Key Points
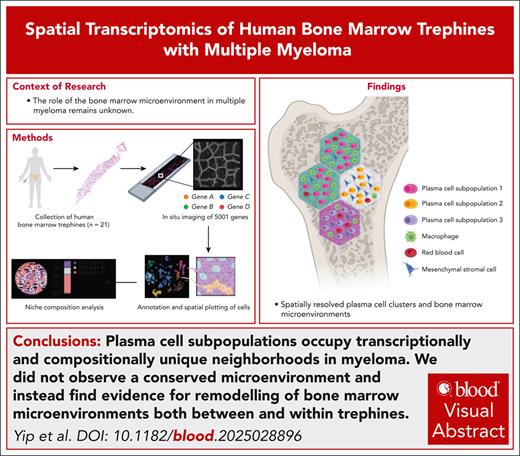
The first map of human bone marrow was generated using single-cell spatial transcriptomics profiling of 5001 genes.
Malignant plasma cells form spatially segregated microenvironments with distinct transcriptional profiles and cell composition.
Visual Abstract
The bone marrow microenvironment is intimately linked to the biology that underpins the development and progression of multiple myeloma. However, the complex cellular and molecular features that form bone marrow niches are poorly defined. Here, we used subcellular spatial transcriptomics to profile the expression of 5001 genes in human bone marrow in the context of multiple myeloma. Using this approach, we explored the plasma cell and stroma ecosystem in bone marrow trephine biopsy specimens (herein referred to as trephines) from 21 individuals, including 7 with premalignant disease and 10 with newly diagnosed multiple myeloma. Using spatial transcriptomics in conjunction with an optimized trephine biobanking methodology, we could resolve major components of the human bone marrow microenvironment and reliably characterize distinct plasma cell populations in samples from healthy, premalignant disease and active myeloma. When plasma cells were visualized in the context of location, we detected spatially restricted subpopulations of plasma cells in 5 of 10 newly diagnosed myeloma trephines. Surprisingly, the composition of hematopoietic and stromal microenvironments varied significantly between newly diagnosed myeloma trephines. Furthermore, these differences in microenvironments were also observed within trephines that had spatially restricted plasma cell subpopulations. Thus, these data are not consistent with the hypothesis that a universal bone marrow microenvironment supports the expansion of malignant plasma cells in myeloma. Instead, we propose that myeloma subpopulations form distinct microenvironments and can vary both between patients and spatial locations.
Introduction
The architecture of the bone marrow (BM) is complex and hypothesized to encompass diverse microenvironments that are critical regulators of both steady-state and malignant hematopoiesis.1-4 Each microenvironment is proposed to have a unique composition of both hematopoietic and nonhematopoietic cells that have been implicated to regulate proliferation, differentiation, and quiescence of surrounding cells. Accordingly, BM microenvironments have been linked to the differentiation of hematopoietic stem cells2,5 and bone metastasis of solid tumors,6,7 as well as disease progression and therapy resistance in hematological malignancies, such as multiple myeloma (MM).8 MM is characterized by the expansion of malignant plasma cells (PCs) in the BM. This PC population is composed of multiple subclones that are detected at diagnosis. Clonal evolution and selection pressure in response to therapy are critical factors that drive both disease progression and relapse.9 The manifestation of MM is preceded by asymptomatic precursor conditions known as monoclonal gammopathy of undetermined significance (MGUS) and smoldering myeloma (SM).10 Despite advances in the clinical criteria defining MGUS and SM, the molecular mechanisms driving MGUS progression to symptomatic myeloma, particularly the contribution of the BM microenvironment, remain poorly understood.
Single-cell RNA sequencing (scRNA-seq) studies in MM have provided valuable insight into genetic heterogeneity as well as changes in hematopoietic, immune, and stromal cells during disease development.11-13 However, experimental variation introduced during tissue digestion affects the characterization of stromal cells and does not capture the spatial distribution of cells and changes in local gene expression.14,15 Thus, the role of cell-cell interactions or ligands in MM BM microenvironments and their interplay with malignant PCs in situ remains unknown.14 The longstanding dogma in hematology has been that malignant cells form universal, disease-specific microenvironments that drive disease progression.16 To answer this question we performed spatial profiling of human BM in the context of MM. Surprisingly, we did not observe a conserved microenvironment and instead find evidence for remodeling of BM microenvironments both between and within trephine biopsy specimens (herein referred to as trephines), driven by malignant PCs themselves.
Methods
Collection and processing of human BM trephines
BM trephines from the posterior superior iliac spine were collected from patients undergoing routine investigation of suspected PC disorders (detailed in supplemental Table 1, available on the Blood website). Control samples were obtained from patients with lymphoma undergoing staging BM biopsy, with no marrow involvement confirmed by immunohistochemistry. Upon collection, trephines were immediately fixed in 10% neutral buffered formalin for at least 24 hours. Samples were then either embedded directly in paraffin (nondecalcified) or decalcified with 10% EDTA pH 7.2 solution (Milestone) for 2 to 4 days before paraffin embedding.
Xenium in situ workflow
Five-micrometer sections of mouse and human BM trephines were mounted on Xenium slides and processed according to the manufacturer’s protocol (10x Genomics). Three predesigned gene panels were used in this study: (1) mouse tissue atlassing panel (379 genes); (2) human multitissue and cancer panel (377 genes); and (3) Xenium Prime 5K human pan-tissue and pathways panel (5001 genes). Run matrices are provided in supplemental Table 2. After Xenium imaging, slides were stained with hematoxylin and eosin and imaged on a Slideview VS200 research slide scanner (Olympus Life Science). Hematoxylin and eosin and Xenium express data were coregistered using a manual key point–based alignment method in Xenium Explorer v3.0 (10x Genomics).
Xenium data preprocessing and downstream analysis
Cells with <60 total transcripts counts or <50 detected genes were removed from the analysis. Downstream analyses of the Xenium data were conducted using R packages Seurat v5.1.017 and edgeR v4.3.17.18 Unsupervised clustering was performed on individual samples using Seurat’s FindClusters function, with a resolution ranging between 0.1 and 0.2. Markers of each cell cluster were detected using Seurat’s FindAllMarkers function. PCs and stromal cells were identified based on cluster-specific marker expression before being extracted for combined analyses. In the combined stromal analyses, cell clusters that expressed blood cell signatures were excluded as contaminants. Cells were also annotated using the SingleR package with a reference of single-cell RNA BM atlas from previous studies.15,19 Pseudobulk differential expression analyses between control, MGUS/SM, and MM samples were conducted in edgeR. Compositional differences between samples were normalized using the trimmed mean of M-values method. Quasi-negative binomial generalized linear models were fitted to each gene using the glmQLFit function in edgeR. Differential expression was assessed using the quasi-likelihood F test. Genes were considered to be differentially expressed if their false discovery rate was <5%. Cell type densities were calculated using a kernel density approach implemented in the scider package.20 Cells were grouped into hexagonal bins, each 80-μm wide, followed by unsupervised clustering on these bins. Differential cell type proportion analyses between clusters of hexagonal bins were performed using a χ2 test.
All materials were obtained after written informed consent and approved by the institutional review boards of the Walter and Eliza Hall Institute of Medical Research and Peter MacCallum Cancer Centre.
Results
Feasibility of subcellular spatial transcriptomics in human BM biopsies
Clinical BM trephines are routinely processed using harsh fixation and decalcification steps. These procedures cause significant RNA degradation, rendering them unsuitable for in situ spatial transcriptomics. Therefore, we developed a protocol for the collection of trephines that was compatible with spatial transcriptomics platforms (Figure 1A). Initially, we compared the RNA integrity of trephines from the diagnostic laboratory processed with historical collection protocols (see “Methods”) to samples that were fixed with neutral buffered formalin and either bypassed decalcification (nondecalcified) or were prepared using an optimized brief EDTA decalcification. These optimized methodologies significantly preserved RNA integrity (as measured by DV200) compared to samples processed using diagnostic collection protocols (Figure 1B). We assessed whether these protocols were compatible with the Xenium spatial transcriptomics platform by analyzing a trephine from a patient with relapsed myeloma (RM-1; supplemental Figure 1) using a 377-gene multitissue and cancer panel. With this approach, we could successfully resolve a broad range of hematopoietic and stromal cells by transferring cell type labels from clusters defined in scRNA-seq data (Figure 1C-D).19 We readily identified vascular structures, megakaryocytes, and erythroid islands (Figure 1E; supplemental Figure 2A). Importantly, PCs could be detected using established markers such as MZB1, SLAMF7, and TNFRSF17 (Figure 1E). When cell lineages were mapped spatially to trephines, the distribution of PCs matched adjacent sections immunostained with the PC marker CD138 (supplemental Figure 2B). Furthermore, this methodology was suitable for murine long bones, allowing the identification of hematopoietic and stromal populations, including eosinophils (Prg2), megakaryocytes (Pf4), pericytes (Rgs5), and osteoblasts (Bgn), localized to the endosteal niche (supplemental Figure 2C-F).15 These findings confirmed the feasibility of our framework to generate spatial molecular profiles for mouse and human BM.
Outline of sample processing and analysis pipeline for spatial transcriptomics. (A) Workflow of preparing human BM trephines for Xenium in situ analysis. (B) DV200 measurements for BM trephines obtained from diagnostic laboratory (n = 5) and formalin-fixed, paraffin-embedded in-house with (n = 8) and without EDTA-based decalcification (n = 16). Error bars represent mean ± standard error of the mean. ∗∗∗P = .0007; ∗∗∗∗P < .0001 (by 1-way analysis of variance [ANOVA] and Dunnett multiple comparisons test). (C-E) Xenium data of a BM trephine collected from a patient with relapsed myeloma (RM-1) using a human multitissue and cancer panel; uniform manifold approximation and projection (UMAP) of Xenium cells (C); spatial plot (scale bar, 1 mm) of panel C with cell type labels derived from published scRNA-seq data (D)19; and H&E images (E) of PCs (i), pericytes and endothelial cells (ii), megakaryocytes (iii), and erythrocytes (iv), overlaid with transcript expression for listed genes. Each dot represents a single transcript (scale bar, 10 μm). (F) Pixel-level analysis of a disease-free BM trephine (Ctrl-1) with FICTURE using a reference single-cell gene expression profile to define cell types.21 Magnification of the white-boxed areas (P1 and P2) focusing on endosteal, vascular, and hematopoietic cell–rich microenvironments. Factors are colored based on cell identity inferred from the reference gene expression profile19 (scale bars, 1 mm [overview] and 100 μm [magnified areas]). Ba, basophil; Eo, eosinophil; H&E, hematoxylin and eosin; Ma, mast cell; RCA, rolling circle amplification; VSMC, vascular smooth muscle cell.
Outline of sample processing and analysis pipeline for spatial transcriptomics. (A) Workflow of preparing human BM trephines for Xenium in situ analysis. (B) DV200 measurements for BM trephines obtained from diagnostic laboratory (n = 5) and formalin-fixed, paraffin-embedded in-house with (n = 8) and without EDTA-based decalcification (n = 16). Error bars represent mean ± standard error of the mean. ∗∗∗P = .0007; ∗∗∗∗P < .0001 (by 1-way analysis of variance [ANOVA] and Dunnett multiple comparisons test). (C-E) Xenium data of a BM trephine collected from a patient with relapsed myeloma (RM-1) using a human multitissue and cancer panel; uniform manifold approximation and projection (UMAP) of Xenium cells (C); spatial plot (scale bar, 1 mm) of panel C with cell type labels derived from published scRNA-seq data (D)19; and H&E images (E) of PCs (i), pericytes and endothelial cells (ii), megakaryocytes (iii), and erythrocytes (iv), overlaid with transcript expression for listed genes. Each dot represents a single transcript (scale bar, 10 μm). (F) Pixel-level analysis of a disease-free BM trephine (Ctrl-1) with FICTURE using a reference single-cell gene expression profile to define cell types.21 Magnification of the white-boxed areas (P1 and P2) focusing on endosteal, vascular, and hematopoietic cell–rich microenvironments. Factors are colored based on cell identity inferred from the reference gene expression profile19 (scale bars, 1 mm [overview] and 100 μm [magnified areas]). Ba, basophil; Eo, eosinophil; H&E, hematoxylin and eosin; Ma, mast cell; RCA, rolling circle amplification; VSMC, vascular smooth muscle cell.
With a robust methodology established, we selected trephines based on the quality of their morphology and RNA integrity (DV200) for spatial analysis using a panel of 5001 genes. Based on these criteria, 17 individual nonlongitudinal samples, including 7 patients with precursor disease (2 with MGUS [MGUS-1 and MGUS-2] and 5 with SM [SM-1 to SM-5]) and 10 with newly diagnosed MM (MM-1 to MM-10), as well as 4 control individuals (Ctrl-1 to Ctrl-4), were identified for spatial analysis. Samples were selected to cover a broad spectrum of common genetic aberrations associated with MM and included decalcification-free (nondecalcified) or EDTA decalcification trephines (supplemental Figure 1). In total, we measured 250 829 044 transcripts across 21 samples (supplemental Table 2). The number of genes and transcripts detected per cell (15-255 genes per cell) was consistent with previous reports of the Xenium Human 5K panel in non-BM samples (44-234 genes per cell). Notably, MM samples exhibited higher transcript counts per cell relative to MGUS, SM, and control samples, consistent with the increased transcriptional activity in malignant PCs (supplemental Figure 3).12,22 These results confirm the technical robustness of our data. We also evaluated the concordance of sample RNA quality and assay sensitivity by quantifying transcripts of canonical marker genes in selected hematopoietic and stromal cell types. We found that transcript detection was not affected by EDTA decalcification and, in general, observed a positive association between sample DV200 and absolute counts per gene (supplemental Figure 4A-H). Low-abundance cell-specific transcripts (such as CAVIN2, SPTA1, and PECAM1) were detected more robustly in samples with high RNA quality. The quality of these data allowed us to resolve complex BM microenvironments with single-cell resolution. To capture all cells (including those with atypical shape or size), we performed a segmentation-free methodology (Factor Inference of Cartographic Transcriptome at Ultra-high REsolution [FICTURE]) to assign distinct transcriptional profiles, approximating cell types from a scRNA-seq reference data set to 0.5 μm × 0.5 μm “pixels.”21 This analysis enabled identification of important structures in normal BM, including the endosteal and vascular niches, as well as a variety of hematopoietic populations (Figure 1F; supplemental Video 1).
We leveraged these expansive data sets to interrogate the spatial organization of malignant PCs within their surrounding BM microenvironment. To perform an unbiased analysis of cell type distribution in different disease states, we annotated cell types using a reference-based label transferring method implemented in the SingleR package.23 We detected a significant expansion of PCs in MM compared to MGUS/SM (MGUS/SM = 9.74%; MM = 45.07%; P = .003 [by quasi-binomial F test]; Figure 2A-B). This was accompanied by a significant reduction in hematopoietic populations normally detected in BM, such as red blood cells (MGUS/SM = 25.50%; MM = 10.99%; P = .000245 [by quasibinomial F test]) and megakaryocytes (MGUS/SM = 7.12%; MM = 3.21%; P = .000526 [by quasi-binomial F-test]; Figure 2B). Spatially mapping the location of assigned cell populations confirmed that PCs localized to areas that corresponded with CD138 immunohistochemistry (Figure 2C). Furthermore, sites of malignant PC expansion corresponded with a significant loss of nontransformed hematopoietic cell lineages, such as megakaryocytes and red blood cells (supplemental Figure 5).
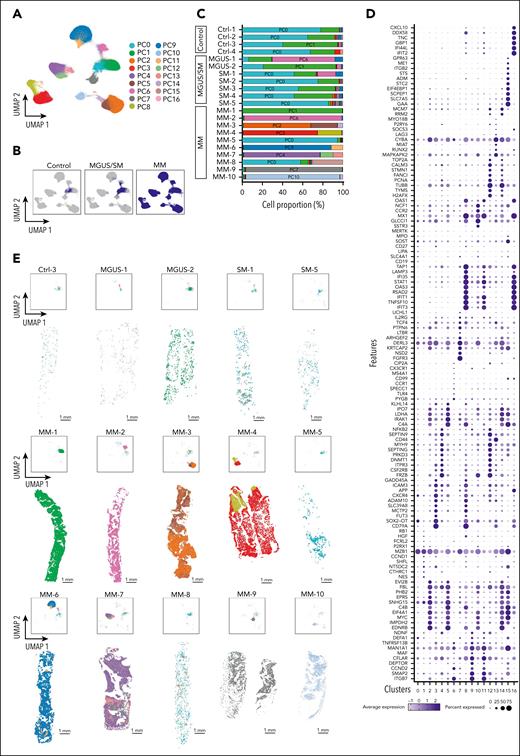
High-throughput subcellular spatial transcriptomics of human BM. Xenium data of BM trephines from controls (Ctrl-1 to Ctrl-4) and patients with MGUS (MGUS-1 and MGUS-2), SM (SM-1 to SM-5), and MM (MM-1 to MM-10) using a Xenium Prime 5K human pan-tissue and pathways panel. UMAP visualization (A) and proportion (B) of Xenium cells annotated using published scRNA-seq data.19 (C) CD138 immunohistochemistry and spatial plots of annotated Xenium cells of SM-1 and MM-3 samples (scale bars, 1 mm [overview] and 10 μm [magnified areas]). Ba, basophil; Eo, eosinophil; Ma, mast cell; Osteo, osteo-lineage cells; VSMC, vascular smooth muscle cell.
High-throughput subcellular spatial transcriptomics of human BM. Xenium data of BM trephines from controls (Ctrl-1 to Ctrl-4) and patients with MGUS (MGUS-1 and MGUS-2), SM (SM-1 to SM-5), and MM (MM-1 to MM-10) using a Xenium Prime 5K human pan-tissue and pathways panel. UMAP visualization (A) and proportion (B) of Xenium cells annotated using published scRNA-seq data.19 (C) CD138 immunohistochemistry and spatial plots of annotated Xenium cells of SM-1 and MM-3 samples (scale bars, 1 mm [overview] and 10 μm [magnified areas]). Ba, basophil; Eo, eosinophil; Ma, mast cell; Osteo, osteo-lineage cells; VSMC, vascular smooth muscle cell.
PC subpopulations occupy distinct spatial locations
We interrogated gene expression in PCs and their spatial distribution to understand the spectrum of healthy BM, precursor disease, and symptomatic MM. PC gene expression from all samples were integrated and used to generate distinct uniform manifold approximation and projection cell clusters (Figure 3A-B; supplemental Table 3). Common PC clusters (PC0 and PC1) were found in all control samples. PC0, in particular, expressed canonical markers of normal PCs, such as CD19 and CD27. Interestingly, we found expansion of PC0 or PC1 in MGUS/SM samples. In addition, we detected smaller populations of malignant clusters; for example, PC6 and PC9 in MGUS-1 and SM-1 and PC7 in SM-5. In contrast, MM samples were characterized by the expansion of distinct cell clusters with patient specific transcriptional state(s) compared to control and MGUS/SM samples (Figure 3C). We could also detect MM samples with transcriptionally distinct PC subpopulations. For example, in MM-4, MM-6, MM-7, and MM-10, we detected PCs with an inflammatory gene signature (clusters PC8, PC11, and PC16; IFI35, STAT1, and IFIT1) and proliferative markers (clusters PC12 and PC14; TOP2A, PCNA, and TUBB; Figure 3D).
Spatial molecular and cellular map of PCs. (A) UMAP plot of the combined data set of PCs from controls (n = 4), MGUS/SM (n = 7), and MM (n = 10). Colors represent PC clusters. (B) UMAP plot of panel A split into controls, MGUS/SM, and MM data sets. (C) Proportion of PC clusters in each sample. (D) Dot plot showing differentially expressed genes between PC populations in panel A. (E) UMAP and spatial plots for representative control (Ctrl-3), MGUS (MGUS-1 and MGUS-2), SM (SM-1 and SM-5), and all MM (MM-1 to MM-10) samples. Scale bars, 1 mm.
Spatial molecular and cellular map of PCs. (A) UMAP plot of the combined data set of PCs from controls (n = 4), MGUS/SM (n = 7), and MM (n = 10). Colors represent PC clusters. (B) UMAP plot of panel A split into controls, MGUS/SM, and MM data sets. (C) Proportion of PC clusters in each sample. (D) Dot plot showing differentially expressed genes between PC populations in panel A. (E) UMAP and spatial plots for representative control (Ctrl-3), MGUS (MGUS-1 and MGUS-2), SM (SM-1 and SM-5), and all MM (MM-1 to MM-10) samples. Scale bars, 1 mm.
We spatially mapped PCs onto trephines and noted that, in the context of MM, PC clusters presented as 1 of 2 scenarios: (1) a single predominant PC cluster that occupied the entire trephine (MM-1, MM-2, MM-9, and MM-10); or (2) a PC cluster with spatially restricted subpopulations that express a distinct gene signature (MM-3, MM-4, MM-5, MM-6, MM-7, and MM-8; Figure 3E; supplemental Figure 6). In the latter scenario, the size of these subpopulations ranged from small, distinct scattered clusters (MM-6, MM-7, and MM-8) to significantly larger populations (MM-3 and MM-4). Small, scattered clusters are seen clearly in MM-6. Here, the trephine is dominated by PC9 that expresses typical malignant PC genes such as ITGB7, CCND2, MAF, and TNFRSF13B. Within the trephine, we also detect distinct clusters of PC11 that share this core transcriptional signature but are distinguished by elevated expression of transcripts that include inflammatory-associated genes IFIT1, IFIT2, IFIT3, and TNFSF10. This spatial phenotype is replicated in MM-8, in which the dominant populations (PC0 and PC1) are similar to PCs found in control trephines. However, small, spatially distinct colonies (PC15) could be found scattered throughout the trephine that not only lacked this transcriptional profile but also had elevated expression of numerous genes including MYC, EIF4A1, ADM, and SOST. In MM-4, the spatial segregation of PC subpopulations was particularly pronounced. Here, we could identify a localized PC subpopulation (PC8) strongly characterized by inflammatory genes (STAT1, IFIT1, and IFIT3). Additionally, we observed the distribution of secreted factors such as IL-16 and NMB, an understudied neuropeptide implicated in various physiological processes (supplemental Figure 7).
Because proliferation markers were differentially expressed in some PC clusters (eg, TUBB, PCNA, and CCND2), we interrogated differences in PC turnover between different types of samples (controls, MGUS/SM, and MM; supplemental Figure 8) and within trephines (supplemental Figure 9) by measuring MKI67 expression. Although MKI67 expression did not differ significantly between PCs from control samples and precursor diseases (MGUS and SM; P = .674), MKI67 expression was significantly higher in MM samples (P = .015; supplemental Figure 8). Within MM-3 and MM-7, we could also detect significant differences in MKI67 expression in PC clusters that occupied spatially distinct areas of trephines (supplemental Figure 9).
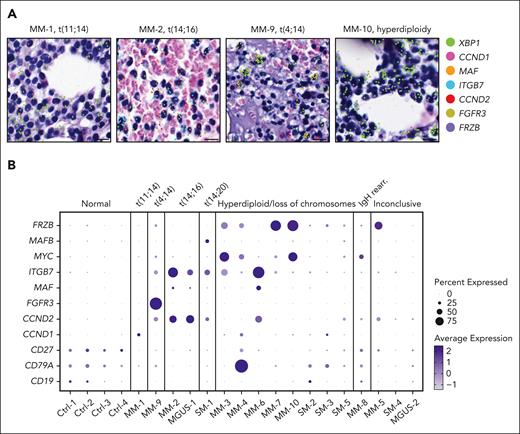
We investigated the relationship between the transcriptional state of PC populations and the underlying genetics of each patient in reference to diagnostic fluorescence in situ hybridization (FISH) results. We could spatially detect specific PC clusters that overexpressed genes associated with translocations identified by each patient’s FISH results (Figure 4A). For example, we observed overexpression of the following genes in a sample-dependent and translocation-specific manner: CCND1 in MM-1, t(11;14); FGFR3 in MM-9, t(4;14); MAF, ITGB7, and CCND2 in MM-2 and MGUS-1, t(14;16); and MAFB in SM-1, t(14;20). Furthermore, we detected elevated expression of FRZB in patients with hyperdiploidy (MM-3, MM-4, MM-7, and MM-10), as well as MM-5, whose FISH results were inconclusive due to insufficient cell numbers (Figure 4B). In addition, we could detect the expression of MAF and CCND2 in MM-6, although diagnostic FISH did not identify an IGH/MAF gene rearrangement.
Spatial mapping of genes associated with translocations detected by diagnostic FISH. (A) H&E images of PCs of representative samples overlaid with XBP1 transcripts (green) to identify PCs and expression of genes commonly upregulated in association with the displayed cytogenetic alteration (scale bar, 10 μm). (B) Samples are grouped by cytogenetic category as determined by diagnostic FISH testing. The size and color intensity of each dot represent the relative expression of selected genes commonly upregulated in association with the specific cytogenetic alteration. H&E, hematoxylin and eosin.
Spatial mapping of genes associated with translocations detected by diagnostic FISH. (A) H&E images of PCs of representative samples overlaid with XBP1 transcripts (green) to identify PCs and expression of genes commonly upregulated in association with the displayed cytogenetic alteration (scale bar, 10 μm). (B) Samples are grouped by cytogenetic category as determined by diagnostic FISH testing. The size and color intensity of each dot represent the relative expression of selected genes commonly upregulated in association with the specific cytogenetic alteration. H&E, hematoxylin and eosin.
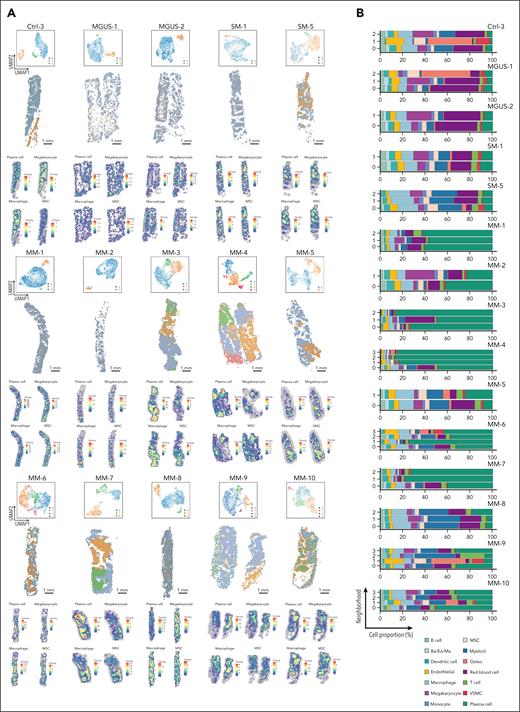
The BM stroma has been implicated as a driving factor in the development, progression, and drug resistance of MM; therefore, we performed an analysis of stromal cell populations. Based on the expression of canonical cell type markers, we identified diverse nonhematopoietic components such as endothelial cells (sinusoidal and arterial), vascular smooth muscle cells, mesenchymal stromal cells (MSCs), osteoblasts, and adipocytes (Figure 5A-B).15,19 In stark contrast to the integrated PC clusters, we did not observe consistent alterations in stromal cell composition across control, MGUS/SM, and MM samples (Figure 5C). Instead, quantification of stromal subsets revealed subtle shifts in composition without a discernible disease-associated trend (Figure 5D). These observations were supported by spatial mapping of stromal populations, which highlighted marked differences both between individuals and within spatial regions of the same trephine (Figure 5E; supplemental Figure 10). This variability highlights the absence of a uniform stromal response to myeloma development.
Spatially resolved neighborhood analysis of stromal cells. (A) UMAP plot of the combined data set of stromal cells from controls (n = 4), MGUS/SM (n = 7), and MM (n = 10). (B) Dot plot showing differentially expressed genes between stromal cell populations in panel A. (C) UMAP plot of panel A split into controls, MGUS/SM, and MM data sets. (D) Proportion of stromal cell clusters in each sample. (E) UMAP and spatial plots for representative control (Ctrl-3), MGUS (MGUS-1 and MGUS-2), SM (SM-1 and SM-5), and all MM (MM-1 to MM-10) samples. Scale bars, 1 mm. AEC, arterial endothelial cell; SEC, sinusoidal endothelial cell; VSMC, vascular smooth muscle cells.
Spatially resolved neighborhood analysis of stromal cells. (A) UMAP plot of the combined data set of stromal cells from controls (n = 4), MGUS/SM (n = 7), and MM (n = 10). (B) Dot plot showing differentially expressed genes between stromal cell populations in panel A. (C) UMAP plot of panel A split into controls, MGUS/SM, and MM data sets. (D) Proportion of stromal cell clusters in each sample. (E) UMAP and spatial plots for representative control (Ctrl-3), MGUS (MGUS-1 and MGUS-2), SM (SM-1 and SM-5), and all MM (MM-1 to MM-10) samples. Scale bars, 1 mm. AEC, arterial endothelial cell; SEC, sinusoidal endothelial cell; VSMC, vascular smooth muscle cells.
Microenvironments develop in a PC subpopulation–specific manner
A prevailing concept in hematological disorders is that malignant cells use a conserved mechanism to create a common protumor microenvironment. To test this theory, we investigated commonalities and differences of tumor microenvironment characteristics in MM trephines. At the whole-sample level, we could detect the expansion of unique PC subpopulations in MM compared to MGUS/SM samples (Figure 3) while observing no obvious trend in stromal cell composition (Figure 5). Therefore, we studied the relationship between malignant PCs and stroma at the neighborhood level to quantify cell composition in MM microenvironments. We adopted a binning strategy implemented in scider (Figure 1A) to group cells into hexagonal grids (a median of 55 cells per grid) across the sample. Unsupervised clustering analysis was then performed at the grid level to identify transcriptionally distinct spatial “neighborhoods.”20
In control samples, neighborhood analysis readily identified major structural components such as bone, when present (eg, Ctrl-3; Figure 6A; supplemental Figure 11). The remaining neighborhoods were characterized by either expansive hematopoietic areas dominated by red blood cell and myeloid signatures or localized regions enriched for transcriptionally active megakaryocytes, scattered throughout the trephine (a common feature of BM). A similar pattern was observed in precursor disease or MM cases dominated by a single PC subpopulation (eg, MM-1, MM-2, MM-5, MM-9, and MM-10), in which red blood cell or myeloid signatures remained conserved in these neighborhoods. In stark contrast, samples with >1 PC subpopulation (eg, MM-3, MM-4, MM-6, MM-7, and MM-8) exhibited multiple spatial neighborhoods with distinct transcriptional profiles (Figure 6A). These neighborhoods aligned with the subpopulation patterning identified in the integrated PC analysis (Figure 3E).
Spatial neighborhood analysis by scider. (A) UMAP and spatial plots of neighborhoods for representative control (Ctrl-3), MGUS (MGUS-1 and MGUS-2), SM (SM-1 and SM-5), and all MM (MM-1 to MM-10) samples. Colors represent transcriptionally distinct neighborhoods. These neighborhoods are deconvoluted with cell labels derived from published scRNA-seq data to generate density heat map for major BM cell types.19 The heat map shows the expected number of cells per hexagonal bin (scale bar, 1 mm). (B) Proportion of annotated cells in distinct neighborhoods for samples in panel A. Ba, basophil; Eo, eosinophil; Ma, mast cell; Osteo, osteo-lineage cells; VSMC, vascular smooth muscle cells.
Spatial neighborhood analysis by scider. (A) UMAP and spatial plots of neighborhoods for representative control (Ctrl-3), MGUS (MGUS-1 and MGUS-2), SM (SM-1 and SM-5), and all MM (MM-1 to MM-10) samples. Colors represent transcriptionally distinct neighborhoods. These neighborhoods are deconvoluted with cell labels derived from published scRNA-seq data to generate density heat map for major BM cell types.19 The heat map shows the expected number of cells per hexagonal bin (scale bar, 1 mm). (B) Proportion of annotated cells in distinct neighborhoods for samples in panel A. Ba, basophil; Eo, eosinophil; Ma, mast cell; Osteo, osteo-lineage cells; VSMC, vascular smooth muscle cells.
Quantification of neighborhood cell proportions, with PCs either included or excluded (Figure 6B; supplemental Figure 12), highlighted significant variation across all sample types (control, precursor disease, or symptomatic myeloma). We found that the proportion of stromal and hematopoietic lineages within each neighborhood varied. In regions heavily infiltrated with PCs such as MM-3 (neighborhoods 0 and 2), MM-4 (neighborhoods 1, 2, and 3), MM-5 (neighborhood 1), MM-6 (neighborhood 1), and MM-7 (neighborhoods 1 and 2), we detected an expected reduction in hematopoietic populations, such as red blood cells, megakaryocytes, and myeloid cells, as discussed previously (supplemental Figure 5). However, these changes were not universal but rather neighborhood specific (Figure 6A heat maps; supplemental Figures 12 and 13). This is highlighted by MM-3, in which neighborhood 0 was enriched for hematopoietic lineages such as macrophages and monocytes, a feature that was not conserved in heavily infiltrated PC regions of other MM samples discussed earlier. Similar differences were also detected in the context of stromal cell populations. For example, endothelial cells and MSCs were more abundant in neighborhoods 0 and 2 of MM-3 and neighborhood 1 of MM-5 than the less PC–infiltrated neighborhoods of the respective samples. In contrast, MM-7 showed a reduction of MSCs in heavily infiltrated PC areas, whereas MM-4 and MM-6 showed no significant differences in the spatial distribution of these cells across regions of the trephine with varying levels of PC infiltration.
Strikingly, the composition of local microenvironments also varied when we compared transcriptionally distinct neighborhoods within a trephine with similar proportion of PCs. For example, the non-PC components of neighborhoods within MM-3 (neighborhoods 0 and 2), MM-4 (neighborhoods 1 and 2), and MM-7 (neighborhoods 1 and 2) all had different proportions of dendritic and endothelial cells (supplemental Figure 12B). Intriguingly, similar differences were also noted in T-cell composition. We detected a higher proportion of T cells within MM-1 (neighborhood 2) and MM-4 (neighborhood 2) that correspond to PC clusters that express an inflammatory gene signature (Figure 3D), highlighting a possible interplay between inflammatory PCs and the adaptive immune system.
An exception to these findings was MM-8, a trephine with less PC infiltration compared to other samples from newly diagnosed patients. Despite small clusters of malignant PCs (PC15) within the trephine, the cellular composition of neighborhood 2 that identified this cluster was relatively similar to neighborhood 0, the predominant nonmegakaryocyte hematopoietic microenvironment. Notably, the general trend in composition of both these neighborhoods was similar to hematopoietic neighborhoods in control samples (Ctrl-1 [neighborhood 0]; Ctrl-2 [neighborhoods 0 and 1]; Ctrl-3 [neighborhood 0]; and Ctrl-4 [neighborhood 0]).
Thus, these data highlight spatial heterogeneity among distinct tumor and hematopoietic microenvironments in MM. Rather than supporting the existence of a conserved myeloma-supportive niche, our findings suggest that distinct PC subpopulations occupy transcriptionally and compositionally unique neighborhoods. Furthermore, the source of these differences could potentially be driven by the identity of PCs themselves, resulting from their ability to instruct changes in local cell composition driven by their transcriptional identity. Our data align with the recent description of evolving in situ clonal microenvironments in solid tumors,24 a finding particularly unexpected in hematological malignancies, in which transformed cells are highly dynamic in the BM microenvironment.25-28
Discussion
The long-standing dogma in hematology has been that malignant cells form universal, disease-specific microenvironments that drive disease progression and evolution of therapy-resistant clones.16 Our finding of diverse microenvironments, often detected within micrometers of each other, challenges this paradigm. Indeed, our study adds to an accumulating body of literature that suggests the MM microenvironment is linked to PC identity, as opposed to a conserved, stepwise evolution of a universal myeloma microenvironment that supports disease progression.
Previous transcriptional analysis of MM samples has characterized the evolution of malignant PCs.12,29-31 Similar to these scRNA-seq studies, we also found that patients with early precursor conditions (MGUS/SM) had expansion of PC clusters found in control samples and malignant clusters with transcriptomic alterations seen in overt MM. Unlike some studies,12 we did not detect a significant difference between MGUS and SM, with the profiles of some SM samples resembling those of patients with MM. We hypothesize this may be due to both technical and biological factors: (1) the reduced gene coverage of the 5001 genes profiled; and (2) the heterogeneity of disease states in SM. Indeed, our SM samples represent those with a low-intermediate International Myeloma Working Group 2/20/20 smoldering myeloma risk and low disease burden (≤20% PCs) and thus may reflect more of a MGUS state.
Although these data have been pivotal in characterizing interpatient genomic and transcriptional variation of malignant PCs in MM,12,29 PC sequencing studies performed at the “macro level” using tissue isolated from focal myeloma lesions and random BM have also shown that significant variation occurs at the intrapatient level and aligns with the site of biopsy.32,33 Our identification of localized PC colonies and corresponding changes to the BM microenvironment at the level of microns, compared to the large, distal foci in previous studies, is fascinating and raises questions about the number and size of distinct colonies and their distribution throughout the skeleton. It was intriguing that this phenotype was detected in MM samples (MM-3, MM-4, MM-6, and MM-7) with hyperdiploidy or tetraploidy identified by diagnostic FISH or microarray. This concept of PC identity–mediated changes in local microenvironments, both between and within patients, is also recapitulated in extramedullary disease and breakout lesions in which variation is seen in malignant PCs, the structural composition of foci, and the composition of infiltrating immune cells, such as T cells, natural killer cells, and myeloid lineages.34,35
In our studies, we were also able to spatially resolve specific previously reported changes in microenvironment phenotypes when measured at the sample level. For example, the localized foci of inflammatory PC signatures we observed spatially within trephines is consistent with previously described interferon-related gene expression in MM that vary both between patients and at focal lesions.29,34 The conservation of this signature within T cells and monocytes isolated from the same patient has been previously suggested to represent a cross talk between malignant PCs and components of the BM microenvironment, a case strongly supported by our spatial transcriptomics data.13,29 Thus, the interplay of inflammatory niches and local changes in microenvironment composition on immune cell function, such as T cells, could be an important modifier of local immune responses. This question is particularly time sensitive, because immunotherapies such as bispecific T-cell engagers and chimeric antigen receptor T cells are rapidly evolving as viable therapeutic options in the treatment of MM. The state of CD4+ and CD8+ T-cell lineages in BM has been demonstrated to evolve significantly during the progression of precursor disease to MM.13,36 In turn, this preexisting T-cell landscape has been directly linked to the efficacy of both B-cell maturation antigen–targeted T-cell engagers and chimeric antigen receptor T-cell therapy.37,38 As recent studies in extramedullary disease have demonstrated, T-cell phenotype can vary significantly, both spatially and between patients34; we propose that understanding how BM PC subpopulations can suppress or modify T cells to facilitate therapy resistance is a pivotal unanswered question. Extrapolating on this idea, linking PC identity to specific oncogenic drivers and their corresponding immunomodulatory BM microenvironments could provide crucial mechanistic insights to inform future therapy design.
Our study demonstrates the feasibility of large-scale spatial transcriptomics to study the functional underpinnings of the BM niche at single-cell level in the development of MM and potentially other BM-related pathologies. Although our work provides a framework for future studies, we are still limited by constraints of current technology. The analysis provided here has limited gene coverage in the context of human BM. Thus, our studies are restricted to the analysis of cell composition rather than a more detailed dissection of transcriptional cell states or comprehensive characterization of rare cell subsets. An alternative approach to circumvent this limitation is the application of high-resolution spatial sequencing methodologies such as Visium HD,39 Slide-tag,40 and Stereo-seq41 that provide transcriptome-wide coverage at near single-cell resolution. However, the current state of in situ transcriptomics technology has significant limitations: (1) small capture areas that prevent profiling of large tissue sections with complex heterogeneity, such as BM; (2) any commonly used technologies are probe based, limiting gene coverage to candidate-based screening similar to the approach we have applied here; and (3) there are currently significant cost restrictions, which paired with small sequencing capture areas means most comprehensive studies are not feasible for most of the research community. However, as technology in spatial transcriptomics is rapidly evolving, overcoming these limitations will be inevitable. An exemplar of this technological development includes recent methodologies that allow PC clones to be resolved spatially via in situ variable, diversity, and joining sequencing.42 Another issue in the field to date has been the scarcity of broad coverage subcellular resolution data sets of human BM. This is even reflected in our own data sets in which most of the 5001 profiled genes were expressed below the detection threshold of the Xenium Prime chemistry. To circumvent these issues, our data sets should be leveraged by the community to inform custom probe design that increases the sensitivity of the platform by approximately threefold compared to the Xenium Prime methodology. This approach will potentially allow more immune and stromal cell subsets to be resolved in a broad range of hematological conditions. In addition, we envisage that integrating our protocols with routine BM trephine collection will significantly improve the quality of samples and aid in the construction of large-scale data sets from multiple spatial platforms that can describe the fundamental biology that underpins BM function in both healthy and diseased states.
Acknowledgments
The authors thank the Walter and Eliza Hall Institute of Medical Research Bioservices for animal husbandry; the Walter and Eliza Hall Institute of Medical Research Advanced Histotechnology Facility for assistance with tissue processing, sectioning, and immunohistochemistry staining; and the Peter MacCallum Cancer Centre hematopathology department for sample collection. The authors are grateful to Kelli Gray for coordination and consenting patients for sample collection. The authors thank Pradeep Rajasekhar and the members of the Hawkins, Bowden, and Rogers laboratories for their reviews and valuable discussion. The authors also thank the patients and their families for consenting to provide tissues for this study.
This work was supported by the National Health and Medical Research Council (grant 2008652; E.D.H.), the Australian Medical Research Future Fund (grant 1176199; Y.C.), Victorian Cancer Agency (grant 21001; M.R.D.), and Hematology Society of Australia and New Zealand and Walking Up the Hill Foundation scholarship (J.E.). The authors acknowledge philanthropic support from the Roebuck family (J.E.), the estate of Judith Corrie Philpots (Y.C.), and the Barrie Dalgleish Centre for Myeloma and Related Blood Cancers (E.D.H.).
E.D.H. dedicates this work to the mentorship of David John Westwick.
Authorship
Contribution: R.K.H.Y., J.E., and E.D.H. conceived the study; R.K.H.Y., J.E., J.S.R., A.L., R.D.M., L.L., C.J.A.A., E.T., and D.A.-Z. conducted the experiments; R.K.H.Y., J.E., L.Q., Q.H.N., A.M., Y.C., and E.D.H. analyzed data; J.E. collected clinical samples; R.K.H.Y., J.E., Y.C., and E.D.H. generated figures; M.R.D., K.L.R., R.B., S.J.H., and E.D.H. contributed to study design and supervised the project; and R.K.H.Y., J.E., Y.C., and E.D.H. cowrote the manuscript, with input from all other authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edwin D. Hawkins, Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Melbourne, VIC 3052, Australia; email: hawkins.e@wehi.edu.au; Yunshun Chen, Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Melbourne, VIC 3052, Australia; email: yuchen@wehi.edu.au; and Raymond K. H. Yip, Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Melbourne, VIC 3052, Australia; email: yip.r@wehi.edu.au.
References
Author notes
R.K.H.Y. and J.E. contributed equally to this study.
K.L.R., R.B., Y.C., S.J.H., and E.D.H. jointly supervised this work.
The data sets generated during this study have been deposited in the Gene Expression Omnibus database (accession number GSE299207).
Data are available on detailed email requests from the corresponding authors, Raymond K. H. Yip (yip.r@wehi.edu.au), Yunshun Chen (yuchen@wehi.edu.au), or Edwin D. Hawkins (hawkins.e@wehi.edu.au).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Outline of sample processing and analysis pipeline for spatial transcriptomics. (A) Workflow of preparing human BM trephines for Xenium in situ analysis. (B) DV200 measurements for BM trephines obtained from diagnostic laboratory (n = 5) and formalin-fixed, paraffin-embedded in-house with (n = 8) and without EDTA-based decalcification (n = 16). Error bars represent mean ± standard error of the mean. ∗∗∗P = .0007; ∗∗∗∗P < .0001 (by 1-way analysis of variance [ANOVA] and Dunnett multiple comparisons test). (C-E) Xenium data of a BM trephine collected from a patient with relapsed myeloma (RM-1) using a human multitissue and cancer panel; uniform manifold approximation and projection (UMAP) of Xenium cells (C); spatial plot (scale bar, 1 mm) of panel C with cell type labels derived from published scRNA-seq data (D)19; and H&E images (E) of PCs (i), pericytes and endothelial cells (ii), megakaryocytes (iii), and erythrocytes (iv), overlaid with transcript expression for listed genes. Each dot represents a single transcript (scale bar, 10 μm). (F) Pixel-level analysis of a disease-free BM trephine (Ctrl-1) with FICTURE using a reference single-cell gene expression profile to define cell types.21 Magnification of the white-boxed areas (P1 and P2) focusing on endosteal, vascular, and hematopoietic cell–rich microenvironments. Factors are colored based on cell identity inferred from the reference gene expression profile19 (scale bars, 1 mm [overview] and 100 μm [magnified areas]). Ba, basophil; Eo, eosinophil; H&E, hematoxylin and eosin; Ma, mast cell; RCA, rolling circle amplification; VSMC, vascular smooth muscle cell.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/146/15/10.1182_blood.2025028896/2/m_blood_bld-2025-028896-gr1.jpeg?Expires=1769163465&Signature=fPn8ImPuakf0m-KkVu05RbBR3rVcR8c4zCPykTom0pH~bS~HFiu0lCMGePa0nC62HHlDwhniVG96RKb5nmknMdv8vikeODZMKppyIgbxecjf645yo2D60PIVCbsdESGuwsEXGvGcX0Zg470PWu9Y3EfDyQ8EPz7QFMiPgNkXjpP-kJ0xR5yK8YwUfrIFtRENffijI-54TnT4GcPvJZJm70f8HZ519G7dGRSoUW81k646zi-6vp67ZC0CmeCcmqtGXUxCorDJ7U6GBbW0MlzZdWoWdK2vK-G0cGfjJKzfEO-2CCvjwd-c591rAOOOx5VtUPUSkIyztz~GTyufzPFe-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![High-throughput subcellular spatial transcriptomics of human BM. Xenium data of BM trephines from controls (Ctrl-1 to Ctrl-4) and patients with MGUS (MGUS-1 and MGUS-2), SM (SM-1 to SM-5), and MM (MM-1 to MM-10) using a Xenium Prime 5K human pan-tissue and pathways panel. UMAP visualization (A) and proportion (B) of Xenium cells annotated using published scRNA-seq data.19 (C) CD138 immunohistochemistry and spatial plots of annotated Xenium cells of SM-1 and MM-3 samples (scale bars, 1 mm [overview] and 10 μm [magnified areas]). Ba, basophil; Eo, eosinophil; Ma, mast cell; Osteo, osteo-lineage cells; VSMC, vascular smooth muscle cell.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/146/15/10.1182_blood.2025028896/2/m_blood_bld-2025-028896-gr2.jpeg?Expires=1769163465&Signature=IxnqEKYJcf-36N~x8Aoggu32Br~RTwnEWL1dpYdLr1u6KA5aoDnoH4u9LUoUXQ10v0L1E3kqQJmAuIbwEWnocDVntabEw9-Q6o9CK1XEHjOpZuLYGVVS1Gw9PCHMUTPFwZJoWQVQCGP~RC5h5-eouZr4STDBgpcS87OXkSKyvnkG0JzeQZ4nSoKrmz2MzTsMxUYuJGllWlOBWCjHObk3G~3iKvg163npgT7dPzzWXVXaOtcDdyQPfjgCSAZ0W2XL1lp6EU5Y3DOGqpKGczwjvbn2Pr5g0z1E8-uKO3-zHGLgphMk6JJAb2-fa1e4ml5ubEZuKYcogVvR73gNo~mY2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)