Abstract
The microenvironment is a key regulator of hematopoietic stem cells (HSCs) and is a likely source of extracellular factors that control stem cell fate. A better understanding of these microenvironmental factors may come from investigations of developmental cell fate determination in which the critical roles of cell-cell interactions of multipotential cells have been shown. The Wnt gene family is known to regulate the cell fate and cell-cell interactions of multipotential cells in a variety of tissues. Expression of Wnts and of their putative receptors encoded by murine homologs of the Drosophila frizzled gene in hematopoietic tissues was examined by reverse transcriptase-polymerase chain reaction. Wnt-5a and Wnt-10b were expressed in day-11 murine yolk sac, day-14 fetal liver, and fetal liver AA4+ cells. The expression profiles of four murine frizzled homologs, Mfz3-7, were nearly identical to that of Wnt-5a and Wnt-10b. Notably, Wnt-10b was expressed in the fetal liver AA4+ Sca+ c-kit+ (flASK) HSC population. A role for Wnts in HSC fate determination was studied by treatment of HSC populations in culture with soluble WNT proteins. The addition of conditioned media from cells transfected with Wnt-1, Wnt-5a, or Wnt-10b cDNAs to cultures of flASK cells stimulated a sevenfold, eightfold, and 11-fold expansion in cell number, respectively, relative to control media. Removal of WNT-5a from this media by immunodepletion depleted the stimulatory activity from the media, whereas addition of a partially purified WNT-5a stimulated a fivefold expansion relative to control cells. Transduction of flASK cells with a retrovirus bearing a Wnt-5a cDNA enhanced proliferation. We conclude that WNTs stimulate the survival/proliferation of hematopoietic progenitors, demonstrating that WNTs comprise a novel class of hematopoietic cell regulators.
THE HEMATOPOIETIC stem cell (HSC) is a pluripotent cell with the capacity to provide for the life-long production of all blood lineages. This is accomplished by a balance between the plasticity of the HSC, ie, the production of committed progenitor cells that generate specific blood lineages, and the replication of the HSC in the undifferentiated state (self-renewal). The mechanisms regulating HSC plasticity and self-renewal in vivo have been difficult to define. However, the major contributory factors represent a combination of cell intrinsic and environmental influences.1,2 The importance of the hematopoietic microenvironment has been established through the use of long-term bone marrow culture systems in which hematopoietic cells cultured on stroma allow for the maintenance of HSCs, albeit at low frequencies.3 4
The demonstration of HSC maintenance in culture has lead to efforts to identify candidate stem cell factors. The role of hematopoietic cytokines in HSC maintenance has been studied by direct addition of purified factors to in vitro cultures of HSC populations followed by transplantation of the cultured cells.4-6 Most of the known early acting cytokines such as interleukin-3 (IL-3), IL-6, and Kit ligand (KL) have been shown to stimulate proliferation of committed progenitor cells while concurrently allowing maintenance, but only limited expansion, of HSCs capable of long-term multilineage repopulation.7 8 Although these data are encouraging from the perspective that HSC plasticity and repopulating function can be preserved by cytokine treatment, the molecules that promote self-renewal of HSCs remain unknown.
Transplantation studies have shown that the signals that regulate HSC fate may be similar in the embryo and adult bone marrow. Cells from the day-11 fetal liver, yolk sac, or aorta/gonad/mesonephros (AGM) region can repopulate the adult marrow and appropriately respond to extrinsic cues to sustain long-term multilineage hematopoiesis.9 Although embryonic hematopoiesis is largely devoted to the erythroid lineage, the embryonic microenvironment clearly contributes to the maintenance of HSCs in the undifferentiated state. Moreover, given that HSC populations are cycling during embryogenesis,10-12 novel self-renewal factors might be uncovered from investigations on the embryonic and fetal hematopoietic microenvironment.
In mammals, hematopoietic precursors are found in the extraembryonic and ventral mesoderm, yolk sac, or AGM region.13,14 In amphibian embryos, the equivalent regions are the ventral blood island mesoderm and the dorsal lateral plate mesoderm.14-16 Secreted factors that potentially regulate cell fate determination of ventral mesoderm in Xenopus include Wnts, FGFs, and BMP-4.14 17 Embryonic expression of XWnt-818 and XWnt-1119 is localized to the area of prospective ventral and lateral mesoderm and XWnt-8 expression can be induced by ventralizing factors such as FGFs and BMP-4.
Wnts comprise a large gene family whose members have been found in round worms, insects, cartilaginous fish, and vertebrates.20Wnts may function in a variety of developmental and physiologic processes because many diverse species have multiple conserved Wnt genes.21,22Wnt genes encode secreted glycoproteins that are thought to function as paracrine or autocrine signals active in several primitive cell types.21,22 A role for Wnts in local cell signaling is likely, because biochemical studies have shown that much of the secreted WNT protein can be found associated with the cell surface or extracellular matrix rather than freely diffusible in the medium.23,24 Putative cell surface receptors for secreted WNTs are proposed to be encoded by the frizzled gene family.25
Studies of mutations in Wnt genes have indicated a role for Wnts in growth control and tissue patterning. In Drosophila,wingless (wg ) encodes a Wnt gene26 and wg mutations alter the pattern of embryonic ectoderm, neurogenesis, and imaginal disc outgrowth.27-29 In Caenorhabditiselegans,lin-44 encodes a Wnt that is required for asymmetric cell divisions.30 Knock-out mutations in mice have shown Wnts to be essential for brain development31,32 and the outgrowth of embryonic primordia for kidney,33 tail bud,34 and limb bud.35 Overexpression of Wnts in the mammary gland can result in mammary hyperplasia21,22 and precocious alveolar development.36 Collectively, these studies show that Wnts are involved in the growth and differentiation of a variety of primitive cell types.
Although a role for Wnts in mammalian hematopoiesis has not previously been considered, in situ hybridization studies have shown that Wnt-3a, Wnt-5a, and Wnt-5b are expressed in the posterior mesoderm during the egg cylinder and primitive streak stages.34Wnt-5a and Wnt-5b are expressed in extraembryonic mesoderm of the day-7 to -8 murine embryo.34 These embryonic domains contribute to the AGM region and yolk sac tissues from which multipotent hematopoietic precursors and HSCs are derived.13,14,37Wnt-5a is also expressed in murine38,39 and human40 limb bud mesenchyme and may play a role in the development or patterning of the limb mesenchymal microenvironment. The localized expression of Wnts in these embryonic regions led us to investigate a role in hematopoietic cell fate regulation or progenitor cell expansion as in the Xenopus system.
In this study, we show that Wnt-5a and Wnt-10b are expressed in the murine yolk sac and fetal liver microenvironment and that Wnt-5a is expressed in fetal liver stromal cells. Furthermore, we provide evidence that Wnts can function as hematopoietic regulatory factors. Wnts directly stimulate the proliferation of hematopoietic stem/progenitor cell populations (HSCPs) in culture and trigger the formation of multicellular aggregates or foci of primitive blast cells. Replating of the cultured cells into methylcellulose under myeloid or lymphoid conditions shows that Wnts expand the total number of multipotential colony-forming cells (CFCs).
MATERIALS AND METHODS
Antibodies.Phycoerythrin-conjugated antibodies (Ly6A/E, TER-119, CD14), fluorescein-conjugated antibodies (c-kit [clone 3C1], CD13, CD31, CD44, CD45, CD49d, CD49e, GR1, VCAM-1, ICAM-1, L-selectin), CD11a, and CD29 antibodies were purchased from Pharmingen (San Diego, CA). Phycoerythrin-conjugated Mac-1, fluorescein-conjugated antibodies (CD4, CD8a, and B220), and all secondary and Lin cocktail antibodies were purchased from Caltag (South San Francisco, CA). Anti-E cadherin antibodies (clone DECMA-1; Sigma, St Louis, MO) were purified from ascites fluid. Anti-P cadherin antibodies were from Transduction Laboratories (Lexington, KY). The AA4.1 antibody was purified from ascites.10
Preparation of hematopoietic progenitor cell populations.Murine fetal liver and bone marrow hematopoietic/stem progenitor cell populations were prepared essentially as described.10 Briefly, day-14 to -15 fetal livers were made into a single-cell suspension and AA4+ cells were positively selected by immunoadherent panning. Sca+ c-kit+ dual-positive cells were recovered from the AA4+ cell population by flow cytometric sorting. We refer to this population as flASK cells (fetal liver AA4+ Sca+ kit+). Linlo Sca+ bone marrow cells were recovered by magnetic depletion of lineage-antigen–expressing cells from total bone marrow and selection of Linlo Sca+ cells by flow cytometric sorting, as described.10 Visceral yolk sacs from day-10.5 to -11 embryos were dissected away from the placenta, embryo, and amnion. Pooled yolk sacs were washed free of maternal blood and made into a single-cell suspension.
Culture of hematopoietic progenitor cells.Suspension culture of sorted cells was performed in 24-well Costar dishes (Costar, Cambridge, MA) with 5,000 cells/well seeded into 0.5 mL of HSC media and cultured at 37°C with 5% CO2 . HSC media contains 50% F12/50% low glucose Dulbecco's modified Eagle's medium, 10% heat-treated fetal bovine serum (Hyclone, Logan, UT), 1 mmol/L glutamine, and murine KL as indicated (R&D Systems, Minneapolis, MN). Conditioned media (4% to 10% vol/vol) was added at the time of plating. For foci formation, cells were plated in HSC media with 25 ng/mL murine KL onto Lab-Tek chamber slides (Nunc, Naperville, IL) coated with 50 μg/mL of human plasma fibronectin (GIBCO, Gaithersburg, MD).
Colony assays.Methylcellulose cultures were initiated in 35-mm plates with 1,000 cells in 1 mL complete myeloid methylcellulose (Stem Cell Technologies, Inc, Vancouver, British Columbia, Canada) or in B-cell conditions consisting of base methylcellulose containing 50 ng/mL murine KL and 50 ng/mL murine IL-7 (R&D Systems). Conditioned media was added at the time of plating. Plates were read at day 12 after plating.
Molecular cloning of Wnt cDNAs.Poly A+ RNA was prepared by the Fast Track method (Invitrogen, San Diego, CA). Reverse transcription was performed by denaturing RNAs and dT18 primers in the presence of 0.1 mol/L methyl mercuric hydroxide, followed by quenching with 20 mmol/L β-mercaptoethanol and extension in 20 μL total with Superscript II reverse transcriptase as recommended (GIBCO). Polymerase chain reactions (PCRs) were performed with Taq polymerase (Perkin Elmer, Foster City, CA) on day-14 fetal liver AA4+ or AA4+ Sca+ cDNAs or a 7-4 cell line cDNA library using one of seven sense primers (LL1 through LL7) with a consensus antisense primer, Wnt-AS, to the conserved sequence MCCGRG [sense primers: LL1, 5′ CAA GAG TGC AAA TGC CAC GGG ATG TCC GGC TCC TGC 3′; LL2, 5′ CAA GAG TGC AAA TGC CAC GGG GTG TCC GGC TCC TGC 3′; LL3, 5′ CTC AAG TGC AAA TGC CAC GGG CTA TCT GGC AGC TGT 3′; LL4, 5′ GTG GAG TGC AAG TGC CAC GGG GTG TCC GGC TCC TGC 3′; LL5, 5′ GTA GCC TGT AAG TGC CAT GGA GTG TCT GGC TCC TGT 3′; LL6, 5′ ACC GGG TGT AAG TGC CAT GGG CTT TCG GGT TCC TGC 3′; LL7, 5′ CTG GAG TGT AAG TGC CAT GGT GTG TCA GGC TCC TGT 3′; Wnt-AS, 5′ GCC (C/G)CG GCC (G/A)CA (G/A)CA CAT 3′]. The PCR reactions were performed with an initial denaturation of 95°C for 2.5 minutes, and then at 94°C for 0.5 minutes, 48°C for 1.0 minute, and 72°C for 2.0 minutes for 60 cycles, with fresh Taq added at the midpoint. PCR products were cloned as a blunt-ended fragments into Sma I-linearized pGEM7 (Promega, Madison, WI) and screened for Wnt sequences by hybridization with the oligonucleotides Liem1 [5′ GAC CTG GTG TAC 3′] or Liem2 [5′ TG(T/C) TG(T/C) GGC CG(G/C) GGC 3′]. A Wnt-5a PCR fragment was used to screen a cDNA library made from the 7-4 cell line and ligated into the pSVSPORT-1 vector (GIBCO). The Wnt-5a coding region was sequenced from one clone, Wnt5a.13.pSVSPORT-1, and the predicted amino acids matched those previously reported,38 except for (H207 > Y). A Wnt-10b cDNA was cloned by reverse transcriptase-PCR (RT-PCR) from flASK cells using the primers wn10b.5ri (5′ GGA ATT CCG GGC TTC GAC ATG CTG GAG GA 3′) and wn10b.3kpn (5′ GGG GTA CCC CAG GCT CAC CTT CAT TTA CAC A 3′) and cloned into pGEM7. The predicted coding sequence matched exactly to that reported elsewhere.41
RT-PCR analysis of Wnt and frizzled gene expression.Total RNA was prepared by the guanidinium thiocyanate/phenol method.42 Reverse transcription was performed as above with 5 μg of DNase-treated RNA. PCR was performed using 1.0 μL RNase-treated cDNA with Extend polymerase (Boehringer Mannheim, Indianapolis, IN) in a total of 50 μL. Equal amounts of template were used for each population or tissue. The PCR reactions were performed with an initial denaturation of 95°C for 3.0 minutes, and then at 94°C for 1.0 minute, 64°C for 2.0 minutes, and 72°C for 3.0 minutes for 35 cycles. To detect Wnt-10b expression, a second PCR was performed on 5.0 μL amplified cDNA as described above with nested primers. Ten microliters of each PCR reaction was fractionated by agarose gel electrophoresis. The primer sets used are as follows: Wnt-3a yielding products of ∼1,170 bp (wn3a.1, 5′ CCC AGC GCC ACT GCA GCC GC 3′; wn3a.3, 5′ GGA ATG AAC CCT GCT CCC GT 3′); Wnt-5a, ∼1,255 bp (wn5a.5, 5′ GAT TGT CCC CCA AGG CTT AAC CCC GAC GCT TC 3′; wn 5aBSTEII, 5′ GAA GGG CAG GCA CAC GGT GAC CTT GCA CAC 3′); Wnt-10b, primary amplification product of ∼1,230 bp (W1065, 5′ TGA CTG ACT CGC CCA CCG GAG CCT CC 3′; MW10.31, 5′ CGT CGT GCC TAA GCT TGA CTC ACC TTC ATT 3′), amplification product of ∼317 bp with nested primers (W1066, 5′ GGG CTT CGA CAT GCT GGA GGA GCC CC 3′; W10G3, 5′ AGC CGC CGC CGC CCT CCA GT 3′); Mfz3 (GenBank accession no. MMU43205; sense primer, bp 1674-1699; antisense primer, bp 2350-2366); Mfz4 (GenBank accession no. MMU43317; sense primer, bp 1716-1740; antisense primer, bp 1961-1985); Mfz5 (sequence provided by A. Rosenthal, Genentech, Inc; sense primer, bp 2355-2379; antisense primer, bp 2676-2700); and Mfz7 (GenBank accession no. MMU43320; sense primer, bp 1866-1890; antisense primer, bp 2101-2125).
Overexpression of murine Wnts in mammalian cells.The Wnt-1, Wnt-5a, and Wnt-10b cDNAs were cloned as EcoRI/Hind III fragments into pRK5tkneo. For production of conditioned media, 293 cells were transfected by the calcium phosphate method.43 The media was conditioned for 48 hours, centrifuged at 3,000g, and sterile filtered. A chimeric Wnt-5a gene was made encoding the first 55 amino acids (AA) of herpes simplex virus glycoprotein D followed by Wnt-5a AA 38-379 in gDCT-1pRK5b.44 The control vector used in experiments involving gDWnt5a was made from gDCT-1pRK5b by excising the CT-1 cDNA as a Xho I-Xba I fragment, filling in the overhanging nucleotides, and closing the gDpRK5b vector with T4 DNA ligase. The resulting construct is expected to encode the first 54 AA of gD followed by D and a stop codon. The gDWnt5aHis6 construct was made by appending a fragment encoding six histidine residues in-frame to the carboxyterminus of Wnt-5a in the gDpRK5b vector by PCR.
Immunoprecipitations.293 cells transfected with gDpRK5b or gDWnt5apRK5b were labeled overnight in serum-free media containing 10 μCi/mL [35S]methionine/[35S]cysteine (Amersham, Arlington Heights, IL). Conditioned media was collected, and the cells were washed twice in phosphate-buffered saline (PBS) and lysed in 50 mmol/L triethanolamine, pH 7.4, 100 mmol/L NaCl, 0.4% sodium dodecyl sulfate, 2% Triton X-100, 1 mmol/L phenylmethylsulfonyl fluoride, and 10 ng/mL each of chymotrypsin, leupeptin, antipain, and pepstatin A. Proteins from lysates or conditioned media were precipitated with 1 μg/mL 5B6 monoclonal antibody (MoAb) plus protein A Sepharose (Pharmacia, Uppsala, Sweden). The precipitates were washed, electrophoresed, and fluorographed as described in Zeigler et al.10
Immunodepletions.Conditioned media was prepared as described above from 293 cells transfected with gDpRK5b or gDWnt5apRK5b. The media was precipitated with 5B6-CPG as described above.
Virus construction and transduction.Wnt5a.13.pSVSPORT-1 was digested with EcoRI/BamHI, and the insert was blunted with T4 DNA Polymerase and cloned into blunted Bgl II/BamHI sites of the pLNL6 vector.45 Wnt5a/LNL6 or the parental LNL6 vector were transfected by a calcium phosphate method into Bosc 23 cells.46 Viral supernatants were collected after 48 to 72 hours and stored at −20°C. Transductions of flASK cells were performed at 100,000 cells/mL for 48 hours in viral supernatants supplemented with the murine cytokines IL-3 (25 ng/mL), IL-6 (50 ng/mL), and KL (10 ng/mL). Transduction efficiency of cells giving rise to methylcellulose colonies was assayed by PCR analysis essentially as described in Gerard et al.47
Purification of gD.Wnt5a.His6 .Stable lines of CHOdp12 cells transfected with gD.Wnt5a.His6pSVi.del.d were selected and maintained in glutamine-, hypoxanthine-, and thymidine-free media. Extracts were made from gD.Wnt5a.His6 CHO cells as described above, and WNT-5a protein was affinity purified using 5B6-CPG essentially as described in Paborsky et al.48 In brief, cell lysates were prepared as described above and were bound to the 5B6-CPG; the resin was washed extensively in PBS and eluted with acid. The eluate was neutrilized, dialyzed against PBS, and refolded in 8 mol/L urea. The refolded WNT-5a protein was diluted in HSC media to a final concentration of less than 60 mmol/L urea in the flASK cell suspension culture assay.
RESULTS
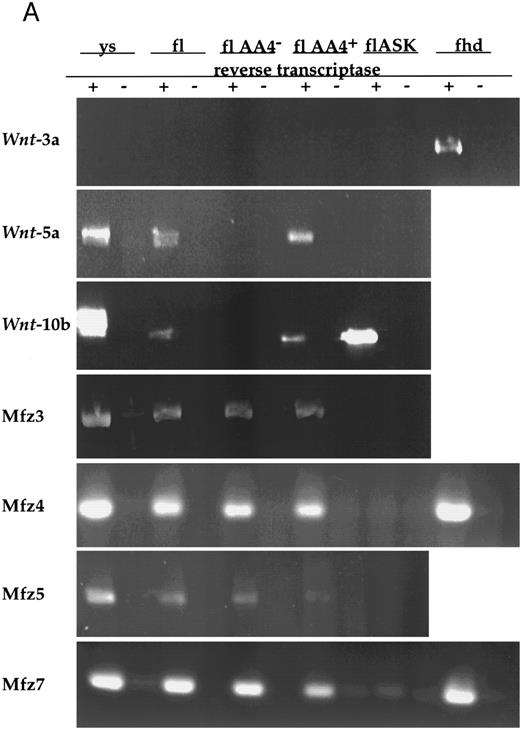
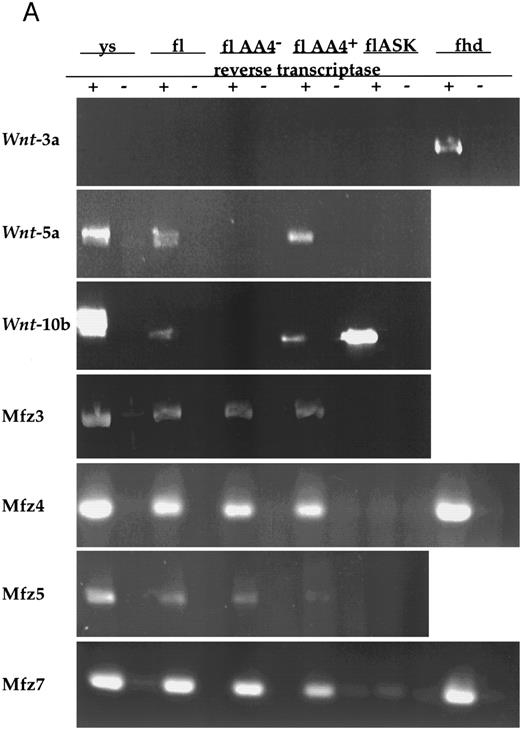
Wnt gene expression in hematopoietic stem/progenitor cell populations.Given the potential for paracrine or autocrine signaling by Wnts, we surveyed both HSCPs and stromal cell lines that support hematopoietic stem/progenitor cell growth for Wnt expression by RT-PCR with degenerate primer sets (see the Materials and Methods). This survey of fetal liver AA4+ and AA4+ Sca+ cells and a fetal liver stromal cell line, 7-4,10 detected expression of only Wnt-5a and Wnt-10b (data not shown). These observations were extended by RT-PCR with specific primers on total RNAs from several hematopoietic tissues. Although Wnt-3a is expressed in posterior mesoderm of earlier embryonic stages and could be amplified from fetal heads, its expression was not detected in any of the hematopoietic tissues assayed (Fig 1). Embryonic yolk sac, fetal liver, and fetal liver AA4+ hematopoietic progenitors expressed both Wnt-5a and Wnt-10b mRNAs. Wnt-10b, but not Wnt-3a or Wnt-5a, was detected in flASK cells (fetal liver AA4+ Sca+ kit+), which are highly enriched for HSCPs capable of long-term engraftment of lethally irradiated animals.10 It is likely that, at the level of detection of the RT-PCR assays, HSCPs express only a subset of the possible Wnt genes. Importantly, the expression of Wnt-10b mRNAs in flASK cells, of Wnt-5a and Wnt-10b in yolk sac and fetal liver AA4+ cells, and of Wnt-5a in fetal liver stromal cells suggests that the fetal liver microenvironment can potentially serve as a source of these Wnts. These data offer strong support for a role of these ligands in the local microenvironment of HSCPs.
(A) Analysis of Wnt and frizzled gene expression in fetal hematopoietic tissues. Reverse transcription was performed with (+) or without (−) the addition of reverse transcriptase. PCR was performed on cDNAs from day-11 visceral yolk sacs (ys), day-14 fetal liver (fl), fetal liver AA4− cells (flAA4−), fetal liver AA4+ cells (flAA4+), fetal liver AA4+ Sca+ c-kit+ cells (flASK), and day-14 fetal heads (fhd). Equal quantities of template were used for the PCR analysis of each cell population or tissue. The primer sets for amplification span known intron sequences of Wnt-3a34 and Wnt-10b (GenBank accession no. MMU30464, MMWNT10B261). The primer sets for Wnt-5a span introns found in the human gene.40 The genomic structures of the murine frizzled homologs Mfz3-7 are not known. In all reactions, no products were observed when reverse transcriptase was omitted.
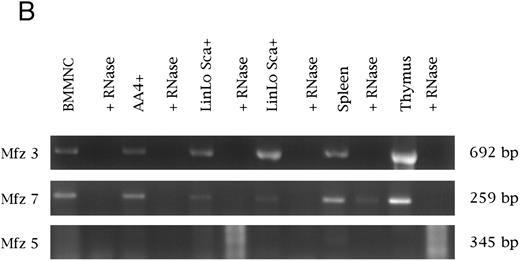
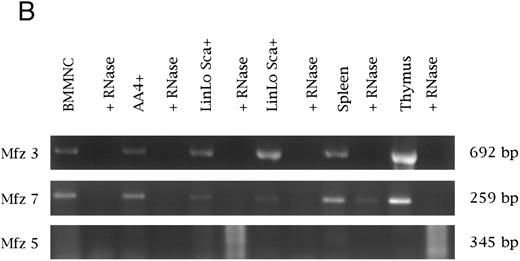
(B) Analysis of frizzled gene expression in bone marrow and adult hematopoietic tissues. Reverse transcription was performed with or without the presence of RNase. PCR was performed on cDNAs from bone marrow mononuclear cells (BMMNC); AA4+ cells from the fetal liver, spleen, and thymus; and two independent samples of Linlo Sca+ cells from bone marrow. Equal quantities of template were used for all reactions. The primer sets for amplification were the same as in (A). Transcripts were detected for frizzled 3 and 7, with no expression detected for frizzled 5 and 4 (data not shown for frizzled 4). No expression of Wnt 3a, 5a, and 10b was detected in these samples.
(A) Analysis of Wnt and frizzled gene expression in fetal hematopoietic tissues. Reverse transcription was performed with (+) or without (−) the addition of reverse transcriptase. PCR was performed on cDNAs from day-11 visceral yolk sacs (ys), day-14 fetal liver (fl), fetal liver AA4− cells (flAA4−), fetal liver AA4+ cells (flAA4+), fetal liver AA4+ Sca+ c-kit+ cells (flASK), and day-14 fetal heads (fhd). Equal quantities of template were used for the PCR analysis of each cell population or tissue. The primer sets for amplification span known intron sequences of Wnt-3a34 and Wnt-10b (GenBank accession no. MMU30464, MMWNT10B261). The primer sets for Wnt-5a span introns found in the human gene.40 The genomic structures of the murine frizzled homologs Mfz3-7 are not known. In all reactions, no products were observed when reverse transcriptase was omitted.
(B) Analysis of frizzled gene expression in bone marrow and adult hematopoietic tissues. Reverse transcription was performed with or without the presence of RNase. PCR was performed on cDNAs from bone marrow mononuclear cells (BMMNC); AA4+ cells from the fetal liver, spleen, and thymus; and two independent samples of Linlo Sca+ cells from bone marrow. Equal quantities of template were used for all reactions. The primer sets for amplification were the same as in (A). Transcripts were detected for frizzled 3 and 7, with no expression detected for frizzled 5 and 4 (data not shown for frizzled 4). No expression of Wnt 3a, 5a, and 10b was detected in these samples.
Very recently, members of the frizzled gene family have been proposed to serve as wingless receptors in Drosophila. with Mfz4 and Mfz7 being implicated in wingless binding.25 We examined the expression of several murine frizzled homologs in hematopoietic tissues (Mfz3, Mfz4, and Mfz749; Mfz5, A. Rosenthal, unpublished data). Expression of Mfz3, Mfz4, Mfz5, and Mfz7 was detected in yolk sac, fetal liver, fetal liver AA4−, and fetal liver AA4+ cells (Fig 1). Similarly, Mfz3 and Mfz7 were detected in both bone marrow mononuclear cells and the bone marrow stem cell population LinloSca+. Transcripts for these genes were also detected in thymus and spleen. Although our RT-PCR experiments have not examined the expression of the full repertoire of frizzled gene family members, clearly a subset of the murine frizzled homologs are expressed hematopoietic tissues.
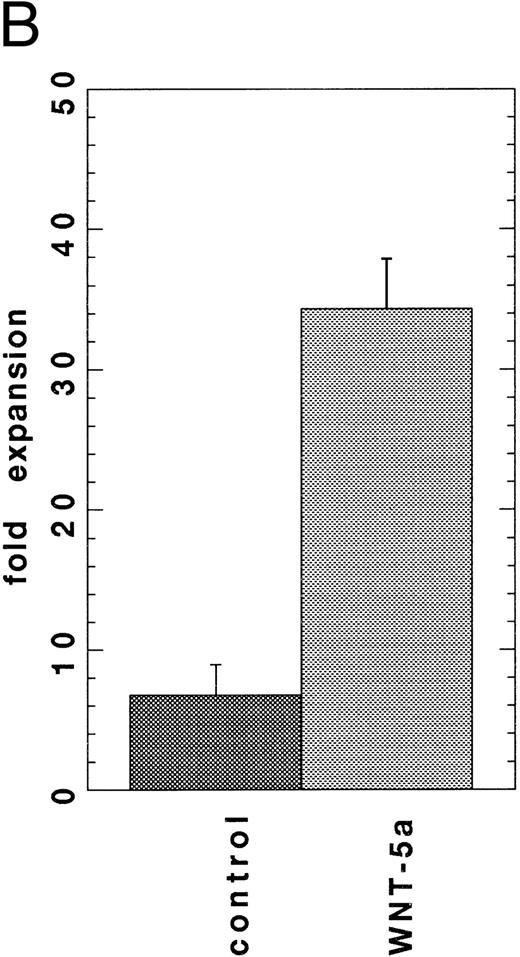
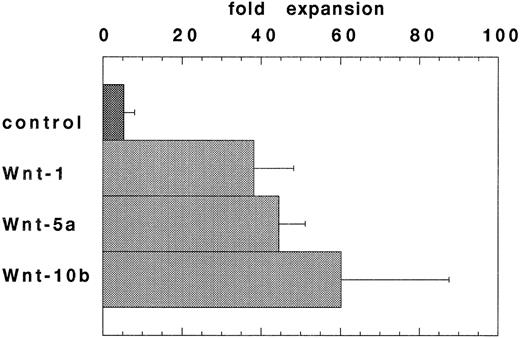
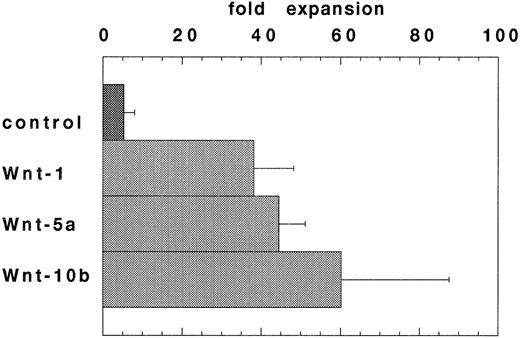
Expansion of HSCPs stimulated by conditioned media from Wnt-transfected cells. Wnt expression in the hematopoietic microenvironment may allow for Wnt-mediated regulation of hematopoietic stem/progenitor cell fate. To enable a further analysis of Wnts in hematopoiesis, Wnt-5a cDNAs were cloned from a 7-4 fetal liver stromal cell line cDNA library and Wnt-10b cDNAs were cloned using RT-PCR from flASK cell mRNAs. An in vitro stroma-free suspension culture system was developed to study the function of WNT proteins on highly enriched HSCPs. In preliminary experiments, most of the known cytokines failed to support the survival of flASK cells in suspension cultures when added as single factors in the presence of 10% fetal bovine serum (data not shown).50 However, the addition of KL at 100 ng/mL provided a potent stimulus for cell survival and proliferation of granulocytic progenitors.51 52 Further experiments showed that titration of KL to 25 ng/mL provided a lower expansion in cell number, but the cultures displayed a reduced level of granulocytic proliferation/differentiation. The addition of conditioned media (CM) from 293 cells transfected with Wnt-1, Wnt-5a, or Wnt-10b cDNAs to the suspension cultures plus 25 ng/mL KL stimulated cell proliferation sevenfold, eightfold, and 11-fold (respectively) over that of control CM after 7 days in culture (Fig 2). Interestingly, control 293 CM provided an approximately twofold stimulatory activity when added to suspension cultures with KL. The proliferative response of flASK cells in WNT CM plus KL was striking and compares well against the early acting cytokines. For example, the addition of the potent cytokines IL-3 or granulocyte-macrophage colony-stimulating factor (GM-CSF ) as single factors to cultures with KL evoked a 1.5-fold to twofold expansion compared with controls. A synergistic effect between KL and WNTs is evident because WNT CM alone triggered very little cell survival/proliferation. Although we have thus far failed to detect Wnt-1 expression in hematopoietic tissues, WNT-1 CM was active in this assay. These results show that signaling by several WNT ligands leads to a similar proliferative response in HSCPs. WNT signaling in flASK cells is potenially mediated by frizzled-related gene products or by other distinct signaling pathways.
WNTs promote cell proliferation in suspension cultures of flASK cells. The fold expansions in cell number after culture for 7 days are shown. Cultures were initiated with flASK cells (5,000/well), 25 ng/mL KL, and CM from 293 cells transfected with control plasmid, Wnt-1, Wnt-5a (gDWnt5aHis6 ), or Wnt-10b. Assays were performed in duplicate and repeated in two independent experiments.
WNTs promote cell proliferation in suspension cultures of flASK cells. The fold expansions in cell number after culture for 7 days are shown. Cultures were initiated with flASK cells (5,000/well), 25 ng/mL KL, and CM from 293 cells transfected with control plasmid, Wnt-1, Wnt-5a (gDWnt5aHis6 ), or Wnt-10b. Assays were performed in duplicate and repeated in two independent experiments.
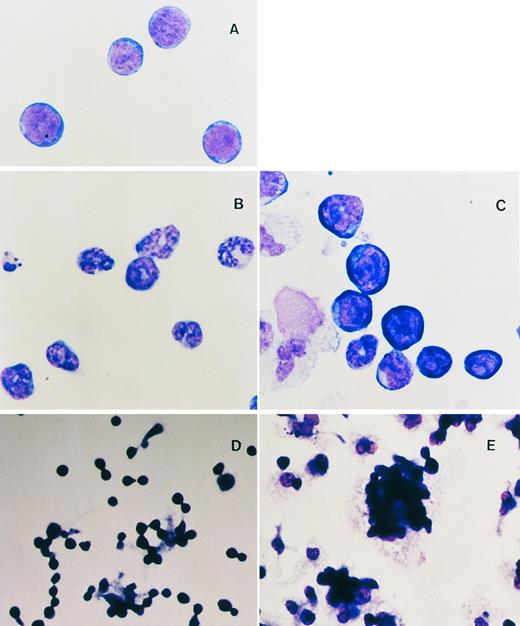
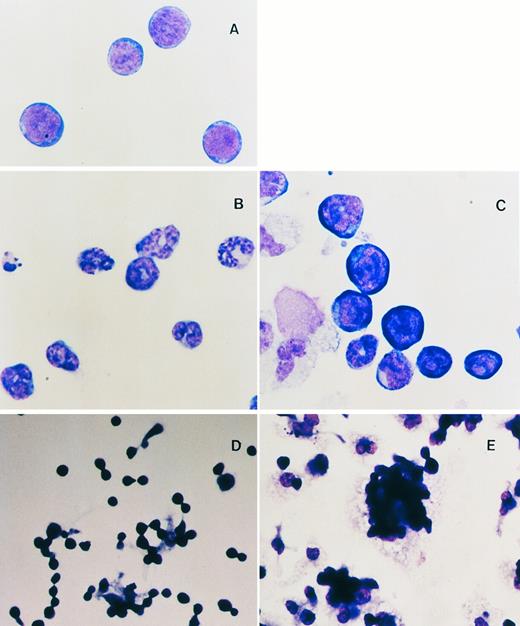
WNTs enhance the maintenance of the blast cell phenotype and foci formation of HSCPs.As described above, in mice Wnts are essential for the development of several primitive cell types and in Xenopus they are thought to be involved in cell fate determination. We evaluated the role of WNTs on the differentiation potential of pluripotent HSCPs by examining the morphology of cultured cells in cytostained preparations. Although addition of KL to the suspension cultures was essential, even in reduced concentrations, the effect of KL alone is to promote the differentiation and proliferation of granulocytic cells from HSCPs (Fig 3B, compare with freshly isolated flASK cells in Fig 3A). However, cells cultured in WNT-5a or WNT-10b CM or partially purified recombinant WNT-5a (see below) generally gave rise to a greater diversity of cell lineages with less commitment towards granulocytic lineages. Myeloid cells (macrophages and neutrophils), megakaryocytes, and early erythroid cells were observed by cytospin analysis of suspension cultures after treatment with WNT-5a CM (Fig 3C). Notably, the ratio of primitive blasts to differentiated mononuclear cells was elevated greater than fourfold (29% ± 3.4% compared with 7% ± 0.8% for the control) in cultures with recombinant WNT-5a. The effect of WNTs plus KL is to promote extensive cell expansion, ie, the net increase in cell number from the initial HSCP inoculum, while also maintaining a fourfold greater proportion of cells with a primitive blast cell morphology.
(A through C) Cytospin analysis of flASK cells. (A) Cytospin preparations of flASK cells immediately after cell sorting or after suspension culture in (B) control CM plus 25 ng/mL KL or (C) WNT-5a CM plus KL. (D and E) Formation of adherent hematopoietic foci after cells were plated onto fibronectin-coated glass chamber slides and cultured for 4 days. Cells were fixed and stained in situ to preserve the intercellular organization of the foci. (D) Control CM plus 25 ng/mL KL. (E) WNT-5a CM (gDWnt5a) plus 25 ng/mL KL. Notice that, in (E), WNT-5a CM results in a dramatic enhancement of foci formation in which blast cells are found as adherent clusters overlying adherent cells.
(A through C) Cytospin analysis of flASK cells. (A) Cytospin preparations of flASK cells immediately after cell sorting or after suspension culture in (B) control CM plus 25 ng/mL KL or (C) WNT-5a CM plus KL. (D and E) Formation of adherent hematopoietic foci after cells were plated onto fibronectin-coated glass chamber slides and cultured for 4 days. Cells were fixed and stained in situ to preserve the intercellular organization of the foci. (D) Control CM plus 25 ng/mL KL. (E) WNT-5a CM (gDWnt5a) plus 25 ng/mL KL. Notice that, in (E), WNT-5a CM results in a dramatic enhancement of foci formation in which blast cells are found as adherent clusters overlying adherent cells.
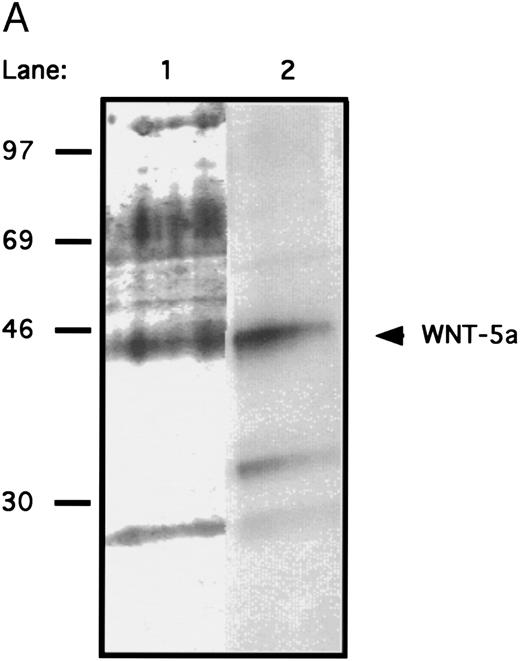
(A) Immunoprecipitation of gDWnt5a from transfected 293 cells. 293 cells were transiently transfected and metabolically labeled with [35S]methionine and [35S]cysteine 48 hours after transfection. Cell lysates and conditioned media were immunoprecipitated with MoAb 5B6 bound to protein A sepharose. The immunoprecipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by fluorography. The arrow denotes the migration of gDWnt5a monomers. Note that the majority of WNT-5a is present in the cell lysate fraction. (B) Immunoprecipitation of gDWnt5aHis6 with the 5B6 MoAb depletes the stimulatory activity of WNT-5a CM towards flASK cells. (1) CM from 293 cells transfected with control plasmid (gDpRK5b), (2) control CM incubated with 5B6-CPG (CPG), (3) Wnt-5a CM (gDWnt5aHis6 ), and (4) Wnt-5a CM depleted with 5B6-CPG. The flASK cells were cultured in duplicate wells containing HSC media supplemented with 25 ng/mL KL for 7 days. The mean cell number was calculated from duplicate wells and repeated in two independent experiments.
(A) Immunoprecipitation of gDWnt5a from transfected 293 cells. 293 cells were transiently transfected and metabolically labeled with [35S]methionine and [35S]cysteine 48 hours after transfection. Cell lysates and conditioned media were immunoprecipitated with MoAb 5B6 bound to protein A sepharose. The immunoprecipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by fluorography. The arrow denotes the migration of gDWnt5a monomers. Note that the majority of WNT-5a is present in the cell lysate fraction. (B) Immunoprecipitation of gDWnt5aHis6 with the 5B6 MoAb depletes the stimulatory activity of WNT-5a CM towards flASK cells. (1) CM from 293 cells transfected with control plasmid (gDpRK5b), (2) control CM incubated with 5B6-CPG (CPG), (3) Wnt-5a CM (gDWnt5aHis6 ), and (4) Wnt-5a CM depleted with 5B6-CPG. The flASK cells were cultured in duplicate wells containing HSC media supplemented with 25 ng/mL KL for 7 days. The mean cell number was calculated from duplicate wells and repeated in two independent experiments.
Wnts have been implicated in the regulation of cell adhesion systems, and it has been proposed that cell-cell interactions may be important in cell fate determination.53 In particular, Wnts are thought to enhance the stability of intracellular β-catenin pools and thereby promote intercellular adhesion through increased loading of β-catenin onto cadherins at the plasma membrane.54 During the first 4 to 5 days of suspension culture in WNT CM, we observed a dramatic increase in the number of loosely adherent cell aggregates or foci. To analyze the spatial organization and morphology of these foci, HSCPs were plated onto glass chamber slides coated with fibronectin, cultured for 4 to 5 days as described above, and then cytostained in situ. Fibronectin was chosen as an adhesive substrate in this assay because it can mediate adhesion of spleen colony-forming cells in vitro.55 After 3 to 5 days in culture, clusters of 5 to more than 30 blasts with low cytoplasm to nucleus ratios were typically found in contact with 1 or more underlying adherent myeloid cells (compare the control culture in Fig 3D with WNT-5a–treated cells in Fig 3E). The formation of these blast cell foci is dramatically enhanced in response to WNT CM and is suggestive of a role for WNTs in cell expansion via the regulation of cellular interactions.
In light of the enhanced proliferation and cell-cell adhesion of cells cultured in WNT CM, the lineage phenotypes and adhesion systems of the cultured cells were analyzed by flow cytometry. The flASK cells were cultured in WNT-5a CM for 7 days and scored for the expression of cell surface antigens. The expression of many antigens found on lineage-commited hematopoietic cells, including CD4, CD8a, CD13, CD14, CD29, CD31, B220, VCAM-1, and integrin β-7, was negative or low on freshly sorted flASK cells. The expression of these antigens remained at low levels after culture in the presence or absence of WNT CM, again showing that WNTs do not promote the commitment of progenitors to particular hematopoietic lineages. Similarly, no staining was observed for E- or P-cadherin on flASK cells before or after culture in any of the conditions. Little or no change was observed after culture in either condition for expression of CD11a, MAC-1, CD44, CD45, CD49d, CD49e, GR1, L-selectin, and ICAM-1. However, cultures supplied with WNT-5a CM did have an increase in the number of cells that were Sca+ (154% ± 22.6%), c-kit+ (158% ± 36%), Sca+ c-kit+ (131% ± 9.4%), or Ter119+ (237% ± 25.8%) relative to the controls. These cell surface antigen profiles compare well with the cytospin analysis and strengthen the view that WNTs plus KL promote the maintenance of a greater proportion of primitive blast cells than KL alone during the ex vivo culture of HSCPs.
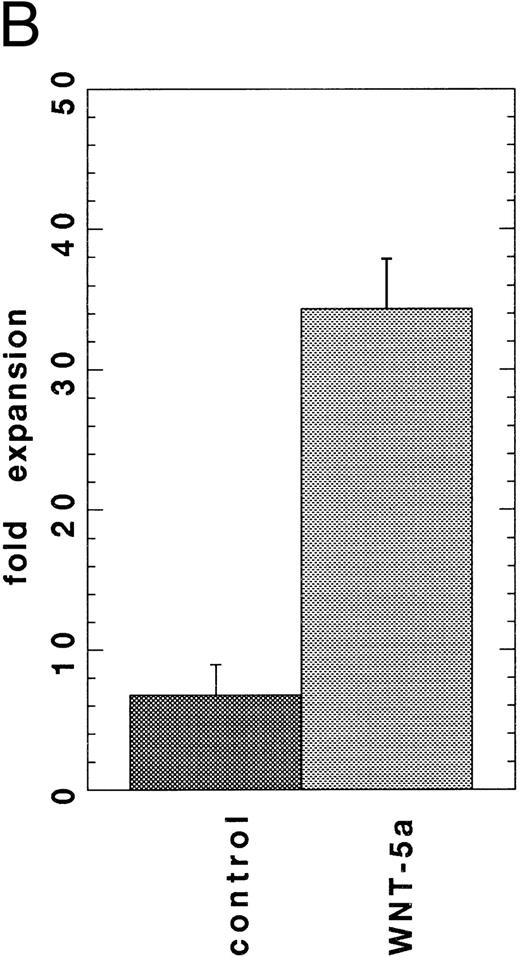
Secreted WNT protein in WNT-conditioned media is required for proliferation of HSCPs.Several approaches were taken to distinguish a direct role of WNTs on HSCPs from the alternative that WNTs stimulate the production of other growth factors in the transfected cells. Antibody depletion experiments were performed to confirm that the proliferation was mediated by secreted WNT proteins present in the media. An epitope-tag was engineered onto WNT-5a to enable depletion with readily available antibody reagents. Chimeric proteins were constructed that encoded the signal sequence and an N-terminal domain of the herpes virus glycoprotein D (gD) followed by Wnt-5a (gDWnt5a, gDWnt5aHis6 ; see the Materials and Methods). Transfection of the gDWnt5a construct into 293 cells directed the expression of 43- to 46-kD polypeptides that were specifically precipitated from lysates and conditioned media by an MoAb (5B6) recognizing an N-terminal epitope of gD (Fig 4A). Incubation of gDWNT5aHis6 CM in MoAb 5B6 coupled to controlled-pore glass (CPG) reduced cell expansion to control values (Fig 4B). Collectively, these data strongly support the hypothesis that secreted WNTs mediate the observed cell expansions in vitro. To test whether WNTs could directly stimulate cell proliferation, we evaluated the activity of partially purified WNT-5a protein in the suspension culture assay. Cell extracts provided a richer source of WNT-5a than CM (Fig 4A), and gDWNT5aHis6 was purified from cell lysates through binding of the gD epitope to 5B6-CPG. Gel analysis showed that the protein migrates as a 43- to 46-kD monomer under reducing conditions (Fig 5A). When added to the suspension culture assay, recombinant WNT-5a was found to stimulate cell expansion by fivefold at approximately 40 to 80 ng/mL or 1 to 2 nmol/L (Fig 5B).
(A) SDS-PAGE analysis of affinity-purified WNT-5a. Total proteins shown by silver-staining (lane 1) and gDWnt5aHis6 detected by Western blotting with the 5B6 MoAb (lane 2). Notice that the prominent 47- to 49-kD band in lane 1 is recognized by the 5B6 MoAb (arrow) and corresponds in size to mature gDWnt5aHis6 monomers. Other cross-reactive bands in lane 2 correspond to proteolytic products of gDWnt5aHis6 . (B) Purified WNT-5a promotes the proliferation of flASK cells. Affinity-purified WNT-5a (gDWnt5aHis6 ) was added to flASK cells in HSC media supplemented with 25 ng/mL KL. The mean cell number was calculated from duplicate wells and repeated in two independent experiments.
(A) SDS-PAGE analysis of affinity-purified WNT-5a. Total proteins shown by silver-staining (lane 1) and gDWnt5aHis6 detected by Western blotting with the 5B6 MoAb (lane 2). Notice that the prominent 47- to 49-kD band in lane 1 is recognized by the 5B6 MoAb (arrow) and corresponds in size to mature gDWnt5aHis6 monomers. Other cross-reactive bands in lane 2 correspond to proteolytic products of gDWnt5aHis6 . (B) Purified WNT-5a promotes the proliferation of flASK cells. Affinity-purified WNT-5a (gDWnt5aHis6 ) was added to flASK cells in HSC media supplemented with 25 ng/mL KL. The mean cell number was calculated from duplicate wells and repeated in two independent experiments.
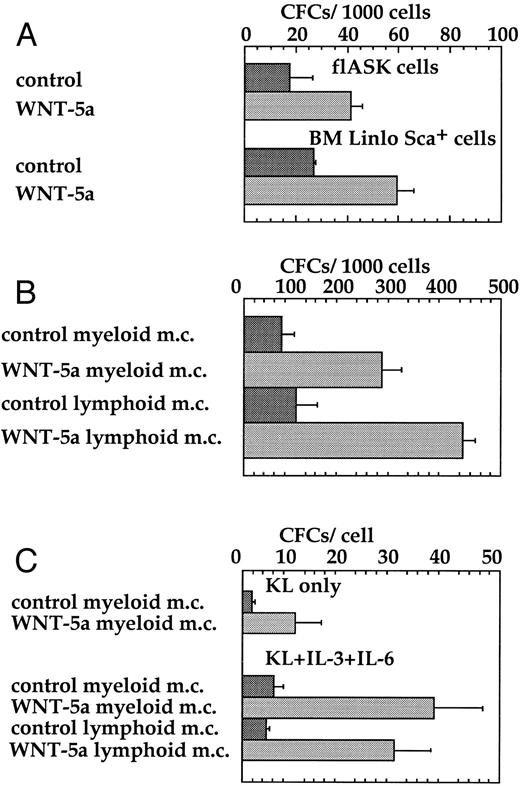
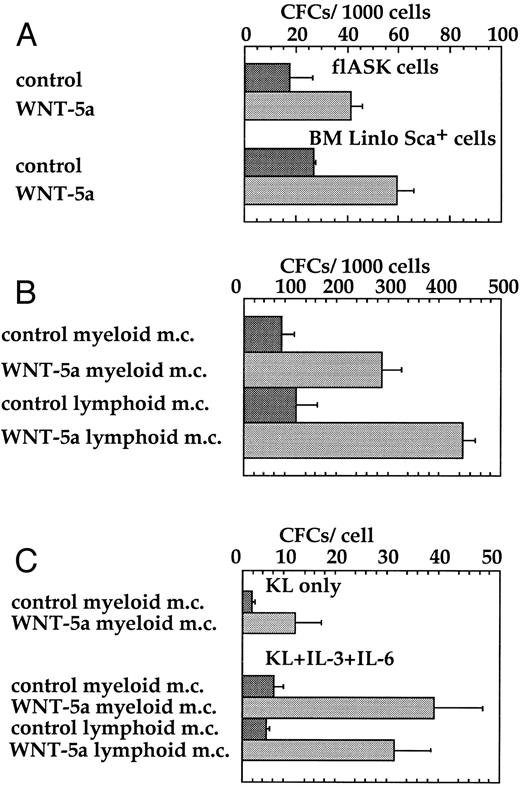
WNT-5a stimulates CFC expansion from HSCPs.KL56,57 and WNTs21 22 are expressed in a variety of mesodermally derived cells and are required in vivo for the outgrowth of nonhematopoietic cells. We therefore performed several in vitro assays to test the ability of WNTs to promote the expansion of hematopoietic progenitors, rather than other mesodermal cell types, from flASK cells. A crucial measure of hematopoietic progenitors is the ability to form colonies in semisolid media in response to lineage-specific cytokines and multilineage colony-stimulating factors. The addition of WNT-5a CM directly to freshly isolated fetal liver AA4+ Sca+ cells or bone marrow Linlo Sca+ cells stimulated colony formation twofold to threefold, respectively, in myeloid methylcellulose (Fig 6A). Thus, WNTs can enhance the survival or frequency of colony formation of hematopoietic progenitors from both fetal liver and bone marrow.
WNT-5a stimulates colony formation of flASK cells. (A) Freshly sorted flASK cells or Linlo Sca+ bone marrow cells were plated into myeloid methylcellulose with control or WNT-5a CM. Colony number was from quadruplicate plates evaluated after day 12 of culture and repeated in three independent experiments. (B) flASK cells expanded for 11 days on stromal layers (fetal liver cell line 10-6) with control or WNT-5a CM and the resultant cells were replated into myeloid or lymphoid methylcellulose and scored for colony growth as described above. (C) flASK cells expanded for 11 days in suspension culture containing c-kit ligand (KL) with control or Wnt-5A CM or KL, IL-3, and IL-6 with control or Wnt-5A CM were replated into myeloid or lymphoid methylcellulose. KL was supplied at 25 ng/mL. IL-3 and IL-6 were supplied at 2.0 ng/mL each. Colony growth was scored as described above. For methylcellulose assays, myeloid cell growth was obtained using medium-containing sreum, GM-CSF, IL-3, IL-6, erythropoietin, and KL and lymphoid cell growth was obtained using medium-containing serum, KL, and IL-7.
WNT-5a stimulates colony formation of flASK cells. (A) Freshly sorted flASK cells or Linlo Sca+ bone marrow cells were plated into myeloid methylcellulose with control or WNT-5a CM. Colony number was from quadruplicate plates evaluated after day 12 of culture and repeated in three independent experiments. (B) flASK cells expanded for 11 days on stromal layers (fetal liver cell line 10-6) with control or WNT-5a CM and the resultant cells were replated into myeloid or lymphoid methylcellulose and scored for colony growth as described above. (C) flASK cells expanded for 11 days in suspension culture containing c-kit ligand (KL) with control or Wnt-5A CM or KL, IL-3, and IL-6 with control or Wnt-5A CM were replated into myeloid or lymphoid methylcellulose. KL was supplied at 25 ng/mL. IL-3 and IL-6 were supplied at 2.0 ng/mL each. Colony growth was scored as described above. For methylcellulose assays, myeloid cell growth was obtained using medium-containing sreum, GM-CSF, IL-3, IL-6, erythropoietin, and KL and lymphoid cell growth was obtained using medium-containing serum, KL, and IL-7.
The ability of WNTs to support CFC expansion during in vitro culture was further evaluted in two different assays. In the first assay, flASK cells were plated onto preformed stromal layers of the fetal liver cell line 10-6. WNT-5a CM was added at the time of plating and CFC expansion on the stromal layer was measured by replating the hematopoietic cells into myeloid or lymphoid methylcellulose. WNT-5a supported an approximately fourfold greater expansion of myeloid and lymphoid CFCs over the control (Fig 6B). In the second assay, the CFC content of HSCPs after expansion in suspension culture for 11 days was measured by subsequent colony formation in methylcellulose. WNT-5a plus KL supported a sixfold expansion of myeloid CFCs after suspension culture. Similarly, WNT-5a plus the combination of KL, IL-3, and IL-6 supported a sixfold expansion of both myeloid and lymphoid CFCs after initial suspension culture (Fig 6C). The proliferative potential of hematopoietic progenitors measured in these assays was further demonstrated by replating cells from myeloid methylcellulose. Analysis of the tertiary plating assays showed that the cumulative expansion of cells brought forth by WNT-5a CM was more than 235-fold greater than that for controls. These results provide compelling functional evidence that secreted Wnts enhance the survival/proliferation of multipotent hematopoietic CFCs.
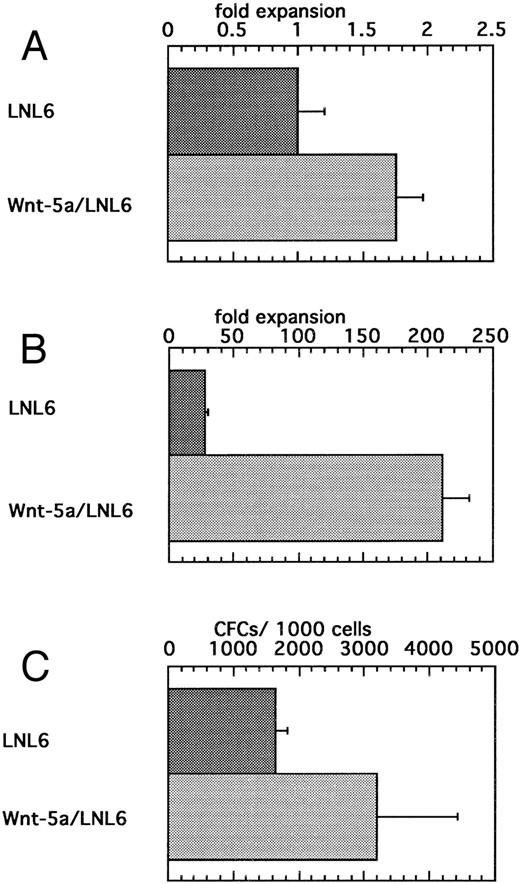
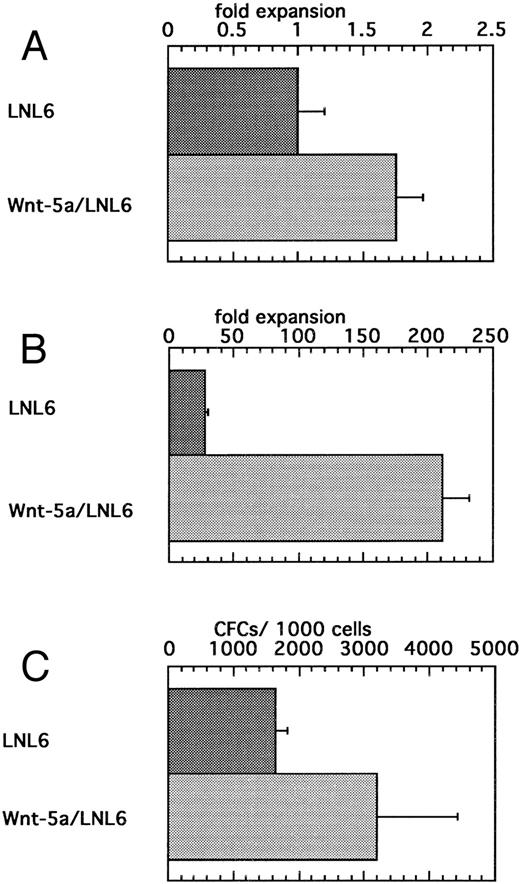
Expansion of HSCPs after transduction with a retrovirus bearing Wnt-5a.To further examine the direct effects of Wnt expression on HSCs, we introduced Wnt-5a via retroviral transduction. A Rous sarcoma virus-based bicistronic LNL6 vector was constructed so that Wnt-5a was placed 3′ to the gag region and LacZ was downstream of the encephalomyocarditis virus internal ribosome entry site. Viral supernatants were recovered from transfected BOSC cells and used to transduce flASK cells. Expression of the biscistronic mRNA in transduced flASK cells was confirmed by measuring LacZ activity using the FACS-Gal method.58 The cytokines IL-3, IL-6, and KL were added to increase the transduction efficiency, which was estimated to be approximately 20% by FACS-Gal analysis 48 hours posttransduction. A potential early acting effect of Wnt-5a on cell survival/proliferation was measured by counting cell numbers 48 hours after transduction. Despite variation in response at this early time point, Wnt5a/LNL6-transduced cells showed a small yet significant twofold expansion over controls (Fig 7A). Notably, this cell survival/proliferation was impressive in that an additional benefit was observed over the potent effects of the early acting cytokines IL-3, IL-6, and KL. Culture of the Wnt5a/LNL6-transduced cells for 7 days showed extensive proliferation compared with that of control cells (Fig 7B). Cells from 2-day transductions were also replated into myeloid methylcellulose. Transduction with Wnt5a/LNL6 stimulated a twofold greater expansion of CFCs than the control vector (Fig 7C). The efficiency of CFC transduction was estimated to be 14% to 47% by PCR analysis of colonies plucked from the methylcellulose cultures.
(A) Enhanced survival/proliferation of flASK cells after transduction with the Wnt5a/LNL6 retrovirus. Transductions were initiated with 100,000 cells/mL in IL-3, IL-6, and c-kit ligand (KL). The fold expansion for LNL6- or Wnt5a/LNL6-treated cells was determined from cell counts at the end of the transduction period (48 hours) and repeated four times. (B) Suspension culture of Wnt5a/LNL6-transduced cells for 7 days results in extensive expansion compared with LNL6-treated cultures. (C) Colony formation from flASK cells after 48 hours of transduction with LNL6 or Wnt5a/LNL6. Cells were plated in quadruplicate under myeloid methylcellulose conditions using medium-containing serum, GM-CSF, G-CSF, IL-3, IL-6, erythropoietin, and KL. Colony growth was evaluated after day 12 of culture and repeated in four independent experiments.
(A) Enhanced survival/proliferation of flASK cells after transduction with the Wnt5a/LNL6 retrovirus. Transductions were initiated with 100,000 cells/mL in IL-3, IL-6, and c-kit ligand (KL). The fold expansion for LNL6- or Wnt5a/LNL6-treated cells was determined from cell counts at the end of the transduction period (48 hours) and repeated four times. (B) Suspension culture of Wnt5a/LNL6-transduced cells for 7 days results in extensive expansion compared with LNL6-treated cultures. (C) Colony formation from flASK cells after 48 hours of transduction with LNL6 or Wnt5a/LNL6. Cells were plated in quadruplicate under myeloid methylcellulose conditions using medium-containing serum, GM-CSF, G-CSF, IL-3, IL-6, erythropoietin, and KL. Colony growth was evaluated after day 12 of culture and repeated in four independent experiments.
DISCUSSION
We have taken a new approach to the question of HSC plasticity and self-renewal through an examination of putative regulators of HSC cell fate. A central role of Wnts in the establishment/maintenance of cell fates has been shown in vertebrates and invertebrates. To examine whether Wnts are involved in the regulation of hematopoietic precursors, we have studied Wnt and frizzled gene expression in hematopoietic tissues and the response of HSCPs to WNTs. Using RT-PCR, we established that Wnts and murine frizzled homologs are expressed in a number of hematopoietic tissues. Expression of Wnt-5a, Wnt-10b, and Mfz3 through Mfz7 was detected in yolk sac, fetal liver, and fetal liver AA4+ cells, with Mfz3 and Mfz7 also being expressed in both mature marrow and hematopoietic and stem cell populations. Moreover, we detected by RT-PCR, cloned, and sequenced Wnt-10b cDNAs from HSCPs and Wnt-5a cDNAs from the 7-4 stromal cell line. Stromal cells and the hematopoietic microenvironment have long been implicated as a source of locally acting secreted factors for HSC maintenance or growth regulation.8 Expression of Wnt-5a and Wnt-10b and of putative Wnt receptors in hematopoietic tissues, of Wnt-10b in HSCPs, and of Wnt-5a in fetal liver stromal cells is strongly suggestive of a role for Wnt signaling in the hematopoietic microenvironment. Currently, we have not detected Wnt 3a, 5a, or 10b gene expression in marrow hematopoietic cells. However, we have not yet conducted an extensive study of marrow stroma or of activated subsets of marrow hematopoietic cells. Consequently, further studies will examine these potential sources.
Previous studies have examined the role of WNT proteins (using CM) as regulators of cell fate and differentiation. These studies have shown that Wnt CM can elicit an increase in protein stability59 or [3H]thymidine uptake accompanied by a partial transformation60 in cell lines. In this work, we have shown that secreted WNTs activate a proliferative response in primary hematopoietic cells. We consider that this proliferative response is a direct consequence of WNT signaling within HSCPs. We base these conclusions on the following observations. WNT-5a CM induced proliferation, whereas immunodepletion of this molecule from the CM effectively reduced the proliferation and formation of blast cell foci to control levels. Furthermore, the addition of partially purified WNT-5a protein stimulated cell expansion. Additionally, transduction of HSCPs with Wnt5A/LNL6 retroviral supernatants elicited a proliferative response measurable within 48 hours. In assays to determine the proliferative potential of HSCPs, WNTs increased both myeloid and lymphoid CFC output. Notably, this proliferative role was accompanied by an increased population of cells with a primitive blast morphology and cell surface antigen profile. We have considered that WNTs are acting as mitogens to stimulate the proliferation of HSCPs. However, we should point out that an alternative explanation is that WNTs play a role in self-renewal events that result in more progenitors being available to respond to the proliferative effects of KL, IL-3, or IL-6 added to the cultures. Further work is needed to address the potential of WNTs to expand multilineage progenitors capable of engraftment and long-term hematopoietic reconstitution of irradiated animals.
Previous studies have shown that Wnt signaling is a critical feature of cell fate determination and patterning of a wide diversity of tissues and cell types. Our work has shown that signaling that underlies developmental patterning may be reiterated in the continuous processes of hematopoietic progenitor cell maintenance and differentiation. WNT signaling in hematopoietic progenitors throughout the lifetime of an organism could provide a key to understanding the local, regenerative, self-organizing properties of the hematopoietic hierarchy.
Wnts have been shown to regulate cell adhesion and cell-cell interactions, perhaps as a means to elaborate the fine-scale patterning of tissues in which they are expressed. The cellular interactions promoted by WNTs may reflect the normal associations that characterize primitive blasts and stromal cells in the fetal liver and bone marrow. Further study of WNTs may provide important clues to the interactions of hematopoietic progenitors and elements of the stromal cell microenvironment. Analysis of the engraftment potential of WNT-treated HSCPs may aid the development of novel gene therapy and bone marrow transplantation protocols.
ACKNOWLEDGMENT
Many thanks to Richard Vandlen for advice; to Sunita Sohrabji for assistance in the preparation of this manuscript; to J. Chin, C. Donohue, and J. Hooley (flow cytometry); and to K. Azuzian and M. Vasser (oligonucleotide synthesis).
Dr Austin's current address is Systemix, Inc, 3155 Porter Dr, Palo Alto, CA 94304.
Address reprint requests to Gregg P. Solar, Genentech, 460 Pt San Bruno Blvd, South San Francisco, CA 94080.
Address correspondence to William Matthews, MD, Deltagen, Inc., 1031 Bing St, San Carlos, CA 94070.

![Fig. 4. (A) Immunoprecipitation of gDWnt5a from transfected 293 cells. 293 cells were transiently transfected and metabolically labeled with [35S]methionine and [35S]cysteine 48 hours after transfection. Cell lysates and conditioned media were immunoprecipitated with MoAb 5B6 bound to protein A sepharose. The immunoprecipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by fluorography. The arrow denotes the migration of gDWnt5a monomers. Note that the majority of WNT-5a is present in the cell lysate fraction. (B) Immunoprecipitation of gDWnt5aHis6 with the 5B6 MoAb depletes the stimulatory activity of WNT-5a CM towards flASK cells. (1) CM from 293 cells transfected with control plasmid (gDpRK5b), (2) control CM incubated with 5B6-CPG (CPG), (3) Wnt-5a CM (gDWnt5aHis6 ), and (4) Wnt-5a CM depleted with 5B6-CPG. The flASK cells were cultured in duplicate wells containing HSC media supplemented with 25 ng/mL KL for 7 days. The mean cell number was calculated from duplicate wells and repeated in two independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/10/10.1182_blood.v89.10.3624/3/m_bl_0047f4a.jpeg?Expires=1769110376&Signature=fSKnOjNTqhhhDHih23DY8mVsC0dSECzMvFj65kSG1UAiMstHMWxEHGjAKCpIFu0E8Td~v9wffmBwohgbHqk415xjcsBUaSCxuVp25slbUBzc8~jAuJ985SMQ0DIwdJh8APBbeEFXGKSRR~eCIAmg1IxccL7QT4atorFhPSvJTiMeWIC-PJSxalpIMWVBqrA5ckNaHO6xdT0AVS72BN3Zvmypp~DJNs2gPxRVP-LRE5tr3uqrqz4vnCvYz6vQTBDIqyxM4hGgPo-UeOkfVbx8kJZOUWwd6~9toxSTIdfyfNiecOyfvsS2O8DduyFnPky994cfLDE9GgsPSLsU7-A2yQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. (A) Immunoprecipitation of gDWnt5a from transfected 293 cells. 293 cells were transiently transfected and metabolically labeled with [35S]methionine and [35S]cysteine 48 hours after transfection. Cell lysates and conditioned media were immunoprecipitated with MoAb 5B6 bound to protein A sepharose. The immunoprecipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by fluorography. The arrow denotes the migration of gDWnt5a monomers. Note that the majority of WNT-5a is present in the cell lysate fraction. (B) Immunoprecipitation of gDWnt5aHis6 with the 5B6 MoAb depletes the stimulatory activity of WNT-5a CM towards flASK cells. (1) CM from 293 cells transfected with control plasmid (gDpRK5b), (2) control CM incubated with 5B6-CPG (CPG), (3) Wnt-5a CM (gDWnt5aHis6 ), and (4) Wnt-5a CM depleted with 5B6-CPG. The flASK cells were cultured in duplicate wells containing HSC media supplemented with 25 ng/mL KL for 7 days. The mean cell number was calculated from duplicate wells and repeated in two independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/10/10.1182_blood.v89.10.3624/3/m_bl_0047f4b.jpeg?Expires=1769110376&Signature=vS1drBRUotXOY9Wr1bgZBwgULc10~vUP~9ZmTZwTCzcpDELGfwelRYKpz53dXI65jaCigjtRIGMqhhsral1D28oWIZZregZRkqYMAuwGwtJ4JskjP-WzxsO-9-MwGpBmLBy02Y9aE0pP6301SvOupvG3dZ1OglnRQq~waUE~VExJXre~PQvyAqnoXfhR5HKRWeZTwCUbhCbOdF7J8~z0GN0yzLHdz~Q6-XTRJICNt-0~NSC4sP0zIV-PbdWhHdlRfkrGR27ESeceAuU0E5iYByn-Ssv8E9QzTkPu5NQ8O7knriSCsUwF~5-J0fDAcawvW32uNDdv4xCpu20gcDja5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 4. (A) Immunoprecipitation of gDWnt5a from transfected 293 cells. 293 cells were transiently transfected and metabolically labeled with [35S]methionine and [35S]cysteine 48 hours after transfection. Cell lysates and conditioned media were immunoprecipitated with MoAb 5B6 bound to protein A sepharose. The immunoprecipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by fluorography. The arrow denotes the migration of gDWnt5a monomers. Note that the majority of WNT-5a is present in the cell lysate fraction. (B) Immunoprecipitation of gDWnt5aHis6 with the 5B6 MoAb depletes the stimulatory activity of WNT-5a CM towards flASK cells. (1) CM from 293 cells transfected with control plasmid (gDpRK5b), (2) control CM incubated with 5B6-CPG (CPG), (3) Wnt-5a CM (gDWnt5aHis6 ), and (4) Wnt-5a CM depleted with 5B6-CPG. The flASK cells were cultured in duplicate wells containing HSC media supplemented with 25 ng/mL KL for 7 days. The mean cell number was calculated from duplicate wells and repeated in two independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/10/10.1182_blood.v89.10.3624/3/m_bl_0047f4a.jpeg?Expires=1769190190&Signature=DC34OSP0VDBAAM~zAbEk13gtu4MAasOMk8fzLwnXfhd5da--BR6o04J~vxqoXJ3UymnccNjeK1HHMCcgKBMBYi~So-Glp-oSOPxVbpLVxo~yp5j5tBFuOjAYabtZxteLZZ18WPX8k8jbmrO8V~YmqNfuVunrM5lT4O12uVZtf-LP5~R5-5Kdql-x12OgS2WAMNjgCG-U2jG6CSoNg8i8JwXBupgbafPD75rKtU1FMU-ks8YUppp2df1qTH9db73q5FzLu1utcBxM4YjIW2r4ga-C0~y30UweO~EBnCcgqRK~xKJn6MsN1n17w~xVO-FldANDl4acFgakyJnnQqY99A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. (A) Immunoprecipitation of gDWnt5a from transfected 293 cells. 293 cells were transiently transfected and metabolically labeled with [35S]methionine and [35S]cysteine 48 hours after transfection. Cell lysates and conditioned media were immunoprecipitated with MoAb 5B6 bound to protein A sepharose. The immunoprecipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by fluorography. The arrow denotes the migration of gDWnt5a monomers. Note that the majority of WNT-5a is present in the cell lysate fraction. (B) Immunoprecipitation of gDWnt5aHis6 with the 5B6 MoAb depletes the stimulatory activity of WNT-5a CM towards flASK cells. (1) CM from 293 cells transfected with control plasmid (gDpRK5b), (2) control CM incubated with 5B6-CPG (CPG), (3) Wnt-5a CM (gDWnt5aHis6 ), and (4) Wnt-5a CM depleted with 5B6-CPG. The flASK cells were cultured in duplicate wells containing HSC media supplemented with 25 ng/mL KL for 7 days. The mean cell number was calculated from duplicate wells and repeated in two independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/10/10.1182_blood.v89.10.3624/3/m_bl_0047f4b.jpeg?Expires=1769190190&Signature=fpdfxqAtW8twOyCjDsGwyu6QSFH60JvYFEEe5uMh6LEXwnprpUElUdOruFu~H-aR1mstEg6tyNlEPPCO6ic3MAJmxG7p~rbZ7lcT~ISIZ-KFiwnKbgJVHnVGyl2856j7tuYY502WlrCBs2uQmOMv7x~TfqEcB2T6lYiyGecfsGYs-SXG85EjXd7yid6MfomgpEk4ryNrpTAUWGdvVTwqu2vYaZ4EQE3~Ty8cQ5uTVfIROk4ghb25JOfq6-SErfufJFpRtIA094DUnC57cL5c2tT52hVNihPG5SqBSZuMWqLwltg87RMRAdPeCQGt1i38qh2CY06aWobJAqDEfxFgoQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)