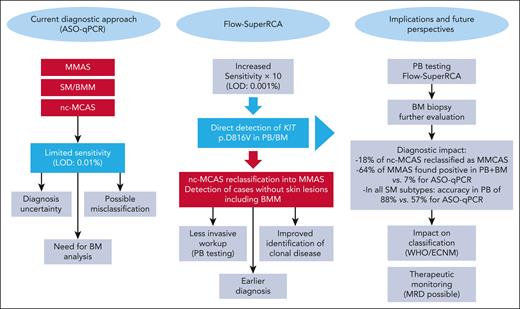
In this issue of Blood, Navarro-Navarro et al1 present compelling evidence that a novel, ultrasensitive molecular assay (Flow-SuperRCA) significantly improves the detection of the KIT p.D816V mutation across a spectrum of clonal mast cell disorders (MCDs). These include systemic mastocytosis (SM), bone marrow mastocytosis (BMM), monoclonal mast cell activation syndrome (MMAS), and even subsets of patients previously classified as having nonclonal mast cell activation syndrome (nc-MCAS). The clinical implications of these findings are summarized (see figure), and are substantial, with potential to reshape current diagnostic algorithms and reclassify patients previously considered nonclonal.
Transformative impact of Flow-SuperRCA on the diagnostic landscape of clonal MCDs. This figure illustrates the transformative impact of Flow-SuperRCA technology compared with the conventional ASO-qPCR method for detecting KIT p.D816V in MCAS and SM including BMM. The left panel summarizes the current limitations of ASO-qPCR, including its restricted sensitivity (LOD: 0.01%), the frequent need for BM biopsy, and the potential for diagnostic uncertainty or misclassification, particularly in patients with low mast cell burden, such as MMAS, BMM, and nc-MCAS. The middle panel emphasizes the key advantages of Flow-SuperRCA, which offers a 10-fold increase in sensitivity (LOD: 0.001%) and enables direct detection of KIT p.D816V in PB and BM samples. This enhanced performance results in reclassification of 18% of previously diagnosed nc-MCAS cases as clonal MCAS, and in detection of BMM cases without skin involvement, cases often missed by ASO-qPCR. The right panel outlines the downstream clinical implications of implementing Flow-SuperRCA, including earlier diagnosis, reduced need for invasive procedures (PB-based screening), improved identification of clonal MCD, and potential for MRD monitoring. In clinical terms, Flow-SuperRCA increased detection rates in MMAS (64% vs 7% with ASO-qPCR), and achieved a diagnostic accuracy of 88% in PB across all SM subtypes, substantially higher than ASO-qPCR (57%). These results support its integration into updated diagnostic algorithms and WHO/ECNM classifications. ECNM, European Competence Network on Mastocytosis; LOD, limit of detection; MRD, minimal residual disease; WHO, World Health Organization.
Transformative impact of Flow-SuperRCA on the diagnostic landscape of clonal MCDs. This figure illustrates the transformative impact of Flow-SuperRCA technology compared with the conventional ASO-qPCR method for detecting KIT p.D816V in MCAS and SM including BMM. The left panel summarizes the current limitations of ASO-qPCR, including its restricted sensitivity (LOD: 0.01%), the frequent need for BM biopsy, and the potential for diagnostic uncertainty or misclassification, particularly in patients with low mast cell burden, such as MMAS, BMM, and nc-MCAS. The middle panel emphasizes the key advantages of Flow-SuperRCA, which offers a 10-fold increase in sensitivity (LOD: 0.001%) and enables direct detection of KIT p.D816V in PB and BM samples. This enhanced performance results in reclassification of 18% of previously diagnosed nc-MCAS cases as clonal MCAS, and in detection of BMM cases without skin involvement, cases often missed by ASO-qPCR. The right panel outlines the downstream clinical implications of implementing Flow-SuperRCA, including earlier diagnosis, reduced need for invasive procedures (PB-based screening), improved identification of clonal MCD, and potential for MRD monitoring. In clinical terms, Flow-SuperRCA increased detection rates in MMAS (64% vs 7% with ASO-qPCR), and achieved a diagnostic accuracy of 88% in PB across all SM subtypes, substantially higher than ASO-qPCR (57%). These results support its integration into updated diagnostic algorithms and WHO/ECNM classifications. ECNM, European Competence Network on Mastocytosis; LOD, limit of detection; MRD, minimal residual disease; WHO, World Health Organization.
Detection of the KIT p.D816V mutation is pivotal to the diagnosis of clonal MCDs.2 Current consensus guidelines from the World Health Organization and from the European Competence Network on Mastocytosis recognize this mutation as a minor diagnostic criterion for SM, and as a hallmark of clonality in MCAS,3,4 a recommendation recently consolidated by the EU-US Cooperative Group,5 which provided detailed standards for genetic testing in SM. Although allele-specific oligonucleotide-quantitative polymerase chain reaction (ASO-qPCR) and digital PCR (dPCR) have dramatically improved the sensitivity of KIT p.D816V detection in peripheral blood (PB) and bone marrow (BM) in the past 15 years, a significant fraction of patients with clonal disease still remain negative in PB or BM by these conventional methods.6-8 This diagnostic gap is particularly critical in patients presenting with MCAS and anaphylaxis, without skin involvement or overt BM mast cell infiltration.
The Flow-SuperRCA assay evaluated by Navarro-Navarro et al represents a significant technological advance. It combines rolling circle amplification using padlock probes with flow cytometry–based detection, allowing the assessment of a high copy load (∼106 copies), and lowering detecting variant allele frequency down to 0.001% (10× more sensitive than the standard ASO-qPCR, where 200 ng gDNA, ∼5 × 104 copies, are loaded). By analyzing over 500 samples from 337 patients, the authors demonstrate that Flow-SuperRCA achieves consistently higher detection rates for KIT p.D816V both in PB and BM samples.1
Most striking is the assay’s performance in MMAS and in BMM, 2 diagnostic entities known for subtle or absent histopathologic features. In PB samples, Flow-SuperRCA detected KIT p.D816V in 28% of patients with MMAS vs 0% with ASO-qPCR, and in 80% of patients with BMM vs only 30% with ASO-qPCR. This improvement also holds true for BM. These results suggest that many clonal MCDs remain underdiagnosed due to the relatively low sensitivity of conventional tools. Importantly, all ASO-qPCR–positive samples were also positive by Flow-SuperRCA, underscoring the latter’s high sensitivity and specificity.
Perhaps the most clinically transformative finding provided in this article is the reclassification of 18% of patients with nc-MCAS as clonal, based solely on the detection of KIT p.D816V by Flow-SuperRCA. These data challenge the existing dichotomy between clonal and nc-MCAS, and highlight the need to revise our diagnostic framework, although the possibility that a significant fraction of so-called “idiopathic anaphylaxis” may in fact have a clonal basis has long been suspected.
These findings prompt critical reconsideration of future classification systems for MCDs. Should these newly identified clonal cases continue to be categorized as MCAS, or would they be more appropriately defined as an early or minor form of SM (pre-SM)? In addition, with increasingly sensitive methods for the detection of KIT mutations, it is conceivable that virtually all patients with SM currently diagnosed as KIT wild-type will reveal KIT-mutated in the future. Thus, one could wonder if the presence of a KIT-activating mutation will become a major criterion for SM diagnosis in the forthcoming years. Conversely, with such a level of sensitivity, the clinical relevance of a KIT-activating mutation in potential relationship with clonal hematopoiesis of indeterminate potential is an issue to be addressed. The implications are not merely academic: diagnosis determines access to specialized care, prognostic evaluation, and eligibility for clinical trials. The next World Health Organization and International Consensus Classification iterations will have to address these gray zones, particularly in light of new molecular technologies that challenge conventional thresholds of clonality. Beyond diagnosis, Flow-SuperRCA may become an essential tool for noninvasive screening and therapeutic monitoring. Given its high sensitivity, the method could be used for minimal residual disease assessment in patients with SM treated with KIT inhibitors, as its ability to detect the mutation in PB opens avenues for patient-friendly sampling strategies. This aligns with current trends in hematology toward liquid biopsy approaches,9 which minimize the burden of invasive procedures while offering robust molecular insight.
Nonetheless, several limitations must be underlined. The assay is currently restricted to expert laboratories and requires both advanced molecular biology and flow cytometry platforms. Its reproducibility across different centers remains to be demonstrated in large-scale multicentric studies. Quantitative aspects of variant allele frequency evaluation by Flow-SuperRCA, as well as its added-value against other methods, such as dPCR, where copy load could theoretically be increased to the magnitude of 105 to 106 copies, also remain to be evaluated. Furthermore, the cost-effectiveness of implementing this method in daily routine remains an open question, particularly in low-resource settings. Finally, the clinical utility of reclassifying patients based on low-level KIT p.D816V detection, particularly regarding prognosis and treatment decisions, requires further prospective validation.
Despite these caveats, the study by Navarro-Navarro et al is a real milestone in the diagnosis of MCDs. Indeed, it validates the concept that increasing analytical sensitivity can reveal previously unrecognized clonal disease in subsets of patients and supports the integration of highly sensitive tools into diagnostic workups. Their findings reinforce prior studies showing the inadequacy of standard ASO-qPCR in certain contexts and propose a feasible solution with immediate clinical relevance.10
To conclude, Flow-SuperRCA substantially enhances our capacity to detect clonality in MCDs. Its implementation could redefine disease boundaries, improve early diagnosis, and ultimately guide more personalized management. If this technique becomes more widely available, then its inclusion in diagnostic algorithms and practice guidelines, especially within expert referral centers, should be strongly considered. The study points also to the fact that our current classifications may not yet fully capture the biological continuum of MCAS and SM.
Conflict-of-interest disclosure: Y.C. has received research grants and honoraria from Blueprint Medicines and Thermo Fisher. M.A. has received research grants from Blueprint Medicines; and honoraria from AB Science, Blueprint Medicines, and Thermo Fisher.