In this issue of Blood, Sundler Björkman et al present data on families with hereditary angioedema (HAE) identified by linking data from the Swedish Multi-Generation Register for establishing familial relationships and the Swedish National Patient Register for the diagnosis of venous thromboembolism (VTE).1 They found that family members with HAE had a 2.5 times higher risk of VTE than relatives without HAE.
Most patients with HAE have either a congenital deficiency (type I) or a congenital dysfunction (type II) of the C1 esterase inhibitor (C1-INH).2 The C1-INH has important functions in the complement and kinin-kallikrein systems and acts as a natural anticoagulant by inhibiting several coagulation factors, including factors XII and XI, and to a lesser extent also thrombin.2
Only recently, evidence was presented that C1-INH deficiency confers hypercoagulability in humans in vitro and in mice ex vivo. Grover et al reported that patients deficient in C1-INH exhibit coagulation system activation as reflected by an increased contact pathway–mediated in vitro thrombin generation. In the same study, C1-INH-deficient mice had higher plasma levels of coagulation activation markers and enhanced venous thrombus formation in an inferior vena cava stenosis model.3
There is now evidence that, in addition to their distinctive clinical features including attacks of cutaneous and submucosal swelling and abdominal pain, patients with HAE indeed also have an increased risk of VTE. In a Swedish registry-based study, individuals with HAE had a more than 1.8-fold higher risk of cardiovascular disease compared with a control cohort from the general population.4 Using the same database, Grover et al reported a more than 3.5-fold increase in the VTE risk.5
In the study reported in this issue of Blood, Sundler Björkman et al went further by estimating the VTE risk of patients with HAE in a large thrombophilia family study. They compared 365 cases of HAE with 1641 controls without HAE from 276 pedigrees and found an incidence rate of VTE of 1.93 per 1000 person-years among patients with HAE compared with 0.82 per 1000 person-years in family members without HAE, which translates into a statistically significant incidence ratio of 2.35. The multivariate hazard ratio for VTE among patients with HAE compared with individuals without HAE amounted to 2.51. The higher risk of VTE was restricted to patients younger than 70 years and was robust, as it was not attenuated by comorbidities or major risk conditions including thrombophilia, obesity, coronary heart disease, cerebrovascular disease, chronic kidney disease, liver disease, or cancer. This article, however, does not provide C1-INH plasma concentrations. Thus, it remains uncertain whether a relationship exists between levels of C1-INH and severity of thrombosis risk.
First-line treatment for patients with HAE includes intravenous or subcutaneous replacement of the C1-INH,2 and it would be important to find out whether this has also an effect on the thrombotic risk of these patients. There are 2 studies that support this notion. In a retrospective study from Hungary, administration of C1-INH preparations was associated with a 10-fold lower incidence of VTE.6 In a nested case-control study from Norway, individuals with C1-INH plasma levels in the highest quartile had a more than 30% lower risk of VTE compared with those with levels in the lowest quartile.7 If this concept holds true, C1-INH replacement therapy would indeed reduce the risk of VTE, at least in those patients who receive prophylactic rather than on demand treatment. Unfortunately, the authors were unable to address this important aspect, as the number of patients with HAE who had received C1-INH replacement was small.
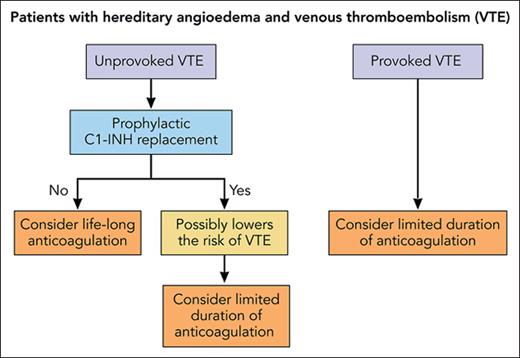
VTE is a chronic disease, which tends to recur.8 The recurrence risk is low in patients in whom the incident VTE has occurred in association with a temporary and removable risk condition including surgery, trauma, or immobilization. These patients are usually treated with anticoagulants for a limited time irrespective of the presence or absence of underlying genetic defects.9 Despite the absence of clinical data, we propose that after an episode of provoked VTE, patients with HAE should not be treated differently compared with patients without HAE (see figure).
Proposal of anticoagulant treatment in patients with HAE and a history of VTE. Professional illustration by Patrick Lane, ScEYEnce Studios.
Proposal of anticoagulant treatment in patients with HAE and a history of VTE. Professional illustration by Patrick Lane, ScEYEnce Studios.
The recurrence risk is much higher in patients with an unprovoked VTE, and a guideline of the American Society of Hematology suggests extended-phase anticoagulation for these patients provided that their bleeding risk is low.8 This implies also that patients with HAE who had experienced an unprovoked VTE are candidates for lifelong anticoagulation. According to a meta-analysis, the incidence of major bleeding during extended-phase anticoagulation is considerable with 1.74 events per 100 person-years for vitamin K antagonists and 1.12 events per 100 person-years for the direct oral anticoagulants.10 To establish the optimal duration of anticoagulation, it is crucial to balance the risk of recurrent VTE against the bleeding risk during anticoagulation. If the hypothesis holds true that replacement with C1-INH lowers the thrombosis risk of patients with HAE, the scale could be tipped from indefinite anticoagulation toward an antithrombotic regimen of limited duration (see figure). Further studies on the risk of recurrent VTE of patients with HAE are therefore warranted.
Conflict-of-interest: The authors declare no competing financial interests.