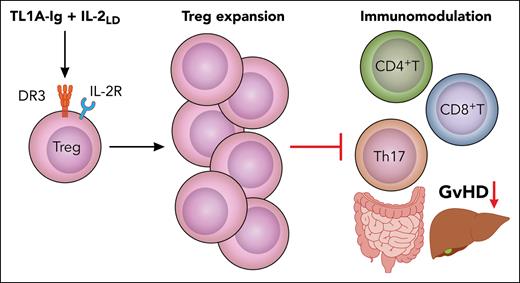
In this issue of Blood, McManus et al1 demonstrate that activating death receptor 3 (DR3) in combination with low-dose interleukin-2 (IL-2LD) effectively activates and expands tissue-resident regulatory T cells (Tregs), ameliorating graft-versus-host disease (GVHD).
Allogeneic hematopoietic cell transplantation (allo-HCT) stands out as a successful therapy for genetically-inherited blood disorders and hematologic malignancies. However, ∼20% of allo-HCT recipients develop acute GVHD, a major cause of transplant-related morbidity and mortality. Adoptive Treg transfer has shown promise in preclinical models and clinical trials for GVHD prevention and treatment.2-4 However, the expansion of donor Tregs for adoptive cellular therapy presents technical challenges including stability, phenotype drift as well as manufacturing and regulatory complexity, which limit their feasibility as an off-the-shelf therapy. Therefore, strategies to expand host Tregs in vivo serve as a promising approach to mitigate GVHD. DR3, a member of the tumor necrosis factor receptor superfamily (TNFRSF), initiates intracellular signaling when 2 or more ligand-induced trimeric receptor complexes cluster at the cell membrane. TNFRSF receptors play critical roles in cell proliferation, differentiation, and survival.5 Notably, TNFR2, GITR, and DR3 are constitutively expressed on resting Tregs and are further upregulated (alongside OX-40 and 4-1BB) after T-cell receptor activation.
McManus et al used a TL1A-Ig fusion protein with IL-2LD to expand host Tregs before allo-HCT. This preemptive approach increased Helios+ host Tregs for up to 2 weeks posttransplantation and significantly improved GVHD outcomes in both major histocompatibility complex (MHC)–matched and MHC-mismatched mouse models without compromising graft-versus-leukemia effects (see figure). Importantly, the treatment spared CD4+ and CD8+ T-cell expansion. The depletion of Tregs or genetic ablation of DR3 abrogated the protective effects, confirming a Treg- and DR3-dependent mechanism. Prior studies using agonistic DR3 antibodies (e.g., clone 4C12) also observed Treg expansion and GVHD mitigation.6,7
TL1A-Ig and IL-2LD treatment expands tissue-resident Tregs and restrains alloreactive T-cell–driven damage in GVHD target organs. Professional illustration by Patrick Lane, ScEYEnce Studios.
TL1A-Ig and IL-2LD treatment expands tissue-resident Tregs and restrains alloreactive T-cell–driven damage in GVHD target organs. Professional illustration by Patrick Lane, ScEYEnce Studios.
During the effector phase of GVHD, Tregs proliferate poorly and are depleted from the colonic lamina propria, allowing effector T cells and myeloid cells to accumulate and fuel inflammation. TL1A-Ig and IL-2LD treatment not only expanded Tregs in secondary lymphoid organs but also promoted a suppressive environment in GVHD target tissues, including the colon, liver, and ocular adnexa. Using fingolimod to block T-cell egress, the authors showed that Tregs expanded locally within affected tissues, rather than migrating from lymphoid sites. This local expansion supported epithelial regeneration and preserved gut barrier integrity.
Strikingly, compared to untreated controls, pretreatment with TL1A-Ig and IL-2LD enriched beneficial gut microbiota,8 including Muribaculum and Lactobacillus, and reduced pathogenic genera such as Escherichia and Enterococcus. The therapy also sustained butyrate-producing and obligate anerobic microbes, which were diminished in controls. GVHD-associated epithelial injury produces reactive oxygen species and hydrogen peroxide (H2O2), whereas the TL1A-Ig and IL-2LD treatment significantly lowered H2O2 levels, helping to restore homeostasis. Collectively, these findings reveal that DR3 agonism not only curbs alloreactive T-cell expansion and tissue injury but also reshapes the intestinal microenvironment, enhancing immune tolerance, stabilizing commensal flora, and reducing oxidative stress.
This study strengthens the growing evidence that TNFRSF members are key regulators of Treg biology. Additional receptors, such as TNFR2, have also demonstrated protective effects in GVHD models.9,10 However, most studies have used pre-emptive strategies to expand Tregs before GVHD onset.1,9,10 Although TNFRSF agonists are effective in controlled settings, it remains unclear whether they can provide therapeutic benefit once acute GVHD is clinically apparent. The rigorous evaluation of these receptors in established disease models will be essential to determine their translational potential and support clinical implementation.
Conflict-of-interest disclosure: The author declares no competing financial interests.