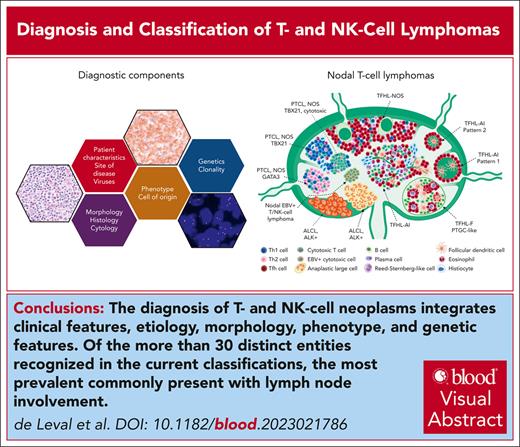
Visual Abstract
T- and natural killer (NK)-cell lymphomas are neoplasms derived from immature T cells (lymphoblastic lymphomas), or more commonly, from mature T and NK cells (peripheral T-cell lymphomas, PTCLs). PTCLs are rare but show marked biological and clinical diversity. They are usually aggressive and may present in lymph nodes, blood, bone marrow, or other organs. More than 30 T/NK-cell–derived neoplastic entities are recognized in the International Consensus Classification and the classification of the World Health Organization (fifth edition), both published in 2022, which integrate the most recent knowledge in hematology, immunology, pathology, and genetics. In both proposals, disease definition aims to integrate clinical features, etiology, implied cell of origin, morphology, phenotype, and genetic features into biologically and clinically relevant clinicopathologic entities. Cell derivation from innate immune cells or specific functional subsets of CD4+ T cells such as follicular helper T cells is a major determinant delineating entities. Accurate diagnosis of T/NK-cell lymphoma is essential for clinical management and mostly relies on tissue biopsies. Because the histological presentation may be heterogeneous and overlaps with that of many benign lymphoid proliferations and B-cell lymphomas, the diagnosis is often challenging. Disease location, morphology, and immunophenotyping remain the main features guiding the diagnosis, often complemented by genetic analysis including clonality and high-throughput sequencing mutational studies. This review provides a comprehensive overview of the classification and diagnosis of T-cell lymphoma in the context of current concepts and scientific knowledge.
Introduction
T-cell lymphoma is a generic term that encompasses malignant neoplasms of immune cells of T and natural killer (NK) lineages. These include tumors of immature T cells, namely T-lymphoblastic lymphoma/leukemia, and of mature T and NK cells, commonly designated as “peripheral” or “mature” T-cell lymphomas (PTCLs).1
In physiological conditions, the human immune system contains many more T cells than B cells, and in general, reactive lymphoid infiltrates in tissues tend to contain a high proportion of T cells. Although most lymphoblastic lymphomas arise from T-precursor cells (85%-90% of the cases), in contrast, PTCLs account for <15% of non-Hodgkin lymphomas worldwide, with substantial geographic variation in incidence and relative prevalence. Despite their rarity, PTCLs are biologically and clinically diverse.2,3 They may present in the lymph nodes, skin, or various extranodal organs, or exclusively involve the blood and the bone marrow (Table 1).
Most PTCLs are clinically aggressive disorders, often challenging to manage with approaches successful in B-cell lymphomas.6 However, some rare conditions characterized by clonal expansion of T or NK cells do not completely meet the pathological criteria for malignancy, and follow a chronic or indolent clinical course. They are now termed “lymphoproliferative disorders” (LPD) to distinguish them from more aggressive PTCLs.4,5
Given the clinical and biological diversity and complexity of T-cell neoplasms, correct diagnosis and precise classification have become essential for accurate prognostication and effective management of these disorders.7
Classification of T- and NK-cell neoplasms
The contemporary principles for the classification of lymphoid neoplasms were established by the publication of the Revised European-American Classification of Lymphoid Neoplasms (REAL) in this journal 30 years ago.8 These principles attempt to identify “real” clinicopathological entities combining clinical features, etiology, implied cell of origin, morphology, phenotype, and genetic features. The contribution of these characteristics that define individual lymphoma entities varies greatly, but multiple attributes are required for diagnosis of virtually all entities. Although few etiologic agents are recognized, there are a number of preexisting conditions, either germ line mutations affecting normal cellular functions (DNA repair machinery, regulation of transcription, immune responses) or acquired conditions (summarized in Table 2), which predispose to the development of T- and NK-cell neoplasms.
Since 2001, the concept developed by the REAL classification has been the basis of the World Health Organization (WHO) classifications of hematolymphoid neoplasms.1,19,20 Until 2022, WHO monographs (Blue Books) have been the main international standard used by pathologists, clinicians, and scientists. In contrast, in 2022, 2 separate updates of the latest 2017 WHO classification were published, 1 led by the WHO Classification of Tumours Editorial Board, and the other by the Clinical Advisory Committee appointed by the American Society for Hematopathology and the European Association for Haematopathology.4,5 The complex reasons for this regrettable development will not be addressed further in this article.21,22 In the context of T/NK-cell neoplasms, both classifications follow the same principles and provide a comprehensive framework integrating the most recent knowledge in clinical, pathological, and genetic features, and will form the basis of further discussion in this review. The 2 classifications are largely similar, but there are several minor and a few significant differences (Table 1). Therefore, professional societies recommend reporting both terminologies in parallel when diagnosing T- and NK-cell neoplasms in hematopathology diagnostic practice.
Cell of origin in T- and NK-cell neoplasms
“Cell-of-origin” is a fundamental attribute and defining feature of many T- and NK-cell neoplasms, defined as the putative physiological counterpart of the neoplastic T- or NK-cell based on phenotype and gene expression profile. In the classification schemes, the first major bifurcation occurs in the separation of immature (precursor) from mature (peripheral) T-cell neoplasms. Two distinct entities are recognized under the umbrella of “T-lymphoblastic lymphoma/leukemia,” based on cell-of-origin: one showing an early T-cell precursor phenotype corresponding to the earliest recognizable T-cell precursors emigrant from the bone marrow to the thymus,23,24 and the other being more heterogeneous with phenotypic features similar to those of differentiating thymocytes.25 Mature T- and NK-cell neoplasms can be separated into 2 distinct groups based on putative cell-of-origin either from innate or adaptive immune cells. Innate-type neoplasms include those derived from NK cells or from unconventional T cells (ie, γδ T cells, αβ NKT cells, or mucosal-associated invariant T cells).26 γδ T cells (CD4−CD8− or CD4−CD8+) comprise <5% of T cells and are preferentially distributed in the skin, mucosae, and the splenic red pulp. Accordingly, lymphomas derived from innate cells tend to occur in the skin (primary cutaneous γδ T-cell lymphoma), mucosae (primary intestinal T-cell lymphomas, extranodal NK/T-cell lymphoma [ENKTCL]), and spleen (hepatosplenic T-cell lymphoma [HSTL]). Innate-type PTCLs usually have a cytotoxic phenotype and are clinically aggressive. Some entities, such as primary cutaneous γδ T-cell lymphoma, are defined by a specific cellular origin. Others, such as HSTL, show diversity in terms of precise cell lineage. This likely reflects the importance of homing and functional properties shared by discrete innate cell subsets.27 In contrast, lymphomas derived from the adaptive immune system are more prevalent and originate from lymph nodes, where most immune cells of the adaptive immune system reside. Adaptive immune responses are characterized by activation-induced differentiation of naïve T cells into antigen-specific effector T cells and T-cell memory. These T cells express T-cell receptor (TCR) αβ and recognize antigen presented in the context of major histocompatibility complex class I or II molecules. Functionally, they comprise 2 categories; CD4+ T cells with primarily helper function and CD8+ cells with mainly cytotoxic function. Upon activation, CD4+ T-helper cells may differentiate into various functional subtypes, such as Th1, Th2, Th17, regulatory (Treg), or T-follicular helper (TFH) cells. T-cell lymphomas assumed to originate from each of these distinct functional subtypes have been described. The best established disease entity defined by its cell-of-origin is follicular helper T-cell lymphoma (TFHL).28 TFHL has several histological subtypes, but all share a common gene expression signature and phenotype of normal TFH cells.4,5,29 Similarly, preliminary data indicate that subsets of PTCL, not otherwise specified (PTCL, NOS), have molecular signatures akin to normal Th1 or Th2 cells, suggesting cell-of-origin from Th1 or Th2 functional subsets.30-33
Samples and diagnostic tools
Diagnosis and monitoring of T- and NK-cell neoplasms remains one of the most challenging areas in pathology. Depending on the clinical presentation, the examination of multiple diagnostic biospecimens from different sources such as lymph nodes, peripheral blood, bone marrow, other extranodal sites, and cerebrospinal or other body fluids is often required. For tissue lesions, a surgical biopsy is the preferred method for adequate histopathological assessment and additional ancillary techniques.34 Where a surgical biopsy is not practical, less invasive core needle or small-volume biopsies may be used, but they offer less sensitivity and specificity compared with surgical biopsies.35-37 A core biopsy complemented by fine-needle aspiration for flow cytometric analysis may fulfill the needs for initial patient management.6,35,37 Importantly, several cores are required to collect sufficient material for ancillary testing and to procure archival biospecimens for future needs such as clinical trial enrollment. In patients with a suspected PTCL relapse, a rebiopsy should be considered because it may disclose proliferation of a different lineage (B-cell or even myeloid), or various reactive or infectious processes, with distinct clinical implications. The neoplastic nature of an NK- or T-cell lymphocytosis (in blood or fluids) or tissue infiltrate is suggested by atypical morphology, aberrant T-cell phenotype, and/or evidence of clonality.38
Histology and cytology
The mainstay of diagnosis remains the morphologic evaluation of smears or tissue sections to establish the differential diagnosis and triage the biospecimens for downstream immunophenotyping and genetic studies.39
Immunophenotyping
Immunophenotyping is essential for the assessment of T- and NK-cell neoplasms. The 2 main clinical methods used for immunophenotyping are multiparameter flow cytometry (MFC) and immunohistochemistry. MFC enables single-cell resolution and the assessment of multiple markers on the same cell and a large number of cells in a short time. The requirement for fresh tissue and loss of tissue architecture limits the utility of MFC primarily to liquid specimens, but valuable information can be obtained if performed in conjunction with histological examination. In contrast, immunohistochemistry is applicable to formalin-fixed paraffin-embedded tissues, and offers, in addition to cell phenotype, architectural information that is essential for the evaluation. Figure 1 and Table 3 summarize the phenotype of the most frequent entities, and the commonly used antibodies. Immunophenotyping allows for determination of cell lineage and differentiation, aberrant antigen expression (loss or dim expression of T-cell markers), phenotypic surrogates of clonality (monotypic TRBC1 expression) or abnormal coexpression of markers of other lineages (eg, CD20), ectopic expression of oncogenes, and assessment of markers reflective of cellular processes and the microenvironment.
Immunophenotypic characteristics in common T and NK-cell neoplastic entities. Each column in the heat map represents a T/NK-cell neoplastic entity, and each row represents a diagnostic marker assessed by immunophenotyping of tissue or cell samples. The prevalence of cases positive for each marker is color-coded, as shown in the legend. ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma kinase; ATLL, adult T-cell leukemia/lymphoma; BIA-ALCL, breast implant–associated ALCL; EATL, enteropathy-associated T-cell lymphoma; HSTL, hepatosplenic T-cell lymphoma; ITCL-NOS, intestinal T-cell lymphoma, NOS; MEITL, monomorphic epitheliotropic intestinal T-cell lymphoma; MF, mycosis fungoides; NA, not available; NK-LGLL/CLPD-NK, NK-large granular lymphocytic leukemia/chronic LPD of NK cells; NK-LPD GI, indolent NK-cell LPD of the gastrointestinal tract; nodal EBV TCL, primary nodal EBV+ T/NK-cell lymphoma; pcALCL, primary cutaneous ALCL; PTCL-NOS, peripheral T-cell lymphoma, NOS; SPTCL, subcutaneous panniculitis-like T-cell lymphoma; SS, Sezary syndrome; TFHL, follicular helper T-cell lymphoma; T-LBL, T-lymphoblastic leukemia/lymphoma; T-LPD GI, indolent clonal T-cell LPD of the gastrointestinal tract; T-LGLL, T-cell large granular lymphocytic leukemia; T-PLL, T-cell prolymphocytic leukemia.
Immunophenotypic characteristics in common T and NK-cell neoplastic entities. Each column in the heat map represents a T/NK-cell neoplastic entity, and each row represents a diagnostic marker assessed by immunophenotyping of tissue or cell samples. The prevalence of cases positive for each marker is color-coded, as shown in the legend. ALCL, anaplastic large cell lymphoma; ALK, anaplastic lymphoma kinase; ATLL, adult T-cell leukemia/lymphoma; BIA-ALCL, breast implant–associated ALCL; EATL, enteropathy-associated T-cell lymphoma; HSTL, hepatosplenic T-cell lymphoma; ITCL-NOS, intestinal T-cell lymphoma, NOS; MEITL, monomorphic epitheliotropic intestinal T-cell lymphoma; MF, mycosis fungoides; NA, not available; NK-LGLL/CLPD-NK, NK-large granular lymphocytic leukemia/chronic LPD of NK cells; NK-LPD GI, indolent NK-cell LPD of the gastrointestinal tract; nodal EBV TCL, primary nodal EBV+ T/NK-cell lymphoma; pcALCL, primary cutaneous ALCL; PTCL-NOS, peripheral T-cell lymphoma, NOS; SPTCL, subcutaneous panniculitis-like T-cell lymphoma; SS, Sezary syndrome; TFHL, follicular helper T-cell lymphoma; T-LBL, T-lymphoblastic leukemia/lymphoma; T-LPD GI, indolent clonal T-cell LPD of the gastrointestinal tract; T-LGLL, T-cell large granular lymphocytic leukemia; T-PLL, T-cell prolymphocytic leukemia.
Molecular genetic tests
Assessment of TR loci rearrangements is an important ancillary technique in the diagnosis of T-cell proliferations. It helps distinguish neoplastic processes from the many reactive mimics, particularly because there is no widely available surrogate immunohistochemical method for determining clonality. Monotypic TRBC1 expression analysis by flow cytometry, now widely available in routine diagnostic laboratories, is mainly used for blood, bone marrow, and other fluid samples, but its application to tissue aspirates is hampered by the fact that the neoplastic cell content may be low. Moreover, its application is restricted to TCRαβ+ populations, and accordingly not to sCD3− nor TCRγδ+ proliferations.40,41 Clonality studies, by PCR-based methods and high-throughput sequencing (HTS) assays, are performed in many cases to confirm a diagnosis of PTCL or T-cell LPD.42,43 Although TR-based clonality methods are overall sensitive, the caveat is that they may generate false-positive results in reactive conditions. Specifically, caution is needed in the interpretation of small clones, and the analyses should in general be run in duplicate to confirm reproducibility. In contrast, ultrasensitive methods for detection of specific pathogenic mutations might be more sensitive and specific for clonality.44
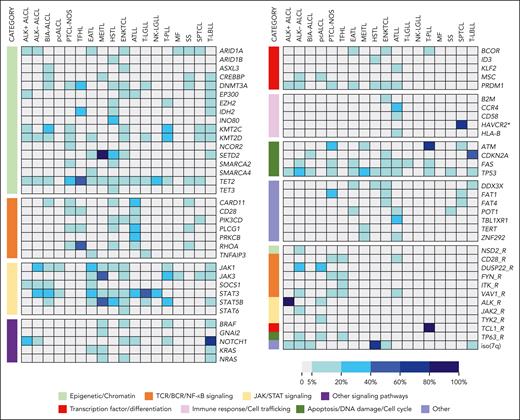
Most mature T-cell neoplasms are characterized by complex genomes, with only rare entities, such as ALK+ anaplastic large cell lymphoma (ALCL) and T-cell prolymphocytic leukemia (T-PLL), carrying disease-defining or highly recurrent genetic alterations. The rest are associated with diverse mutations affecting various cellular pathways, including epigenetics, TCR, and cytokine signaling pathways, genes involved in immunobiology and the cell cycle, and others (Figure 2).45 Fluorescent in situ hybridization assays are performed to assess the most common gene rearrangements or fusions, and selected copy number variations. The detection of gene variants (point mutations or small indels) may rely on targeted assays for certain hotspots but is more commonly achieved by HTS panels (Table 3). In addition to providing diagnostic information, especially for entities associated with frequent hot spot mutations or combinations of genetic alterations (ie, TFHL, T- and NK- large granular lymphocyte proliferations, and intestinal T-cell lymphomas), HTS data may generate information relevant to therapeutic decisions given that a number of PTCL-associated genetic lesions are amenable to targeted intervention.45 Of note, HTS in presumed PTCLs requires a high depth of sequencing and adequate filtering allowing for the identification of variants with low allele frequency (1%-2%) given the often low proportion of neoplastic cells. Moreover, the frequently found mutations in TET2 and DNMT3A, which may in part reflect underlying clonal hematopoiesis, must be interpreted with caution.
Mutational landscape of common T- and NK-cell neoplastic entities. Each column in the heat map represents a T/NK-cell neoplastic entity, and each row represents selected single nucleotide variants/indels and rearrangements (R), grouped according to functional annotations. The prevalence of mutations is color-coded, as shown in the legend. Genes with a mutational frequency of at least 10% observed in 1 or more mature T/NK-cell neoplasm were selected for display. Mutation frequencies 5%-10% were reported in some entities given their diagnostic or clinical value. ∗Refers to germ line mutation. BIA-ALCL, breast implant–associated ALCL; MF, mycosis fungoides; NK-LGLL, NK-large granular lymphocytic leukemia/chronic LPD of NK cells; pcALCL, primary cutaneous ALCL; SPTCL, subcutaneous panniculitis-like T-cell lymphoma; SS, Sezary syndrome; T-LBL, T-lymphoblastic leukemia/lymphoma; T-LGLL, T-cell large granular lymphocytic leukemia.
Mutational landscape of common T- and NK-cell neoplastic entities. Each column in the heat map represents a T/NK-cell neoplastic entity, and each row represents selected single nucleotide variants/indels and rearrangements (R), grouped according to functional annotations. The prevalence of mutations is color-coded, as shown in the legend. Genes with a mutational frequency of at least 10% observed in 1 or more mature T/NK-cell neoplasm were selected for display. Mutation frequencies 5%-10% were reported in some entities given their diagnostic or clinical value. ∗Refers to germ line mutation. BIA-ALCL, breast implant–associated ALCL; MF, mycosis fungoides; NK-LGLL, NK-large granular lymphocytic leukemia/chronic LPD of NK cells; pcALCL, primary cutaneous ALCL; SPTCL, subcutaneous panniculitis-like T-cell lymphoma; SS, Sezary syndrome; T-LBL, T-lymphoblastic leukemia/lymphoma; T-LGLL, T-cell large granular lymphocytic leukemia.
RNA-based gene expression profiling (GEP) has been instrumental in determining PTCL molecular subtypes based on biological signatures of physiological T-cell subsets and their microenvironment.28,30 Broad GEP approaches have not been widely translated to diagnostic practice, but there are new simplified assays in development that may provide clinical utility.33,46
Emerging data indicate that plasma circulating tumor DNA analysis (quantification, targeted sequencing, or methylation profiling) represents a clinically relevant biomarker for diagnostic prediction, assessment of pretreatment load and treatment response, and early relapse detection in patients with PTCLs.47-49
Practical approach to establishing a diagnosis
The diagnosis of T- and NK-neoplasms often requires knowledge of the host background, clinical presentation, laboratory findings, and pathological features. Host factors that could guide the diagnosis include rare genetic and other risk factors predisposing to the development of specific disease entities (Table 2). Sometimes, characteristic clinical findings such as autoimmunity, disseminated lymphadenopathy, skin rashes, and B symptoms as seen in TFH lymphoma may help guide the diagnosis. Other conditions such as hemophagocytic lymphohistiocytosis are less specific but may represent the presenting sign in a small number of patients with PTCL. However, in most cases, a diagnosis of PTCL is considered during pathological examination of lymph node, bone marrow, or extranodal tissue biopsies, performed for suspicion of hematologic disorder, malignancy, or another systemic disorder; or as an “incidental” finding. For cutaneous T-cell neoplasms, the clinical presentation and gross appearances may be so typical that a clinical diagnosis can be made.
In most cases, definitive diagnosis of PTCL requires integration of the pathological findings considering host factors and clinical features. Important clinical characteristics include age, sex, ethnicity, medical history, coexisting disorders (genetic predisposition, immunosuppression, autoimmune disease), (chronic) infections (eg, Epstein–Barr virus [EBV], HIV), site and extent of involvement, and associated symptoms. Serology testing for human T-lymphotropic virus 1 (HTLV-1) should be performed for patients from endemic areas, and is recommended for patients with a working diagnosis of PTCL, especially PTCL, NOS.6,34 The diagnostic approach based on clinicopathological scenarios is summarized in the following sections. Cutaneous presentations of T-cell neoplasms are summarized in Table 1 and will not be further discussed.
CD30+ T-cell lymphomas
A cohesive growth of large anaplastic cells including so-called “hallmark cells” with strong and homogeneous staining for CD30 is the defining feature of ALCLs. The 2 systemic ALCL entities are defined by the presence or lack of anaplastic lymphoma kinase (ALK) expression, and present typically as lymphadenopathy but can also primarily involve any extranodal tissue. The other 2 ALCL types are defined by their presentation in the skin or in the vicinity of a breast implant. With the exception of rare variants of ALK+ ALCL, the morphology is often clearly malignant with abundant mitoses and sometimes necrosis. In lymph nodes, sinusoidal involvement is characteristic but not always present, and may mimic the appearance of metastatic carcinoma or melanoma. The diagnostic workflow shown in Figure 3 considers lymphomas composed of large anaplastic CD30+ cells together with T-cell lymphomas with more heterogeneous CD30 expression, which also require ALK testing, as they may represent variants of ALK+ ALCL. The differential diagnosis of ALCL encompasses several lymphoma entities with overlapping morphology and/or immunophenotype, including EBV+ T- or NK-cell lymphomas, enteropathy-associated T-cell lymphoma (EATL), transformed mycosis fungoides, and PTCL, NOS. The border between ALK− ALCL and PTCL-NOS remains blurry and moving, but the distinction is clinically important given that patients with CD30+ PTCL-NOS appear to have a prognosis inferior to that of patients with ALK− ALCL.58 The recent finding of recurrent JAK2 fusions in PTCLs coexpressing CD30 and CD15 suggests that these cases should be considered in the spectrum of ALK− ALCL rather than be classified as PTCL, NOS, as initially proposed.53
Diagnostic approach to CD30+ T-cell lymphoma. Cohesive growth of large cells including hallmark cells, with strong and homogeneous expression of CD30 at the membrane and in the cytoplasm with a paranuclear “Golgi-like” pattern, suggests anaplastic large cell lymphoma (ALCL). Demonstration of T-cell surface markers or expression of cytotoxic molecules is required to show T-cell lineage and distinguish from potential mimics, such as metastatic melanoma or carcinoma, or a variety of other hematologic neoplasms that may be CD30+, notably classic Hodgkin lymphoma. Clonality studies may be necessary or useful in some instances to show monoclonal TR gene rearrangements because immunohistochemistry results may not accurately represent cell lineages, and some cases of ALCL may be completely negative for T-cell markers (“null” phenotype). EBV testing is recommended in cases of presumed ALCL or CD30+ PTCLs, and HTLV-1 serology may be helpful in selected instances because EBV-associated NK- or T-cell lymphomas (extranodal NK/T-cell lymphoma, nasal type [ENKTCL]; primary nodal EBV+T/NK-cell lymphoma or aggressive NK-cell leukemia) and ATLL can present as tumors with anaplastic morphology and CD30 expression,50-52 and EBV is by definition negative in ALCL. Immunohistochemistry is the routinely used method to detect ectopic ALK expression reflecting ALK rearrangement. In selected cases, FISH analysis or other genetic assays may be useful to confirm an ALK rearrangement or specific ALK fusion transcripts. In the small cell and the lymphohistiocytic variants of ALK+ ALCL, the neoplastic cells tend to be smaller with less numerous hallmark cells and show more heterogeneous CD30 staining, therefore ALK testing should be generously applied to other PTCLs with various levels of CD30 expression, especially in pediatric cases where ALK+ ALCL is most prevalent. Differentiating ALK− ALCL from CD30+ peripheral T-cell lymphoma, NOS (PTCL, NOS), may be difficult or subjective, and there is a number of cases whose classification remains uncertain.53-55 In addition, other lymphomas such as enteropathy-associated T-cell lymphoma (EATL), or transformed mycosis fungoides (MF), may resemble ALCL and involve lymph nodes.51 Therefore, clinical history, topography of the lesion, and staging need to be integrated into the diagnosis. Only cases with strong CD30+ expression can eventually be considered for ALK− ALCL, which may present in various sites. With extremely rare exceptions, those in the vicinity of a breast implant presenting as a periprosthetic effusion or a capsular mass in principle correspond to breast implant-associated (BIA) ALCL. Cutaneous presentation can reflect primary cutaneous (pc) ALCL or cutaneous presentation of a systemic disease, and likewise nodal ALK− ALCL may represent systemic disease or nodal dissemination from a primary cutaneous or breast implant–associated ALCL.56 Staging is essential to the correct diagnosis because there is no single phenotypic or genetic mark that reliably allows their distinction. In (systemic) ALK− ALCL, FISH testing for DUSP22 rearrangement (recommended by the ICC and optional in WHO5) enables the identification of DUSP22-rearranged cases, which represent a biologically distinct subgroup.57 FISH, fluorescent in situ hybridization.
Diagnostic approach to CD30+ T-cell lymphoma. Cohesive growth of large cells including hallmark cells, with strong and homogeneous expression of CD30 at the membrane and in the cytoplasm with a paranuclear “Golgi-like” pattern, suggests anaplastic large cell lymphoma (ALCL). Demonstration of T-cell surface markers or expression of cytotoxic molecules is required to show T-cell lineage and distinguish from potential mimics, such as metastatic melanoma or carcinoma, or a variety of other hematologic neoplasms that may be CD30+, notably classic Hodgkin lymphoma. Clonality studies may be necessary or useful in some instances to show monoclonal TR gene rearrangements because immunohistochemistry results may not accurately represent cell lineages, and some cases of ALCL may be completely negative for T-cell markers (“null” phenotype). EBV testing is recommended in cases of presumed ALCL or CD30+ PTCLs, and HTLV-1 serology may be helpful in selected instances because EBV-associated NK- or T-cell lymphomas (extranodal NK/T-cell lymphoma, nasal type [ENKTCL]; primary nodal EBV+T/NK-cell lymphoma or aggressive NK-cell leukemia) and ATLL can present as tumors with anaplastic morphology and CD30 expression,50-52 and EBV is by definition negative in ALCL. Immunohistochemistry is the routinely used method to detect ectopic ALK expression reflecting ALK rearrangement. In selected cases, FISH analysis or other genetic assays may be useful to confirm an ALK rearrangement or specific ALK fusion transcripts. In the small cell and the lymphohistiocytic variants of ALK+ ALCL, the neoplastic cells tend to be smaller with less numerous hallmark cells and show more heterogeneous CD30 staining, therefore ALK testing should be generously applied to other PTCLs with various levels of CD30 expression, especially in pediatric cases where ALK+ ALCL is most prevalent. Differentiating ALK− ALCL from CD30+ peripheral T-cell lymphoma, NOS (PTCL, NOS), may be difficult or subjective, and there is a number of cases whose classification remains uncertain.53-55 In addition, other lymphomas such as enteropathy-associated T-cell lymphoma (EATL), or transformed mycosis fungoides (MF), may resemble ALCL and involve lymph nodes.51 Therefore, clinical history, topography of the lesion, and staging need to be integrated into the diagnosis. Only cases with strong CD30+ expression can eventually be considered for ALK− ALCL, which may present in various sites. With extremely rare exceptions, those in the vicinity of a breast implant presenting as a periprosthetic effusion or a capsular mass in principle correspond to breast implant-associated (BIA) ALCL. Cutaneous presentation can reflect primary cutaneous (pc) ALCL or cutaneous presentation of a systemic disease, and likewise nodal ALK− ALCL may represent systemic disease or nodal dissemination from a primary cutaneous or breast implant–associated ALCL.56 Staging is essential to the correct diagnosis because there is no single phenotypic or genetic mark that reliably allows their distinction. In (systemic) ALK− ALCL, FISH testing for DUSP22 rearrangement (recommended by the ICC and optional in WHO5) enables the identification of DUSP22-rearranged cases, which represent a biologically distinct subgroup.57 FISH, fluorescent in situ hybridization.
ALK− ALCLs harboring a DUSP22 rearrangement (25%-30% of the cases) are biologically distinct from those lacking DUSP22 rearrangement, as they usually lack STAT3 activation, strongly express LEF-1, less frequently express cytotoxic molecules, and have distinctive transcriptomic and methylation profiles.59 However, the prognostic value of DUSP22 status is more controversial.60,61 The presence of DUSP22-R does not discriminate between systemic and primary cutaneous cases62 but excludes BIA-ALCL. The detection of other rearrangements involving TP63, VAV1, JAK2, and TYK2, which are less common and not specific to ALK− ALCL, may be useful in certain circumstances.63,64
Lymph node involvement by T/NK-cell proliferations
Lymphadenopathy is the most common presentation for PTCL, and the primary nodal entities are ALCLs (discussed above), TFH lymphoma, primary nodal EBV+ T-cell or NK-cell lymphoma, and PTCL, NOS (Table 1). The morphologic spectrum is broad (Figure 4, discussed below). In addition, lymph nodes may be involved by T-lymphoblastic or mature leukemias (especially adult T-cell lymphocytic leukemia [ATLL] and T-PLL) or cutaneous or extranodal NK/T-cell neoplasms. EBV testing is recommended in all PTCL cases.
Histological presentation of primary nodal T- and NK-cell lymphomas. Anaplastic large cell lymphomas (ALCLs), ALK+ or ALK−, tend to invade the peripheral sinuses and produce cohesive sheet-like infiltrates in the lymph node. Peripheral T-cell lymphoma, NOS (PTCL, NOS) is not associated with a characteristic pattern of growth, and can to a variable extent replace the normal lymphoid tissue. Subtypes of PTCL, NOS are composed of mostly CD4+ or CD8+ lymphoid cells with a phenotype resembling that of Th1 or Th2 cells, or of cells expressing cytotoxic molecules. The tumor microenvironment tends to be more abundant in Th1-type PTCL, NOS, and can sometimes comprise abundant epithelioid histiocytes. Primary nodal EBV+ lymphomas derived of cytotoxic T cells, or less commonly NK cells, represent a rare aggressive entity, which is mostly reported in Asians. Lymphomas derived from CD4+ TFH cells (TFHL) represent the most prevalent nodal PTCL with a variety of histological patterns. The most common form of TFHL is the angioimmunoblastic type (TFHL-AI), which in its usual form is a diffuse microenvironment-rich tumor, comprising a proliferation of arborizing vessels and follicular dendritic cells, and an infiltrate of many reactive large (blastic) and small B cells, plasma cells, histiocytes, and non-neoplastic T cells. Less commonly, the neoplastic cells of TFHL-AI concentrate around reactive or regressive germinal centers (patterns 1 and 2), and these may be more difficult to diagnose because of the association with reactive follicles. In the uncommon follicular type of TFHL (TFHL-F), the neoplastic TFH cells grow in follicles, resembling folliicular lymphoma (FL-like), or in clusters within large B-cell nodules resembling progressively transformed germinal centers, which is an uncommon form of reactive follicular hyperplasia (PTGC-like). The NOS subtype of TFHL, defined by the TFH phenotype of the neoplastic cells, does not contain the complete microenvironment of TFHL-AI, grows diffusely, and may preferentially distribute in the paracortex and between preserved follicles. TFHL, in particular the AI and follicular subtypes, may contain large atypical cells resembling Reed-Sternberg cells, a source of frequent diagnostic difficulties in distinguishing them from classic or nodular lymphocyte predominant Hodgkin lymphoma.
Histological presentation of primary nodal T- and NK-cell lymphomas. Anaplastic large cell lymphomas (ALCLs), ALK+ or ALK−, tend to invade the peripheral sinuses and produce cohesive sheet-like infiltrates in the lymph node. Peripheral T-cell lymphoma, NOS (PTCL, NOS) is not associated with a characteristic pattern of growth, and can to a variable extent replace the normal lymphoid tissue. Subtypes of PTCL, NOS are composed of mostly CD4+ or CD8+ lymphoid cells with a phenotype resembling that of Th1 or Th2 cells, or of cells expressing cytotoxic molecules. The tumor microenvironment tends to be more abundant in Th1-type PTCL, NOS, and can sometimes comprise abundant epithelioid histiocytes. Primary nodal EBV+ lymphomas derived of cytotoxic T cells, or less commonly NK cells, represent a rare aggressive entity, which is mostly reported in Asians. Lymphomas derived from CD4+ TFH cells (TFHL) represent the most prevalent nodal PTCL with a variety of histological patterns. The most common form of TFHL is the angioimmunoblastic type (TFHL-AI), which in its usual form is a diffuse microenvironment-rich tumor, comprising a proliferation of arborizing vessels and follicular dendritic cells, and an infiltrate of many reactive large (blastic) and small B cells, plasma cells, histiocytes, and non-neoplastic T cells. Less commonly, the neoplastic cells of TFHL-AI concentrate around reactive or regressive germinal centers (patterns 1 and 2), and these may be more difficult to diagnose because of the association with reactive follicles. In the uncommon follicular type of TFHL (TFHL-F), the neoplastic TFH cells grow in follicles, resembling folliicular lymphoma (FL-like), or in clusters within large B-cell nodules resembling progressively transformed germinal centers, which is an uncommon form of reactive follicular hyperplasia (PTGC-like). The NOS subtype of TFHL, defined by the TFH phenotype of the neoplastic cells, does not contain the complete microenvironment of TFHL-AI, grows diffusely, and may preferentially distribute in the paracortex and between preserved follicles. TFHL, in particular the AI and follicular subtypes, may contain large atypical cells resembling Reed-Sternberg cells, a source of frequent diagnostic difficulties in distinguishing them from classic or nodular lymphocyte predominant Hodgkin lymphoma.
When a PTCL composed of a monotonous population of atypical small/medium-sized T cells is in consideration, a lymphoblastic proliferation must be excluded, especially if the cells are blastoid or a high mitotic rate is observed. By IHC, immature T cells express terminal deoxynucleotidyl transferase and variably cell surface T-cell antigens such as double positive or double negative expression of CD4 and CD8. The differential diagnosis includes indolent T-lymphoblastic proliferations of polyclonal terminal deoxynucleotidyl transferase-positive T-lymphoblasts, which can be associated with various benign or malignant conditions.65 Another benign condition that may be misinterpreted as T-cell lymphoblastic lymphoma or PTCL is the autoimmune lymphoproliferative syndrome, usually encountered in infants or children. Autoimmune lymphoproliferative syndrome cases show an expansion of double-negative CD4−CD8− cytotoxic T cells in tissues and the blood.66,67 Another notable pitfall is the Kikuchi-Fujimoto disease, a disease of young female adults who present with cervical lymphadenopathy, systemic symptoms, and autoimmune manifestations. The lymph nodes comprise a necrotizing lymphohistiocytic infiltrate with large CD8+ cytotoxic T cells and may be confused with PTCL.68
Polymorphous T-cell infiltrates intermixed with eosinophils, histiocytes (sometimes with microgranulomas), B cells, and plasma cells represent one of the most challenging scenarios raising many differential diagnoses, including a variety of benign conditions. These comprise drug-induced, postvaccination reactions, or viral infections such as infectious mononucleosis, which may comprise atypical CD8+ cells and harbor oligo- or monoclonal TR gene rearrangements.69,70 Follicular helper T-cell lymphoma of the angioimmunoblastic type (TFHL-AI or AITL) is the prototypic entity presenting as a polymorphous infiltrate of neoplastic CD4+ T cells and various reactive cells in the background of prominent arborizing vasculature and a proliferation of follicular dendritic cells, highlighted by immunostains for CD21 or CD23.71 Immunohistochemistry for TFH markers (BCL6, CD10, PD1, ICOS, and CXCL13) is useful in this setting to confirm the diagnosis. In addition, testing for TFH markers is required to identify the less common patterns of TFHL-AI with neoplastic cells distributed around germinal centers or regressive follicles,72 and the other subtypes of TFHL, NOS, and follicular. In some instances, early involvement by TFHL-AI can result in a very low burden of neoplastic cells, which may be difficult to identify, even with immunostains. In those cases, flow cytometric analysis and molecular studies on biopsies are useful adjuncts to the diagnosis.73 Specifically, RHOA Gly17Val mutation analysis is valuable in the early detection of TFHL-AI.74 This hot spot mutation, which occurs in ∼60% of the patients with TFHL-AI, is rather specific for TFHL, in contrast to TET2 and DNMT3A mutations, which are highly prevalent not only in TFHL but also PTCL, NOS, and might reflect underlying clonal hematopoiesis.15,16 In addition, flow cytometric analysis of the peripheral blood can detect abnormal CD4+ T cells coexpressing CD10 or ICOS in most patients with TFHL, even when lymphopenic.75 TFHL, NOS is a CD4+ PTCL that does not contain the typical microenvironment of TFHL-AI, but expresses a TFH phenotype, shown by the expression of at least 2, ideally 3 TFH markers. In the follicular subtype of TFHL, the pattern may resemble follicular lymphoma, or progressive transformation of the germinal centers, and the neoplastic T cells usually strongly express several TFH markers. A common feature of TFHL subtypes is the frequent presence of morphologically heterogeneous and often EBV+ and clonal B-cell and plasma cell proliferations including large B cells expressing CD30 and resembling Reed–Sternberg cells. Such B-cell proliferations may be confused with classic B-cell lymphomas or nodular lymphocyte-predominant Hodgkin lymphoma.63,76-78 In difficult cases, the diagnosis may require the use of clonality tests for B and T cells or mutation analysis.45,73 Lymphoepithelioid PTCL is a morphologic descriptor for PTCLs with a high content in epithelioid histiocytes, and a diffuse or paracortical pattern of growth, which may correspond to TFHL or PTCL, NOS.79
PTCL, NOS is a diagnosis of exclusion, once other PTCL entities (including extranodal PTCL entities and T-cell leukemias) have been excluded. It is, in essence, biologically and clinically heterogeneous. Two molecular subtypes of PTCL, NOS are recognized, namely TBX21-PTCL, NOS/Th1 and GATA-3- PTCL, NOS/Th2. These subtypes, defined by GEP, exhibit different levels of genomic complexity (higher in the GATA-3 subtype characterized by biallelic deletion/mutation of TP53, CDKN2A/B, or RB1) and prognosis (better for the PTCL-TBX21 subtype).30,80 The TBX21-PTCL, NOS/Th1 subtype, in addition to CD4+ phenotype, comprises a distinct subset of cases with an activated CD8+ T-cell cytotoxic signature with DNMT3A mutations and an inferior outcome relative to DNMT3A-WT TBX21-PTCL, NOS.81 Molecular subtypes may be reproduced by an immunohistochemical algorithm using 4 markers (TBX21, CXCR3, GATA-3, and CCR4), and a digital NanoString-based assay.32,33 These findings need to be confirmed in larger cohorts, and currently, both the ICC and the WHO classification do not require molecular subtyping of PTCL, NOS in routine clinical practice.
Extranodal presentations of NK/T-cell neoplasms
Extranodal entities (Table 1) are best discussed in the context of anatomical presentation. ENKTCL, nasal type, typically develops in the nasal area, predominantly in male adults, but can be seen in any other extranodal organ, including skin and soft tissues, or even the brain. By definition, the tumor must be EBV+. In contrast, BIA-ALCL exclusively occurs in the breast around the implant capsule. Other specific entities include T-cell and NK-cell lymphoproliferations in the gastrointestinal tract and HSTL.
T-cell and NK-cell lymphoproliferations in the gastrointestinal tract
The gastrointestinal tract is the second most frequent extranodal compartment, after the skin, involved by T/NK-cell neoplasms, which are summarized in Figure 5. EATL and monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) are the 2 main primary intestinal PTCL entities. They are often diagnosed in surgical resections performed for perforation or obstruction caused by a lymphoma mass. EATL and MEITL have distinctive epidemiology, morphology, immunophenotype, and mutation patterns. Notably, SETD2 mutations are almost always found in MEITL but are rarely reported in EATL.83-85 Intestinal T-cell lymphoma, NOS is a rare classification applied to aggressive tumors not qualifying for EATL or MEITL. Refractory celiac disease type II and gastrointestinal T/NK-cell LPDs present with digestive or abdominal symptoms and are usually diagnosed on endoscopic mucosal biopsies. Refractory celiac disease, type II is a neoplastic in situ/intraepithelial proliferation of abnormal IELs showing an aberrant immunophenotype (loss of sCD3 or CD8 or TCR expression) with monoclonal TR gene rearrangement and mutations in JAK1 or STAT3.86 In indolent clonal T-cell LPD of the GI tract, the mucosa contains diffuse infiltrates of small monotonous lymphoid cells. The infiltrate may contain phenotypically aberrant T cells, but demonstration of clonality at the genomic level is required to confirm the diagnosis.87,88 This condition, also named “indolent T-cell lymphoma of the gastrointestinal tract,”5 usually follows a chronic and relapsing course, but rare cases may transform into aggressive PTCL. Similar gastrointestinal indolent LPDs derived from NK cells are also recognized. They are morphologically more atypical, and by definition should be EBV−, in contrast to ENKTCL, which may involve the gastrointestinal tract. Other T/NK-cell neoplastic entities to consider in the differential diagnosis with primary intestinal diseases include ATLL, ALCL, and chronic active EBV disease.
Differential diagnosis of T-cell lymphomas and T/NK-cell LPDs in the gastrointestinal tract (modified from de Leval et al82). CD, celiac disease; CM, cytotoxic molecules; EBV, Epstein-Barr virus; ENKTCL, extranodal NK/T-cell lymphoma, nasal type; IBD, inflammatory bowel disease; ITCL, NOS, intestinal T-cell lymphoma, not otherwise specified; NK-LPD-GI, indolent NK-cell LPD of the gastrointestinal tract; RCD, refractory CD; T-LPD-GI, indolent clonal T-cell LPD of the gastrointestinal tract (ICC), indolent T-cell lymphoma of the gastrointestinal tract (WHO5).
Differential diagnosis of T-cell lymphomas and T/NK-cell LPDs in the gastrointestinal tract (modified from de Leval et al82). CD, celiac disease; CM, cytotoxic molecules; EBV, Epstein-Barr virus; ENKTCL, extranodal NK/T-cell lymphoma, nasal type; IBD, inflammatory bowel disease; ITCL, NOS, intestinal T-cell lymphoma, not otherwise specified; NK-LPD-GI, indolent NK-cell LPD of the gastrointestinal tract; RCD, refractory CD; T-LPD-GI, indolent clonal T-cell LPD of the gastrointestinal tract (ICC), indolent T-cell lymphoma of the gastrointestinal tract (WHO5).
Hepatosplenic T-cell lymphoma
In HSTL, the presentation may mimic inflammatory systemic disorders with B symptoms, splenomegaly without overt tumoral syndrome, or lymphadenopathy. Patients with HSTL lack leukemic cells at diagnosis but present with cytopenia, especially thrombocytopenia and anemia. As splenectomy is rarely performed, the diagnosis is often established by a bone marrow biopsy or liver biopsy. These biopsies typically reveal exclusive intrasinusoidal infiltration of medium-sized lymphoid cells with a CD3+, CD5−, CD4−/CD8−, CD56+ phenotype and a TIA1+/granzyme B−/perforin− nonactivated cytotoxic profile.89,90 The neoplastic T cells usually express the γδ TCR, and rarely an αβ phenotype. HSTL is characterized by isochromosome 7q in most cases and common mutations in SETD2 and STAT5B.91
Peripheral blood and bone marrow involvement
In the presence of lymphocytosis, examination of blood smears and/or marrow by morphology, MFC immunophenotyping, chromosome analysis, or molecular genetic analysis is required to identify T-cell neoplasms. Common leukemic T- and NK-cell neoplasms include T-PLL, Sézary syndrome, ATLL, aggressive NK-cell leukemia, and T-cell large granular lymphocytic leukemia (Figure 6). In addition, rarely PTCLs such as ALCL ALK+ and TFHL may have a leukemic dissemination, either at presentation or during the course of the disease. T-PLL, a rare mature T-cell leukemia, is characterized by significant lymphocytosis, B symptoms, hepatosplenomegaly, and in 25% of the patients, nodal or extranodal involvement. The major diagnostic criteria include >5 × 109/L lymphocytes with a T-PLL phenotype, the demonstration of T-cell clonality, and anomalies of 14q32 or Xq28 or expression of TCL1A/B, or MTCP1.92 The diagnosis of Sézary syndrome, an aggressive form of cutaneous T-cell lymphoma characterized by erythroderma, lymphadenopathy, and circulating atypical lymphocytes (Sézary cells above 1 × 109/L), largely relies on the clinical presentation, the demonstration of clonal CD4+, CD7−, usually KIR3DL2+ Sézary cells in the blood, and the histopathology of the skin biopsy.93,94 ATLL, which arises from clonally expanded T cells infected with by HTLV-1, is often characterized by widespread lymphadenopathy and peripheral blood involvement, sometimes with skin and other extranodal site of involvement.95 ATLL neoplastic cells, referred to as “flower cells” on blood smears, show variable morphology in tissue sections, express CD4 and CD25, but lack CD7.50 The diagnosis of ATLL is confirmed by serologic tests for HTLV-1 antibody or, more specifically, by the demonstration of monoclonal integration of proviral HTLV-1 DNA in the neoplastic cells.96 Aggressive NK-cell leukemia, a rare systemic NK-cell neoplasm associated with EBV in most cases, is more frequent in young to middle-aged adults of Asian ethnicity. The patients usually present with hepatosplenomegaly, B symptoms, cytopenia, hemophagocytic syndrome, variable leukemic spread, and typically follow a fulminant course.97 Examinations of peripheral blood and bone marrow are the most common diagnostic procedures.
Differential diagnosis of leukemic T- and NK-cell neoplasms. ∗NK-LGLL differs from T-LGLL by the absence of expression of the TCR and lack of monoclonal TR rearrangement; the clonal neoplastic nature of this disorder is supported by a restricted activated KIRs expression and the presence of acquired mutations, especially in TET2, STAT3, and CCL22 (mostly exclusive). ANKL, aggressive NK-cell leukemia; BM, bone marrow biopsy; HPS, hemophagocytic syndrome; LN, lymphadenopathy; NK-LGLL, chronic LPD of NK cells/NK large granular lymphocytic leukemia; SS, Sezary syndrome; T-LGLL, T-cell large granular lymphocytic leukemia.
Differential diagnosis of leukemic T- and NK-cell neoplasms. ∗NK-LGLL differs from T-LGLL by the absence of expression of the TCR and lack of monoclonal TR rearrangement; the clonal neoplastic nature of this disorder is supported by a restricted activated KIRs expression and the presence of acquired mutations, especially in TET2, STAT3, and CCL22 (mostly exclusive). ANKL, aggressive NK-cell leukemia; BM, bone marrow biopsy; HPS, hemophagocytic syndrome; LN, lymphadenopathy; NK-LGLL, chronic LPD of NK cells/NK large granular lymphocytic leukemia; SS, Sezary syndrome; T-LGLL, T-cell large granular lymphocytic leukemia.
In contrast, T-cell large granular lymphocytic leukemia is a chronic indolent disorder affecting frequently elderly patients with autoimmune conditions, who are either asymptomatic or present with cytopenia, moderate splenomegaly, and a leukemic picture.98 Blood or bone marrow smear examination, together with MFC, demonstrates an increased number (0.5 × 109/L) of lymphocytes containing azurophilic granules, an activated cytotoxic phenotype (CD3+, CD8+, CD57+, TCRαβ+, CD56−), and clonal TR gene rearrangement. In rare cases with a CD4+, CD4−/CD8−, or TCRγδ+ phenotype, the demonstration of STAT3 or STAT5B mutations may be helpful.99 A subset of patients with similar features but showing an NK-cell phenotype are referred to as NK-LGLL5 or “chronic lymphoproliferative disorder of NK cells.”4,100
Future directions and conclusions
Within the last 2 decades, there has been significant progress in the understanding of the biology and genetics of PTCL, and these findings have formed the basis of modern classifications. Application of these diagnostic paradigms based on new biological knowledge has allowed for novel clinical interventions, such as the use of epigenetic modifying agents in TFHL. However, overall, the development of precision therapy has been limited because of challenges in the application of these advancements to clinical practice. Broader understanding of phenotypic, epigenetic, and genetic features of the neoplastic cells and their microenvironment, achieved through high-resolution genomic tools such as optical genome mapping, whole genome sequencing, assay for transposase-accessible chromatin with sequencing, circulating tumor DNA analysis, and single-cell characterization methods, holds the promise of new insights that may have important therapeutic applications. In this regard, it is our hope that the next generation of lymphoma classification will offer a unified platform integrating all new knowledge to advance patient care.
Acknowledgments
Authorship
Contribution: L.d.L., P.G., and A.D. conceived the structure of the article, conceived and prepared the figures, and wrote the article; and L.d.L. prepared the tables.
Conflict-of-interest disclosure: L.d.L. had a consulting or advisory role for Abbvie, Lunaphore Technologies, Bayer, Blueprint Medicines, Novartis, and Roche (all institutional), and received travel grants from Roche. P.G. received research funding from Alderan, Innate Pharma, Takeda, and Sanofi; had a consulting or advisory role for Takeda and Gilead; and received travel grants from Roche. A.D. received research support from Roche and Astra-Zeneca.
Correspondence: Laurence de Leval, Institute of Pathology, Lausanne University Hospital, 25 rue du Bugnon, 1011 Lausanne, Switzerland; email: laurence.deleval@chuv.ch.


![Diagnostic approach to CD30+ T-cell lymphoma. Cohesive growth of large cells including hallmark cells, with strong and homogeneous expression of CD30 at the membrane and in the cytoplasm with a paranuclear “Golgi-like” pattern, suggests anaplastic large cell lymphoma (ALCL). Demonstration of T-cell surface markers or expression of cytotoxic molecules is required to show T-cell lineage and distinguish from potential mimics, such as metastatic melanoma or carcinoma, or a variety of other hematologic neoplasms that may be CD30+, notably classic Hodgkin lymphoma. Clonality studies may be necessary or useful in some instances to show monoclonal TR gene rearrangements because immunohistochemistry results may not accurately represent cell lineages, and some cases of ALCL may be completely negative for T-cell markers (“null” phenotype). EBV testing is recommended in cases of presumed ALCL or CD30+ PTCLs, and HTLV-1 serology may be helpful in selected instances because EBV-associated NK- or T-cell lymphomas (extranodal NK/T-cell lymphoma, nasal type [ENKTCL]; primary nodal EBV+T/NK-cell lymphoma or aggressive NK-cell leukemia) and ATLL can present as tumors with anaplastic morphology and CD30 expression,50-52 and EBV is by definition negative in ALCL. Immunohistochemistry is the routinely used method to detect ectopic ALK expression reflecting ALK rearrangement. In selected cases, FISH analysis or other genetic assays may be useful to confirm an ALK rearrangement or specific ALK fusion transcripts. In the small cell and the lymphohistiocytic variants of ALK+ ALCL, the neoplastic cells tend to be smaller with less numerous hallmark cells and show more heterogeneous CD30 staining, therefore ALK testing should be generously applied to other PTCLs with various levels of CD30 expression, especially in pediatric cases where ALK+ ALCL is most prevalent. Differentiating ALK− ALCL from CD30+ peripheral T-cell lymphoma, NOS (PTCL, NOS), may be difficult or subjective, and there is a number of cases whose classification remains uncertain.53-55 In addition, other lymphomas such as enteropathy-associated T-cell lymphoma (EATL), or transformed mycosis fungoides (MF), may resemble ALCL and involve lymph nodes.51 Therefore, clinical history, topography of the lesion, and staging need to be integrated into the diagnosis. Only cases with strong CD30+ expression can eventually be considered for ALK− ALCL, which may present in various sites. With extremely rare exceptions, those in the vicinity of a breast implant presenting as a periprosthetic effusion or a capsular mass in principle correspond to breast implant-associated (BIA) ALCL. Cutaneous presentation can reflect primary cutaneous (pc) ALCL or cutaneous presentation of a systemic disease, and likewise nodal ALK− ALCL may represent systemic disease or nodal dissemination from a primary cutaneous or breast implant–associated ALCL.56 Staging is essential to the correct diagnosis because there is no single phenotypic or genetic mark that reliably allows their distinction. In (systemic) ALK− ALCL, FISH testing for DUSP22 rearrangement (recommended by the ICC and optional in WHO5) enables the identification of DUSP22-rearranged cases, which represent a biologically distinct subgroup.57 FISH, fluorescent in situ hybridization.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/18/10.1182_blood.2023021786/2/m_blood_bld-2023-021786-c-gr3.jpeg?Expires=1767812352&Signature=2~MVtLp3n-wL5OaZ03XvndRAfkkxdZNRAQwk520YxpcYykMhsCrGHaAJhIDnWffBsGh8i1u-VCB3i9Ae1ReXid~~ZfMxLxGfA37zaqIO4~Umh0WieJAxsYPDbzWt3UpGN0RcwHkzmjqc8oNQRkjOjIhG2s9ZO1aKlY8eYeA~8o58Rw56cbb1QQ4FdQbvIi-YarHbFG6MhCEOTCjtKW6QAEUihSfQ0IqfDSDRysmlN2OKKqYPlrFwd9g19utPccHmyhRCYJnQDvcDiYH0hxfTkmJUuv5iBUk6Bs-3PAnKo56nhfIwcKPgpkCWqPhwCPduGnjy5pEcQJUulryLAfvTSw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



