Abstract
Human leukocyte antigen CD38, a 45-kD single-chain, transmembrane glycoprotein, is a bifunctional ectoenzyme that participates in signal transduction pathways involved in the regulation of cell growth and differentiation. In this study, we demonstrate the nature of retinoid receptors involved in retinoic acid–induced expression of CD38 protein in the human myeloblastic leukemia cell line HL-60. We used a variant HL-60 cell line, HL-60R, in which retinoid receptor function has been abrogated by a trans-dominant negative mutation. We introduced the normal retinoic acid receptors (RAR)-α, -β, and -γ or retinoid X receptor (RXR)-α into HL-60R cells by retroviral vector-mediated gene transfer. Based on experiments using these cell lines and receptor-specific synthetic retinoids that preferentially bind to one of the RARs or RXRs, we conclude that RAR-α is involved in retinoid-induced CD38 expression in HL-60 cells. Further evidence included our demonstration that blocking of RAR-α with the antagonist Ro 41-5253 completely suppressed the retinoid-induced expression of CD38 mRNA transcript and the production of CD38 protein in HL-60 cells. Various tissues from transgenic mice that expressed an antisense construct of RAR-α lacked or produced very low levels of CD38. As expected, the tissues from transgenic mice contained 50% to 80% reduced levels of RAR-α. These results suggest that regulation of CD38 expression, both in vitro and in vivo, is under the direct control of RAR-α retinoid receptors.
RETINOIDS ARE IMPORTANT regulators of cell growth and differentiation in a wide variety of tissues.1 Study of the molecular mechanisms that lead to retinoid-induced modulation of cell growth and differentiation has been greatly facilitated by the availability of cell lines in which the growth and differentiation is altered in vitro by retinoid treatment. An example is the HL-60 cell line, which was derived from a patient with advanced myeloblastic leukemia. HL-60 cells recapitulate the events of normal hematopoietic granulocytic differentiation following their treatment with all-trans-retinoic acid (ATRA).2 Thus, HL-60 cells provide a convenient in vitro model for studying the action of retinoids in regulating cell differentiation.
Studies using HL-60 and other useful models have shown that the pleiotropic effects of retinoids are mediated by activation of two known families of nuclear receptors, the retinoic acid receptors (RARs) and the retinoid X receptors (RXRs).3 These receptors are members of the steroid-thyroid hormone receptor superfamily of proteins that function as dimeric, ligand-dependent transcription factors. RARs and RXRs represent distinct types of nuclear receptors: they are encoded by distinct genes and bind to specific ligands and response elements. Each RAR and RXR family of receptors consists of three subtypes, -α, -β and -γ, and each subtype is encoded by a distinct gene.1,3 RAR subtypes can bind both ATRA and its naturally occurring double-bond isomer, 9-cis retinoic acid (9-cis RA), whereas RXR subtypes bind only 9-cis RA.4 Expression of each RAR and RXR subtype (-α, -β, and -γ) is developmental stage- and cell type–specific,5 which suggests that each may specifically regulate the expression of subsets of retinoid target genes.
Among the various genes that are upregulated during ATRA-induced granulocytic differentiation of promyelocytic cells, CD38 exhibits an early and rapid expression. CD38 is a type II glycoprotein with a single transmembrane domain, the expression of which is tightly regulated by retinoids.6 The CD38 protein serves as a bifunctional ectoenzyme that catalyzes the synthesis of cyclic adenosine diphosphate-ribose (cADPR) from NAD+ and the hydrolysis of cADPR to ADP-ribose.7-9 cADPR has potent Ca2+-mobilizing activity and is a potential endogenous regulator of calcium-dependent calcium release.10 Using an RAR-α–transfected mutant subclone of the HL-60 cell line, HL-60R, we recently demonstrated that CD38 expression in myeloid cells is mediated by activation of RAR-α.11 In the present study, we confirm and further extend these initial observations, using HL-60R subclones expressing a specific subtype of receptor and receptor-selective retinoids that preferentially activate or block RAR or RXR action. Various tissues from transgenic mice that contained 50% to 80% fewer transcripts for RAR-α lacked or contained significantly lower amounts of CD38 protein than their counterparts from normal animals. The results clearly demonstrate that RAR-α is a general mediator of retinoid-induced CD38 expression.
MATERIALS AND METHODS
Retinoids.ATRA, retinol, and β-carotene were purchased from Sigma Chemical (St Louis, MO). The ATRA metabolites 5, 6-epoxy RA, 4-hydroxy RA, and 4-oxo-RA; the ATRA analogs 9-cis RA and 13-cis RA; and the RAR-α antagonist, Ro41-5253, were kindly provided by Dr Jerry Spinwall (Hoffman-La Roche, Nutley, NJ). The receptor-specific retinoids CD336 (RAR-α), CD417 (RAR-β), CD437 (RAR-γ), CD2608 (RXR), and CD2624 (RXRα) were kindly provided by Dr Uwe Reichert (CIRD/Galderma, Sophia Antipolis, France). Ligand-binding properties of these retinoids have been described previously.12 Retinoids were dissolved in dimethyl sulfoxide (DMSO) at 1 mmol/L concentration. The final concentration of DMSO in culture medium was ≤ 0.01%.
Cells and cell culture.The parental HL-60 clone was obtained from American Type Culture Collection (Rockville, MD). A subclone resistant to the differentiating effects of ATRA, HL-60R, was established by continuous culture of HL-60 cells in the presence of ATRA as described previously.13 Four additional subclones from HL-60R were developed by retroviral vector (LXSN)-mediated transduction of cDNA sequences coding for RAR-α, RAR-β, RAR-γ or RXR-α.14 All cell lines were cultured in RPMI 1640 medium supplemented with 5% fetal calf serum (FCS), 10 mmol/L HEPES, 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. In addition, the four transfected cell lines were cultured at alternative passages in the presence of neomycin (1 mg/mL) to prevent selection against retrovirus-negative clones. Cells were treated with retinoids during the log phase of cell growth.
Flow cytometry.The level of CD38 expression on HL-60 cells in response to retinoid treatment was determined using the monoclonal antibody Leu-17 (phycoerythrin [PE]-conjugated; Becton Dickinson, San Jose, CA) as previously described.11 In brief, the retinoid-treated or untreated cells were stained with anti-CD38 or isotypic control antibody (PE-labeled IgG1). The fluorescence was then detected on a logarithmic scale using a FACScan flow cytometer and LYSIS II Research Software (Becton Dickinson). CD38 levels were expressed as signal to noise (S/N) ratios, defined by the mean fluorescence of CD38-expressing cells divided by the mean fluorescence of cells stained with the isotypic control antibody.
Reverse-transcriptase polymerase chain reaction for CD38 gene expression.Total RNA was isolated from HL-60 cells using TRIzol reagent (GIBCO BRL, Grand Island, NY), according to the manufacturer's instructions. First-strand cDNA was synthesized in the presence of murine leukemia virus reverse transcriptase ([RT] 2.0 U/μL) by incubating 1 μg of total RNA in a reaction mixture that contained 50 mmol/L Tris-HCl (pH 8.3), 40 mmol/L KCI, 6 mmol/L MgCI2 , 10 mmol/L dithiothreitol (DTT), 1 mmol/L deoxynucleotide triphosphate, 25 U RNase inhibitor, and 0.2 μg random hexanucleotide as a primer. The reaction mixture was incubated at 37°C for 1 hour in a DNA thermal cycler (Perkin-Elmer Cetus, Norwalk, CT). The synthetic polymerase chain reaction (PCR) primers were designed to amplify the extracellular domain of human CD38 cDNA, as described by Kontani et al.15 The 5′-sense primer was designed to cover 202 to 217 base pairs, and the 3′-antisense primer covered 956 to 972 base pairs of CD38 cDNA.16 The single-stranded cDNA was amplified by PCR in the presence of 10 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 2 mmol/L MgCl2 , 0.1 μg cDNA template, 0.15 μmol/L primer, 0.2 mmol/L dNTPs, 2.5 U Taq DNA polymerase (Perkin Elmer Cetus), and 50 μL mineral oil. The amplification was performed for 35 cycles in a DNA thermal cycler using a cycle of denaturation for 1 minute at 95°C, annealing for 2 minutes at 55°C, and extension at 75°C for 2 minutes. The PCR-amplified DNA products were subjected to 2% agarose gel electrophoresis and visualized by ethidium bromide staining. The specific amplification product was considered the one that matched the expected size of the fragment (770 base pairs). Equal loading was determined from the intensity of the total RNA bands on an agarose gel.
Assays for enzymatic activity.NAD+ glycohydrolase (NADase) activity of CD38 was determined by incubating the membrane fraction (5 μg protein) from retinoid-treated or untreated cells in a 50-μL volume that contained 0.1 mmol/L (adenyl-32P)NAD+ (specific activity, 0.1 μCi/μmol) in 10 mmol/L Tris-HCl (pH 7.5) at 37°C for 15 minutes. The reaction was stopped by freezing the samples on dry ice, and 5-μL aliquots were spotted onto polythethylenimine cellulose thin layer chromatography (TLC) plates (Aldrich Chemical, Miwaukee, WI). The plates were developed using 0.1 mol/L formic acid as mobile phase, air-dried, and autoradiographed. In some cases, the radioactive bands were scraped out from the developed TLC plates, and the radioactivity was counted. NAD+ (0.05 μCi) was run on each plate as a marker. The relative front (Rf ) values for NAD+, ADP-ribose, and, 5′-AMP were 0.55, 0.94, and 0.4, respectively.
In some experiments, NADase and cyclase activities of CD38 were determined by a fluorometric method, using NAD+ and nicotinamide guanine dinucleotide (NGD) as substrates, essentially as described earlier.17 18
Transgenic mice.A 3.0-kb human RAR-α cDNA in the antisense orientation was inserted at the Sal site of the pMAMneo expression vector that contains the RSV/MMTV-LTR promoter and SV40 splicing/polyadenylation sites (Clontech, Palo Alto, CA). The DNA construct was isolated from the bacterial sequences by digestion with EcoRI and Pvul restriction enzymes and microinjected into fertilized eggs as previously described.19 Briefly, the DNA was microinjected into the male pronucleus of fertilized F1 hybrid eggs from SWRxSJL mice. The DNA-injected eggs were then transplanted into the oviduct of CD-1 pseudopregnant foster mothers. The offspring were screened by PCR for inserted cDNA sequences, which were then confirmed by Southern blot analysis. Six independent transgenic lines carrying the RAR-α antisense construct were generated. These mice were intercrossed and intracrossed to generate homozygous mice.
Western blots.Fresh tissues from normal and transgenic mice and lymphomas from transgenic mice were homogenized in 20 mmol/L Tris-HCl buffer (pH 7.2) containing 1 mmol/L EDTA, 1 mmol/L EGTA, 100 mmol/L NaCl, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF ), 0.1% aprotinin, 0.1% leupeptin, 0.1% pepstatin, and 0.1% Triton X-100. The supernatants obtained by centrifuging the samples at 35,000 rpm for 45 minutes were assayed for protein using the Coomassie blue staining procedure and bovine serum albumin as standard. The samples (100 μg) were subjected to 9.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and the proteins electroblotted onto nitrocellulose membrane. After being blocked with 5% nonfat milk, the membranes were probed with mouse anti–RAR-α monoclonal antibody (Affinity Bioreagent, Neshanic Station, NJ). The immunoreactive bands were detected by using horseradish peroxidase–conjugated antimouse IgG as secondary antibody and an enhanced chemiluminescence detection system according to the manufacturer's instructions (Amersham, Arlington Heights, IL). The membranes were stripped using a procedure recommended by the manufacturer (Amersham) and reprobed with antimouse CD38 monoclonal antibody (kindly supplied by Dr Debra Cockayne of DNAX, Palo Alto, CA) or with mouse anti–RAR-β monoclonal antibody (Affinity Bioreagent).
All experiments were repeated at least twice. Most of the data shown are averages of duplicate or triplicate determinations with less than 10% variations.
RESULTS
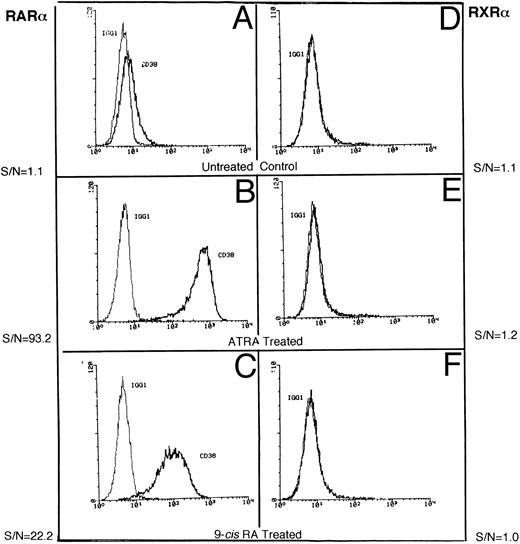
Induction of CD38 by receptor-selective retinoids.We confirmed our earlier observations that the basal expression of CD38 protein in RAR-α– (Fig 1A) and RXR-α–transfected (Fig 1D) HL-60R cell lines is very low.11 Treatment with the RAR agonist ATRA induced high expression of CD38 (40- to 60-fold) in RAR-α–transfected HL-60R cells (Fig 1B), but not in RXR-α–transduced HL-60R cells (Fig 1E). Interestingly, RXR-α–transfected cells failed to show any increase in CD38 accumulation, even when they were treated with the retinoid receptor panagonist, 9-cis RA, which has the ability to activate both RARs and RXRs.20 RAR-α–transduced HL-60R cells showed a moderate, but consistent increase in CD38 expression (Fig 1C) following treatment with 9-cis RA. These results suggest that CD38 expression in HL-60 cells may be regulated by RAR-type nuclear receptors.
Representative flow cytometric histograms show CD38 expression in RAR-α (A-C) and RXR-α (D-F ) –transfected HL-60R cells, incubated in medium alone (A and D) or in medium containing 10 nmol/L ATRA (B and E) or 10 mmol/L 9-cis RA (C and F ) overnight. The cells were stained with either isotypic control IgG1 or anti-CD38 antibody (Leu-17) as described in the Materials and Methods.
Representative flow cytometric histograms show CD38 expression in RAR-α (A-C) and RXR-α (D-F ) –transfected HL-60R cells, incubated in medium alone (A and D) or in medium containing 10 nmol/L ATRA (B and E) or 10 mmol/L 9-cis RA (C and F ) overnight. The cells were stained with either isotypic control IgG1 or anti-CD38 antibody (Leu-17) as described in the Materials and Methods.
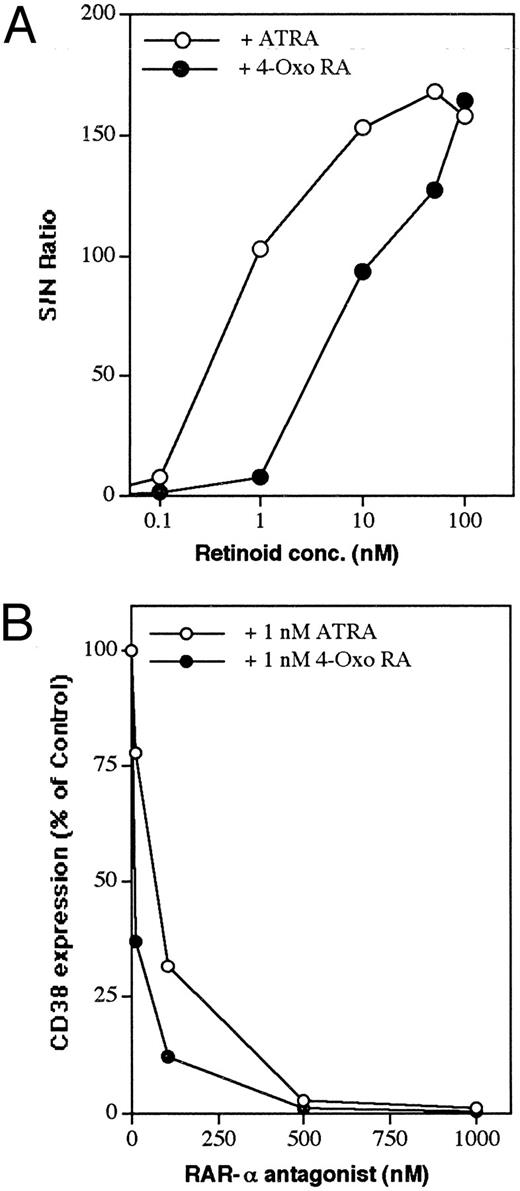
To investigate this further, we tested several receptor-selective synthetic retinoids for their ability to induce CD38 expression in HL-60 cells. Ligand-mediated activation of RAR-α indeed served as a potent signal for CD38 induction (Table 1). Thus, the RAR-α–specific retinoid CD336 induced an approximately 114-fold increase in CD38 protein accumulation, whereas RAR-β (CD417), RAR-γ (CD437), or RXR (CD2608, CD2624)–selective retinoids did not induce CD38. Only wild-type HL-60 cells with a high endogenous RAR-α expression21 or HL-60R cells transfected with functional RAR-α responded to RAR-selective retinoids. RAR-γ–transfected HL-60R cells also responded, although much more poorly than RAR-α–transfected cells, to retinoid treatment. Some of the physiologic retinoids, such as retinol, retinaldehyde, and β-carotene, were virtually inactive in driving CD38 expression. An interesting feature of this study was the observation that polar metabolites of ATRA, particularly 4-oxo RA and 4-hydroxy RA, were highly effective in driving the expression of CD38 in HL-60 cells. The dose-dependent effect showed that 4-oxo RA was approximately 1 log less active than ATRA in inducing CD38 expression (Fig 2A).
Dose-dependent induction of CD38 by ATRA and 4-oxo RA and its downmodulation by RAR-α antagonist. (A) RAR-α–transduced cells were incubated in the presence of increasing doses of ATRA or 4-oxo RA. (B) Cells were incubated overnight with 1 nmol/L ATRA or 4-oxo RA alone or simultaneously with increasing concentrations of RAR-α antagonist (Ro41-5253). At the end of the incubation period, cells were washed and analyzed for CD38 expression by flow cytometry, as described in the Materials and Methods. Data points represent the means of 2 experiments, with a standard deviation < 10% from the mean.
Dose-dependent induction of CD38 by ATRA and 4-oxo RA and its downmodulation by RAR-α antagonist. (A) RAR-α–transduced cells were incubated in the presence of increasing doses of ATRA or 4-oxo RA. (B) Cells were incubated overnight with 1 nmol/L ATRA or 4-oxo RA alone or simultaneously with increasing concentrations of RAR-α antagonist (Ro41-5253). At the end of the incubation period, cells were washed and analyzed for CD38 expression by flow cytometry, as described in the Materials and Methods. Data points represent the means of 2 experiments, with a standard deviation < 10% from the mean.
Effect of an RAR-α antagonist on retinoid-induced CD38 expression.To analyze further the involvement of RAR-α in the regulation of CD38 expression by retinoids, the effect of the RAR-α antagonist Ro41-525322 was studied. RAR-α–transfected cells were cultured simultaneously with a suboptimal concentration of ATRA or 4-oxo RA (1 nmol/L) and increasing concentrations of the RAR-α antagonist. After a 16-hour treatment, the cells were analyzed for cell-surface CD38 expression by flow cytometry. As shown in Fig 2B, increasing concentrations of Ro41-5253 inhibited in a dose-dependent manner the ATRA- and 4-oxo RA–induced expression of CD38 protein. At 0.5 μmol/L concentration, Ro41-5253 almost completely blocked retinoid-mediated induction of CD38. Treatment of RAR-α–transfected HL-60R cells with the antagonist alone did not modulate CD38 expression.
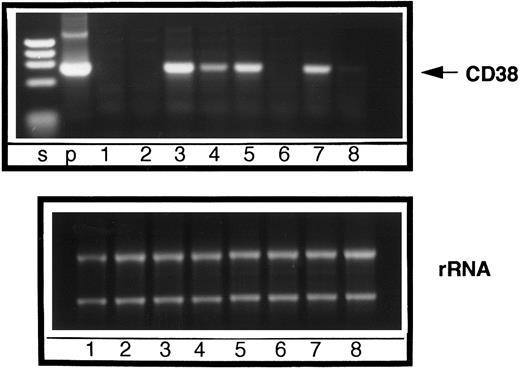
In the next series of experiments, we determined the effect of Ro41-5253 on retinoid-induced expression of CD38 mRNA by PCR analysis. The synthetic PCR primers were designed to amplify the extracellular domain from full-length CD38 cDNA.15 16 We could not detect any CD38 mRNA in untreated cells (Fig 3, lane 1) or in cells treated with 1 μmol/L Ro41-5253 (Fig 3, lane 2). However, treatment with ATRA, 4-oxo RA, or 9-cis RA induced significant expression of CD38 mRNA (Fig 3, lanes 3, 5, and 7, respectively). Simultaneous treatment of cells with Ro41-5253 and either of these three retinoids caused 70% to 95% inhibition in CD38 mRNA expression (Fig 3, lanes 4, 6, and 8, respectively).
Effect of RAR-α antagonist on retinoid-induced expression of CD38 mRNA. RAR-α–transduced HL-60R cells were cultured with medium alone (lane 1) or medium containing Ro41-5253 alone (lane 2), ATRA alone (lane 3), ATRA plus Ro-41-5253 (lane 4), 4-oxo RA alone (lane 5), 4-oxo RA plus Ro41-5253 (lane 6), 9-cis RA alone (lane 7), or 9-cis RA plus Ro41-5253 (lane 8). After appropriate treatment (10 nmol/L of each retinoid and/or 1 μmol/L Ro41-5253 for 16 hours), the PCR product specific for CD38 mRNA was generated and resolved by agarose gel electrophoresis. Lane p shows PCR product generated from a plasmid containing the full-length CD38 cDNA sequence. M, DNA molecular weight markers, gl-EcoT141. Lower panel shows equal loading for total RNA used for different samples in RT-PCR.
Effect of RAR-α antagonist on retinoid-induced expression of CD38 mRNA. RAR-α–transduced HL-60R cells were cultured with medium alone (lane 1) or medium containing Ro41-5253 alone (lane 2), ATRA alone (lane 3), ATRA plus Ro-41-5253 (lane 4), 4-oxo RA alone (lane 5), 4-oxo RA plus Ro41-5253 (lane 6), 9-cis RA alone (lane 7), or 9-cis RA plus Ro41-5253 (lane 8). After appropriate treatment (10 nmol/L of each retinoid and/or 1 μmol/L Ro41-5253 for 16 hours), the PCR product specific for CD38 mRNA was generated and resolved by agarose gel electrophoresis. Lane p shows PCR product generated from a plasmid containing the full-length CD38 cDNA sequence. M, DNA molecular weight markers, gl-EcoT141. Lower panel shows equal loading for total RNA used for different samples in RT-PCR.
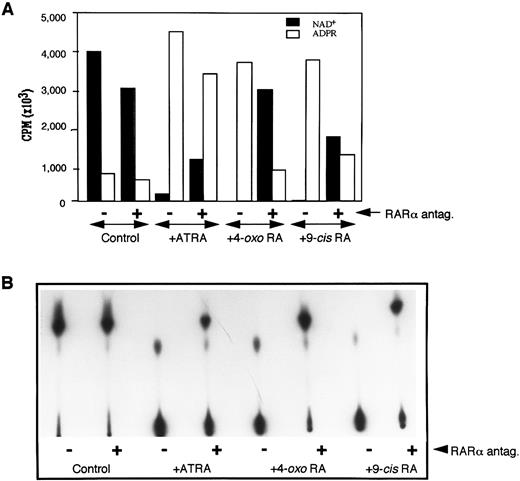
Because the extracellular domain of CD38 has been shown to exhibit NADase activity,6-9 15 we next determined the effect of Ro41-5253 on ATRA-induced ecto-NADase activity. RAR-α–transfected HL-60R cells were treated in the presence of retinoid alone or with retinoid plus Ro41-5253. The membrane fractions from treated and untreated cells were isolated and incubated with (adenylate-32P)NAD. The conversion of NAD+ to its radioactive metabolites was determined by TLC, as described earlier. There was no basal expression of NADase activity in untreated cells, nor could it be induced by treatment with Ro41-5253 alone (Fig 4). However, treatment with ATRA, 4-oxo RA, or 9-cis RA caused a dramatic increase in NADase activity, as was evident by the complete degradation of NAD+ into its metabolites (Fig 4B). The simultaneous presence of RAR-α antagonist and retinoids caused a 60% to 80% decrease in the expression of enzyme activity (Fig 4A). These results further suggested that increased expression of NADase activity in retinoid-treated HL-60 cells is under the control of RAR-α nuclear receptors and that ATRA-induced ecto-NADase activity is due to CD38 protein.
Downregulation of NADase activity by RAR-α antagonist. The membrane fraction from HL-60 cells cultured in medium alone (control) or medium containing 10 nmol/L of appropriate retinoid without (−) or with (+) 1 μmol/L Ro41-5253, was incubated with 32P-labeled NAD, and the reactants were analyzed by TLC for radioactive products as described in the Materials and Methods. Radioactive bands were either scrapped from the TLC plates, counted by scintillation spectroscopy, and cpms from each lane corresponding to NAD or ADP-ribose were plotted (A), or the plates were developed and the radioactive bands visualized by autoradiography (B).
Downregulation of NADase activity by RAR-α antagonist. The membrane fraction from HL-60 cells cultured in medium alone (control) or medium containing 10 nmol/L of appropriate retinoid without (−) or with (+) 1 μmol/L Ro41-5253, was incubated with 32P-labeled NAD, and the reactants were analyzed by TLC for radioactive products as described in the Materials and Methods. Radioactive bands were either scrapped from the TLC plates, counted by scintillation spectroscopy, and cpms from each lane corresponding to NAD or ADP-ribose were plotted (A), or the plates were developed and the radioactive bands visualized by autoradiography (B).
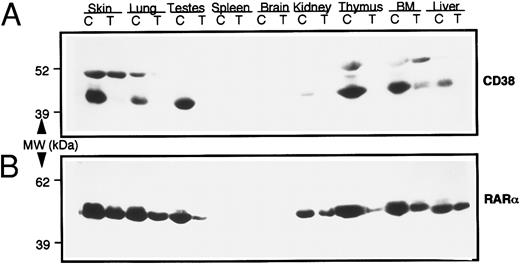
In vivo modulation of CD38 expression by RAR-α.Next we studied the possible involvement of RAR-α in CD38 expression in vivo. For this purpose, transgenic mice that expressed an antisense construct of RAR-α were generated, as described earlier. The mice were intercrossed and intracrossed to generate homozygous mice. Various tissues from homozygous transgenic mice were studied for CD38 and RAR-α protein expression by immonoblotting, using monospecific antimouse CD38 or anti–RAR-α antibodies. Results, seen in Fig 5, showed a 50% to 80% reduction in RAR-α protein in all of the tissues studied from transgenic mice. Even more intriguing was the observation that these tissues exhibited an almost complete absence of CD38 protein. Corresponding tissues from wild-type control mice, especially the skin, lung, testes, thymus, bone marrow, and liver, contained high levels of CD38 protein (45-kD band) (Fig 5A). The identity of another immunoreactive band (51 kD) that reacted with anti-CD38 antibody in some tissues is not known. These results thus suggested that RAR-α plays a critical role in regulating the expression of CD38 protein in vivo as well.
Levels of CD38 and RAR-α proteins in various tissues from normal wild-type (C) and RAR-α homozygous transgenic (T) mice. Soluble extracts from indicated tissues were prepared, and equal amounts of protein (100 μg) were resolved on SDS-PAGE. The proteins were transferred onto nitrocellulose membrane, and the membrane was probed with mouse anti-CD38 monoclonal antibody (A). The membrane was stripped and reprobed with anti–RAR-α antibody (B), as described in the Materials and Methods.
Levels of CD38 and RAR-α proteins in various tissues from normal wild-type (C) and RAR-α homozygous transgenic (T) mice. Soluble extracts from indicated tissues were prepared, and equal amounts of protein (100 μg) were resolved on SDS-PAGE. The proteins were transferred onto nitrocellulose membrane, and the membrane was probed with mouse anti-CD38 monoclonal antibody (A). The membrane was stripped and reprobed with anti–RAR-α antibody (B), as described in the Materials and Methods.
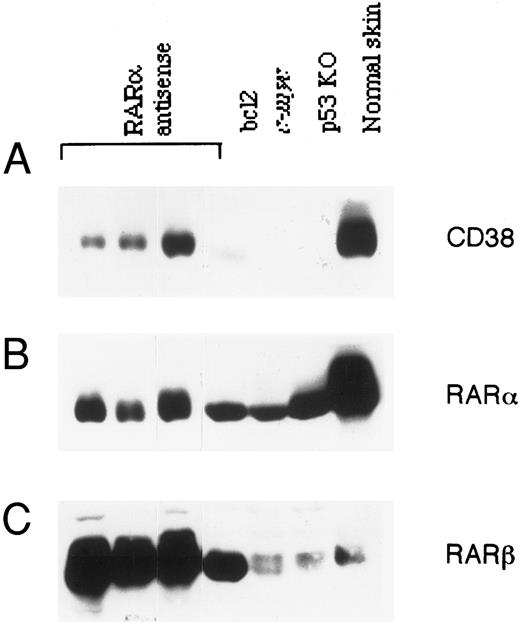
Correlation between RAR-α and CD38 expression in lymphoma cells.A significant number (44%) of RAR-α antisense transgenic mice develop spontaneous lymphoma early in life.19 Since lymphomas in general express relatively high levels of CD38,6 we investigated the status of CD38 protein in lymphomas from RAR-α–deficient mice. Tumor tissue from three homozygous transgenic mice was analyzed for CD38 protein expression by immunoblotting and compared with spontaneous lymphomas in bcl-2 and c-myc transgenic mice and p53 knockout mice. The results are shown in Fig 6. Surprisingly, unlike other tissues from these mice (Fig 5), the tumors from RAR-α antisense transgenic mice contained detectable amounts of CD38, as well as RAR-α proteins. Even more surprising was the observation that tumors from p53 knockout, as well as bcl-2 or c-myc transgenic mice, had no detectable expression of CD38 protein, despite the fact that these tumors contained normal levels of RAR-α protein. The expression of RAR-β protein in various tissues (data not shown), as well as in tumors (Fig 6) obtained from antisense transgenic mice, was significantly increased. A similar increase in RAR-β was observed in lymphomas obtained from bcl-2 transgenic mice. However, tumors from c-myc transgenic and p53 knockout animals, as well as skin tissue from normal mice, contained much lower levels of RAR-β protein.
Expression of CD38, RAR-α, and RAR-β proteins in lymphomas. Lymphomas that developed spontaneously in RAR-α antisense homozygous mice, bcl-2 transgenic mice, c-myc transgenic mice, or p53 knockout mice were homogenized, and the soluble extracts (100 μg) were resolved on SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti-CD38 antibody (A). After stripping, the membrane was reprobed with RAR-α–specific (B) or RAR-β–specific antibody (C). Protein extract of skin from a normal mouse was loaded in parallel as a reference.
Expression of CD38, RAR-α, and RAR-β proteins in lymphomas. Lymphomas that developed spontaneously in RAR-α antisense homozygous mice, bcl-2 transgenic mice, c-myc transgenic mice, or p53 knockout mice were homogenized, and the soluble extracts (100 μg) were resolved on SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti-CD38 antibody (A). After stripping, the membrane was reprobed with RAR-α–specific (B) or RAR-β–specific antibody (C). Protein extract of skin from a normal mouse was loaded in parallel as a reference.
DISCUSSION
Treatment of HL-60 cells with retinoids, particularly ATRA, induces morphologic changes that are characteristic of cells undergoing granulocytic differentiation.2 These and other biologic effects of retinoids are mediated through a number of closely related nuclear receptors that carry unique DNA and ligand-binding domains and regulate the transcription of specific target genes. HL-60 cells contain the mRNAs for RAR-α, RAR-β, RXR-α, and RXR-β nuclear receptors.21,23 RAR-α protein levels are higher than RAR-β levels in HL-60 cells.24 RAR-α has been suggested to be intimately involved in eliciting the differentiation of HL-60 cells, whereas ligand activation of RXRs is implicated in the induction of retinoid-induced apoptosis.25 26
ATRA-induced differentiation and apoptosis in HL-60 cells are associated with expression of several enzymes and cell-surface proteins.27-30 For example, the expression of bcl-2, a protein whose expression is frequently suppressed in cells undergoing apoptosis, is downmodulated following treatment with ATRA.29 Similarly, tissue transglutaminase, an enzyme that frequently accumulates to high levels in apoptotic cells, is induced in response to ATRA.30 An earlier report by Hemmi et al31 suggested that NADase is one cell-surface enzyme that is induced in ATRA-differentiated HL-60 cells. Recent studies showed that the ATRA-induced increase in NADase is due to induction of the membrane antigen CD38.7-9 15 In the present study, we examined the signaling pathways that are involved in the retinoid-induced increase in CD38.
Human CD38 is a 45-kD, single-chain transmembrane protein with complex biologic behavior.6 Besides functioning as an ecto-bifunctional enzyme, whereby it converts NAD+ into cADPR and subsequently to ADP-ribose,7-9 CD38 protein participates in transmembrane signaling pathways32,33; serves as an adhesion molecule34; and regulates intracellular calcium.10,35 ATRA is a potent inducer of CD38 expression in HL-60 and other myeloid cells.14,16,17,36 ATRA-induced upregulation of CD38 is quite specific and does not occur when HL-60 cells are treated with other inducers of differentiation such as DMSO, granulocyte colony-stimulating factor, interferon-gamma, or phorbol ester.11,36 Although the molecular basis of CD38 regulation has not yet been determined, it is likely that retinoid receptors participate in this action. In an earlier study of HL-60R, an HL-60 cell line with a trans-dominant negative mutation, we suggested the possible involvement of RAR-α in retinoid-induced CD38 expression.11 In the present study, we determined the effect of several receptor-selective retinoids, an RAR-α antagonist, and the HL-60R subline transduced to express a specific subtype of retinoid receptor in an effort to define the receptor that is involved in the regulation of CD38. The results obtained support our contention that induction of CD38 antigen is mediated by a specific retinoid signaling pathway that involves the RAR-α receptor.
For example, only wild-type HL-60 cells that harbor endogenous RAR-α receptor20 or RAR-α–transfected HL-60R cells responded to RAR-selective retinoids in terms of induced CD38 expression (Table 1). Moreover, of the RAR-α–, -β–, and -γ–selective retinoids, only the RAR-α–specific retinoid CD336 induced CD38 expression. The RXR-selective retinoids CD2608 and CD2624, which selectively bind and activate RXRs,37 failed to induce the expression of CD38 protein in RXRα–transfected cells, which suggests that CD38 expression is mediated via an RAR-RXR heterodimer, rather than an RXR-RXR homodimer. Indeed, our preliminary studies have shown the presence of retinoic acid response elements in the 5′ upstream sequence of the CD38 gene, which supports our contention that trans-activation of the CD38 gene may be mediated via an RAR-RXR heterodimer (G. Gaikwad and K. Mehta, unpublished results, 1997). RXR-transfected HL-60R cells treated with the panagonist 9-cis RA or the RXR-selective retinoids induced strong expression of another protein, tissue transglutaminase,25 29 suggesting that CD38 induction is highly RAR-specific. Interestingly, RAR-γ–transfected cells showed a moderate, but consistent accumulation of CD38 protein in response to RAR-selective retinoids (Table 1). This may suggest some redundancy between RAR-α and RAR-γ in terms of CD38 regulation.
Another interesting feature of our study was the observation that polar metabolites of ATRA, especially 4-oxo RA and 4-hydroxy RA, were highly active in inducing the expression of CD38 (Table 1). The 4-oxo RA was initially considered to be an inactive metabolite of ATRA, but it recently was shown to be highly active in modulating the positional specification in early Xenopus embryos.38 When compared with ATRA, the binding affinity of 4-oxo RA to recombinant RARs is equal for RAR-α and RAR-β and slightly less for RAR-γ.39 In our studies, dose-dependent response suggested that 4-oxo RA is approximately 10-fold less active than ATRA in inducing expression of CD38 (Fig 2A).
Another piece of evidence that RAR-α receptors are directly involved in regulation of retinoid-mediated expression of CD38 was provided by studies with an RAR-α antagonist. RAR-selective retinoids incubated in conjunction with Ro41-5253 inhibited the expression of CD38 protein (Fig 2) and mRNA (Fig 3), as well as the expression of its catalytic activity (Fig 4). The exact mechanism by which the antagonist exerts its effect is not known, but it has been shown to compete directly with ATRA for binding sites on RARα.22 In the presence of Ro41-5253, the RAR-RXR heterodimer retained its ability to bind to the response element. It is therefore likely that binding of the antagonist to RAR-α does not induce the correct conformation in the receptor protein to allow transactivation.22 The complete inhibition of CD38 induction by ATRA, 9-cis RA, or 4-oxo RA in the presence of antagonist (Figs 2, 3, and 4) suggested that all three retinoids mediate CD38 induction via activation of RAR-α. Since the antagonist has much lower binding affinity to RAR-α than ATRA22 or 4-oxo RA, this might account for the 500-fold higher concentration required to inhibit CD38 induction (Fig 2B).
Finally, the RAR-α nuclear receptors seemed to play a critical role in the regulation of CD38 protein in vivo also. Various tissues from homozygous transgenic mice that harbored 50% to 85% reduced levels of RAR-α protein showed no detectable expression of CD38 protein (Fig 5). The major abnormality in RAR-α antisense transgenic mice is their coarse fur and the infertility of males.19 Microscopically, the skin sections from homozygous transgenic mice showed significant atrophy in sebaceous glands and hair follicles, and examination of the testes showed some atrophy in seminiferous tubules, which in some cases resembled those described in incomplete spermatocystic arrest syndrome.40 Interestingly, skin and testes from normal mice expressed very high levels of CD38 protein (Fig 5). To our knowledge, this is the first report that describes the expression of CD38 in these tissues. From these results it is tempting to speculate that CD38 may play a role in maintaining the normal functions of the skin and testes. Convincing evidence for such a notion should be provided by a more relevant model, such as CD38 knockout mice.
Another important abnormality in antisense homozygous transgenic mice was the development of tumors at an early stage of life. Ninety percent of the tumors that develop in these mice are lymphomas.19 Analysis of these tumors for CD38 and RAR-α expression showed some unexpected results. Unlike other tissues from transgenic mice (Fig 5), lymphomas showed appreciable levels of CD38 and RAR-α proteins when compared with lymphomas of different etiology (Fig 6). For example, lymphomas that were developed spontaneously in bcl-2 and c-myc transgenic mice, as well as in p53 knockout mice, showed a complete absence of CD38, despite detectable expression of RAR-α. The RAR-β expression in tissues (data not shown), as well as in lymphomas (Fig 6) from RAR-α antisense transgenic mice, was upregulated, whereas no such change was observed in tumors derived from other transgenic (bcl-2 and c-myc) or knockout (p53) mice. Whether phenotype of the lymphoma and CD38 expression correlates more to their cell of origin or to the level of RAR-α expression remains to be determined. Direct proof of this and other premises on the potential role of CD38 in normal biologic functions will be provided by more relevant models, such as RAR-α–41 and CD38-deficient mice.
ACKNOWLEDGMENT
We thank Dr Tim McDonald for providing the lymphoma samples from bcl-2 and c-myc transgenic and p53 knockout mice. We also thank Walter Pagel for editorial assistance.
Supported by grants from the Texas Higher Education Coordinating Board (ATP000015-012) and the US Food and Drug Administration (FDR-000923).
Address reprint requests to Kapil Mehta, PhD, Department of Bioimmunotherapy, Box 60, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030.