a contributor to the underlying disease process. This concept, however, began to change with the observation in the 1990s that intraluminal thrombus thickness predicted rupture.2 Later, platelets were found to be a source of inflammatory factors and adhesion molecules that either directly contributed enzymes that broke down collagen and elastin in the aortic wall or contributed to the recruitment of leukocytes that released proteolytic enzymes.3 In response to these findings, investigators began to evaluate the effect of antiplatelet agents in rodent models of AAA progression. These models showed, for example, that P2Y12 inhibition,4-6 aspirin,6 or αIIbβ37 inhibition slowed AAA progression. More recently, observational studies suggested some efficacy of antiplatelet agents in improving outcomes in patients with AAA.8 A randomized controlled trial, however, showed that ticagrelor did not inhibit the growth of AAA.9 Thus, whether platelets are an important driver of AAA progression, whether platelet markers can be used to monitor AAA growth, and whether platelets can be targeted to slow AAA growth are unresolved questions in the field.
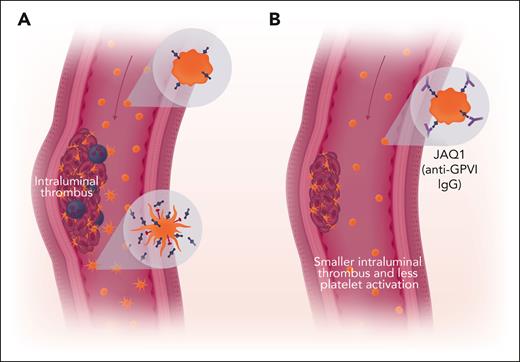
Inhibition of GPVI impairs growth of AAA. (A) Resting platelets expressing GPVI (blue) circulate in the bloodstream (upper insert). Some platelets interact with the intraluminal thrombus and become activated. These platelets shed their GPVI and can express P-selectin (red, lower inset). These platelets can either incorporate into the thrombus and recruit leukocytes (purple) or circulate as partially activated platelets. Plasma levels of soluble GPVI fragments shed from activated platelets serve as a biomarker for fast-growing aneurysms. (B) Anti-GPVI IgG interferes with platelet incorporation into intraluminal thrombi and decreases thrombus size. Red arrow indicates blood flow. Professional illustration by Somersault18:24.
Inhibition of GPVI impairs growth of AAA. (A) Resting platelets expressing GPVI (blue) circulate in the bloodstream (upper insert). Some platelets interact with the intraluminal thrombus and become activated. These platelets shed their GPVI and can express P-selectin (red, lower inset). These platelets can either incorporate into the thrombus and recruit leukocytes (purple) or circulate as partially activated platelets. Plasma levels of soluble GPVI fragments shed from activated platelets serve as a biomarker for fast-growing aneurysms. (B) Anti-GPVI IgG interferes with platelet incorporation into intraluminal thrombi and decreases thrombus size. Red arrow indicates blood flow. Professional illustration by Somersault18:24.
Benson et al now make a significant advance in addressing the role of platelets in AAA by focusing on GPVI as a molecular linchpin between platelet activation and AAA progression. Evaluation of surgical aneurysm repair samples showed a greater than ninefold increase in GPVI transcript in intraluminal thrombi compared with transcript levels in the AAA wall. This result was of interest since GPVI is the major collagen receptor responsible for platelet activation in response to collagen binding. Evaluation of platelets from patients with AAA showed somewhat increased expression of GPVI at baseline and, more impressively, marked platelet activation in response to the GPVI agonist convulxin. Platelet activation was detected using P-selectin as a marker, which is relevant given the role of P-selectin in recruiting leukocytes into thrombi. These observations led to the hypothesis that GPVI promotes disease progression in AAA.
To evaluate this premise, the authors determined whether sGPVI measurements correlated with the growth rate of AAA in patient cohorts. GPVI is cleaved by the metalloproteinase ADAM10 on the platelet surface. sGPVI levels thus correlate with both GPVI expression and the extent of platelet activation. In 2 population-based case-controlled cohort studies (1 European cohort [n = 264] and 1 American cohort [n = 128]), Benson et al evaluated sGPVI levels in patients with slow- or fast-growing AAAs and their matched controls. In the European group, sGPVI predicted the occurrence of fast-growing aneurysms with a specificity of 77% and a sensitivity of 81%. The sensitivity and specificity of sGPVI for predicting fast-growing aneurysms in the American studies was 83% and 66%, respectively. Like sGPVI, D-dimer has also been studied in the context of AAA and has been shown to be elevated in AAA.10 However, when comparing D-dimer with sGPVI, the authors found that only sGPVI was able to predict accelerated rate of AAA growth.
To address the question of whether GPVI is causative in the setting of AAA growth progression, 2 well-controlled mouse models of AAA were used. In the first model, either elastase or heat-inactivated elastase was applied to the infrarenal aorta. After 2 weeks, mice were blindly randomized to receive either nonimmune immunoglobulin G (IgG) or JAQ1, a neutralizing antibody directed at GPVI. Aortic diameter was tracked by ultrasound. An advantage of this design is that it provides an opportunity to evaluate whether JAQ1 inhibits aortic diameter growth, reverses growth, or prevents rupture. Mice treated with JAQ1 had no significant growth in the diameter of the aorta following JAQ1 administration and no aortic rupture. In contrast, elastase-treated mice in the IgG group showed substantial increases in aortic wall diameter, and 13.3% of these mice experienced rupture and died. Although JAQ1 is well known to cause thrombocytopenia in mice, under the conditions of these studies, platelet counts were reduced in JAQ1 mice only on days 3 and 5 of the 28-day experiment and only by 20%. In a second murine model of AAA, Ldlr−/− mice received 28 days of angiotensin II infusion. Mice were then randomized to either JAQ1 or nonimmune IgG. Again, JAQ1-treated mice showed less aortic diameter growth and no aortic rupture, whereas one-third of the control mice died of aortic rupture. In both models, platelets from mice with AAA were hyperreactive in response to thrombin, and this effect was inhibited in AAA mice receiving JAQ1. In addition, mice receiving JAQ1 showed more type I collagen and fewer infiltrating macrophages compared with control mice.
The confluence of evidence from their 2 independent case-controlled studies and 2 separate mouse models strongly implicates GPVI in progression of AAA. Establishment of this mechanistic link is important since, as suggested by the authors, it could potentially form the basis for both new diagnostics and therapeutics. Of course, whether an understanding of the role of GPVI in AAA development can be leveraged to improve the management of patients with AAA remains to be seen. Monitoring sGPVI to detect those with faster-growing aneurysms will need to be studied in prospective longitudinal trials before this marker can be incorporated into the care of AAA patients. Such studies will need to define thresholds and show that measurement of sGPVI improves management. Similarly, GPVI antagonists, which are currently in clinical trials for other indications, will need to be tested in randomized, prospective trials. Nonetheless, the work by Benson et al provides strong incentive to pursue GPVI-targeted strategies to improve management of this common and insidious threat.
Conflict-of-interest disclosure: R.F. is founder of and has equity in PlateletDiagnostics, is a consultant for Xap Therapeutics and Tessera Therapeutics, and is on the scientific advisory board of Function Therapeutics and Porosome Therapeutics.