Abstract
The cytomegalovirus (CMV)-specific CD8+ cytotoxic T-lymphocyte (CTL) and CD4+ T-helper cell (Th) functions were characterized in 15 CMV seropositive recipients of autologous peripheral blood stem cell or bone marrow transplants. These immune functions were evaluated in peripheral blood specimens obtained before and at 1, 2, and 3 months after transplant. For study of CTL activity, blood mononuclear cells were cocultured with CMV-infected autologous fibroblasts for 2 weeks and then tested for cytotoxicity against CMV-infected or mock-infected autologous and HLA-mismatched fibroblasts. The Th response to CMV antigen was assessed by standard lymphoproliferative assay. CMV-specific CD8+ CTL and CD4+ Th responses were detectable in 12 (80%) and 14 (93%) patients, respectively, in the first 3 months after transplantation. A Th response to CMV was always present by the time of first CTL detection. During the posttransplant period, CMV infection occurred in 6 (40%) patients, and detection of CMV-specific CD8+ CTL activity was associated with protection from subsequent CMV infection (P = .002). Among CMV seropositive autograft recipients, CMV-specific CD8+ CTL and CD4+ Th responses are restored in a large proportion of patients in the first 3 months after transplantation, and the presence of a specific CD8+ CTL activity affords protection from CMV infection.
DURING THE PROFOUND immunodeficiency early after allogeneic or autologous bone marrow transplantation (BMT), patients are at risk for cytomegalovirus (CMV) infection and serious CMV disease.1-3 In recent years, several antiviral drugs against CMV became available for clinical use, and efficient strategies for the prevention of CMV disease in immunocompromised hosts were developed.4-9 However, potent anti-CMV agents, such as ganciclovir, foscarnet, and cidofovir, are associated with significant toxicity that limits their use in BMT recipients.8,10-14 In contrast, prophylactic high-dose intravenous acyclovir is generally well tolerated after BMT, but is only partially effective in preventing CMV disease in allograft recipients15,16 and does not appear to protect CMV seropositive autograft recipients.17 Thus, no drug currently available for prevention of CMV disease after BMT is both highly efficacious and safe.
Additional factors may limit the efficacy of antiviral drugs against CMV after BMT. Long-term prophylaxis with agents that efficiently suppress viral replication can cause delayed recovery of CMV-specific cellular immunity that contributes to the occurrence of late CMV disease.18 Moreover, drug-resistant CMV strains may emerge and cause disease refractory to therapy.19-21 Hence, alternative approaches to the prevention and therapy of CMV disease in BMT recipients need to be investigated. A potential option is the use of CMV-specific immunotherapy.
In the past decade, important advances were made in understanding the immune mechanisms responsible for the control of CMV infection and disease. Studies in murine models of CMV infection showed that CD8+ cytotoxic T-lymphocytes (CTL) specific for CMV play a predominant role in the host defense against CMV.22-24 Among patients after allogeneic BMT, the recovery of a CMV-specific HLA class I-restricted CD8+ CTL response was shown to be crucial for protection from severe CMV disease, such as CMV pneumonia.18,25 26
More recently, the restoration of virus-specific cellular immunity by the adoptive transfer of specific CD8+ CTL clones to immunodeficient patients was shown to be safe,27-29 and ongoing studies are aimed at determining the efficacy of this strategy in preventing CMV infection and disease after allogeneic BMT. Thus, it becomes important to define other populations of immunocompromised hosts who might benefit from such an immunotherapeutic approach in the future.
Recipients of autologous BMT or peripheral blood stem cell transplants (PBSCT) carry a substantial risk for CMV infection, and CMV pneumonia although infrequent is associated with a high case-fatality rate in these patients.2,3 17 Data on T-cell immunity to CMV in autograft recipients have not been reported to date. The present study was performed to characterize the CMV-specific CD8+ CTL and CD4+ T-helper cell (Th) activity in autologous PBSCT or BMT recipients and to correlate the restoration of these immune functions with the occurrence of CMV infection during the posttransplant course.
PATIENTS AND METHODS
Patient population.The study was conducted prospectively among 15 patients at the University Hospitals in Tübingen, Germany (10 patients) and Geneva, Switzerland (5 patients). Patients were selected for study if they received autologous PBSCT or BMT and if they were seropositive for CMV IgG antibody before transplantation. Informed consent was obtained from each patient, and the study protocol was approved by the Institutional Ethical Committees at the two participating centers. Characteristics of the study population are summarized in Table 1. At the transplant center in Geneva, all patients received CMV seronegative blood products, whereas patients in Tübingen received unscreened blood products. Low-dose acyclovir was administered to 13 of the 15 study patients for prevention of herpes simplex reactivation after transplantation. Prophylactic ganciclovir or foscarnet was not used in any patient.
Expansion of CMV-specific CTL in vitro.The presence of HLA class I-restricted CD8+ CTL specific for CMV in the peripheral blood of patients was assessed before transplantation and at 1, 2, and 3 months after transplantation. CMV-specific CTL lines were generated in an in vitro culture system as described elsewhere.26 In brief, skin biopsies were obtained from each patient to establish fibroblast lines for use as stimulator and target cells. Fibroblasts were propagated in Waymouth's medium supplemented with 20% heat-inactivated fetal calf serum, 2 mmol/L of L-glutamine, 50 U/mL of penicillin, and 50 mg/mL of streptomycin. Peripheral blood mononuclear cells (PBMC) were cocultured with autologous fibroblast stimulators at a ratio of 20:1, which were infected for 2 hours with the CMV AD169 strain at a multiplicity of infection (MOI) of 5 before initiation of culture. The lymphocyte culture medium was RPMI-HEPES supplemented with 10% CMV seronegative human AB serum, 2.5 × 10−5 mol/L of 2-mercaptoethanol, 2 mmol/L of L-glutamine, 50 U/mL of penicillin, and 50 mg/mL of streptomycin. After 7 days of incubation at 37°C in a humidified 5% CO2 atmosphere, the cultured cells were restimulated with fresh CMV-infected fibroblast stimulators and supplemented with autologous irradiated (3,500 cGy) PBMC as filler cells. Two days later, recombinant interleukin-2 was added to the cultures at 2 U/mL. Under these culture conditions, fibroblast stimulators carry high amounts of HLA class I molecules but no detectable levels of HLA class II molecules, which results in preferential activation and expansion of HLA class I-restricted CD8+ CTL specific for CMV.18,26,30-33 As demonstrated by selective depletion of T-cell subsets in our previous study that used the identical CTL culture method, the phenotype of the effector cells mediating CMV-specific lysis in this system is CD3+, CD8+, CD4−.26
Cytotoxicity assay.One week after restimulation, the cytotoxicity of the cultured cells was assessed in a 4-hour chromium release assay against a panel of target cells that included autologous and HLA class I-mismatched CMV-infected and mock-infected fibroblasts as previously described.26 CMV-infected fibroblast lines used as HLA-mismatched targets were all lysable by autologous effector cells. Before use, fibroblast targets were incubated for 48 hours with recombinant interferon-γ at 800 U/106 cells to enhance HLA class I expression and thereby increase the sensitivity of the assay.31 The targets were then labeled overnight with Cr51 (100 mCi/106 cells; Amersham Laboratories, Amersham, UK), and an aliquot was infected with CMV AD169 at an MOI of 5. Fibroblast targets were subsequently harvested and 100 mL (104 cells) was dispensed in triplicate into 96-well round-bottom plates together with 100 mL of effector cell suspension at an effector to target (E:T) ratio of 15:1. Cytotoxicity was also evaluated in parallel against fibroblast targets that were preincubated with the anti-class I monoclonal antibody W6/32 (kindly provided by Gennaro De Libero, Department of Research, University Hospital, Basel, Switzerland) to confirm HLA class I restriction of target cell lysis.26 34 Maximum chromium release was obtained from targets incubated with 1% Nonidet P40-solution, and spontaneous release, which never exceeded 30% of maximum release, was determined from incubation of target cells with medium alone. Specific cytotoxicity was calculated by the standard formula. A patient was considered to have a positive CMV-specific CTL response if lysis of autologous CMV-infected fibroblast targets was greater than 5% above the level of lysis obtained with autologous mock-infected and HLA-mismatched CMV-infected and mock-infected targets. This cut-off was selected based on results obtained before study onset in 4 CMV seronegative healthy volunteers and in 1 CMV seronegative autograft recipient whose CTL response was assessed once pretransplant and on three occasions during the posttransplant course; these 8 CTL cultures from CMV seronegative individuals yielded levels of lytic activity against autologous CMV-infected fibroblast targets that never exceeded the cytotoxicity measured against autologous mock-infected and HLA class I-mismatched CMV-infected and mock-infected fibroblasts (data not shown).
Lymphoproliferative assay.Lymphoproliferation to soluble CMV antigen reflects the CMV-specific HLA class II-restricted CD4+ Th cell response.18,35 The proliferative response to soluble CMV antigen obtained by glycine extraction and to phytohemagglutinin (PHA; Murex Diagnostics Benelux BV, Schaffhausen, Switzerland) was assessed each time a CTL culture was initiated as reported.26 Briefly, PBMC were suspended at 106 cells/mL in lymphocyte culture medium and 100 mL dispensed in triplicate into 96-well round-bottom plates. CMV antigen or PHA was added at final concentrations of 1:100 and 10 mg/mL, respectively. The plates were then incubated at 37°C in a humidified 5% CO2 atmosphere for 96 hours. During the last 16 hours of the incubation period, the cells were pulsed with 1 mCi per well of 3H-thymidine (Amersham Laboratories). Wells were harvested, and samples were measured in a β-scintillation counter. Results are expressed as a stimulation index calculated by dividing the mean counts per minute (cpm) of cells exposed to CMV antigen or PHA by the mean cpm of cells incubated with medium alone. A stimulation index of 4 or greater was considered to indicate a positive lymphoproliferative response. This definition was based on results obtained from 5 CMV seronegative healthy blood donors in whom the median stimulation index with the soluble CMV antigen used was 1.6 (range, 1.1 to 3.7).
Virologic monitoring and definition of CMV infection and CMV disease.During the first 3 months after transplantation, the presence of CMV was evaluated in specimens from urine and throat by shell-vial culture and was assessed in blood specimens by polymerase chain reaction (PCR) every 1 to 2 weeks during the inpatient period and at least once monthly after discharge from hospital.7,36,37 The diagnosis of CMV infection was based on evidence of CMV in clinical specimens by culture, histology, or PCR. CMV disease was defined as detection of CMV in tissue specimens and, in the case of CMV pneumonia in broncho-alveolar lavage fluid, associated with clinical symptoms and signs compatible with CMV organ disease.38
Statistical analyses.Continuous variables were compared by the Wilcoxon rank-sum test and dichotomous variables by Fisher's exact test. P values less than .05 were considered significant.
RESULTS
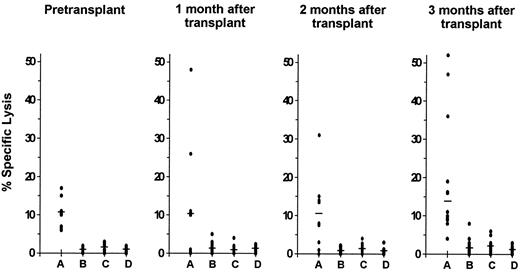
CMV-specific CD8+ CTL response.Before transplantation, the CTL response to CMV was investigated in 12 of the 15 study patients. A CMV-specific CD8+ CTL activity was detectable in 8 of these 12 patients (67%). The lytic activity against autologous CMV-infected fibroblast targets was significantly higher than the level of cytotoxicity against autologous mock-infected or HLA class I-mismatched CMV-infected and mock-infected targets (P = .008; Fig 1).
Cytolytic activity of cells from CMV seropositive autograft recipients with detectable CMV-specific HLA class I-restricted CD8+ CTL responses. Class I-restricted CTL specific for CMV were demonstrable in 8 patients pretransplantation and in 12 patients during the first 3 months after transplantation. Cytotoxicity was assayed at an E:T ratio of 15:1 against autologous CMV-infected (A) and mock-infected (B) fibroblast targets as well as against HLA-mismatched CMV-infected (C) and mock-infected (D) fibroblast targets. Lysis of the autologous CMV-infected targets was significantly higher than lysis of each of the other three targets before transplantation (P = .008) and at 1 month (P = .04), 2 months (P = .04), and 3 months (P = .005) after transplantation. The median is indicated by the horizontal bar.
Cytolytic activity of cells from CMV seropositive autograft recipients with detectable CMV-specific HLA class I-restricted CD8+ CTL responses. Class I-restricted CTL specific for CMV were demonstrable in 8 patients pretransplantation and in 12 patients during the first 3 months after transplantation. Cytotoxicity was assayed at an E:T ratio of 15:1 against autologous CMV-infected (A) and mock-infected (B) fibroblast targets as well as against HLA-mismatched CMV-infected (C) and mock-infected (D) fibroblast targets. Lysis of the autologous CMV-infected targets was significantly higher than lysis of each of the other three targets before transplantation (P = .008) and at 1 month (P = .04), 2 months (P = .04), and 3 months (P = .005) after transplantation. The median is indicated by the horizontal bar.
Within the first 3 months after transplantation, the presence of a CMV-specific CD8+ CTL response was demonstrable in 12 of the 15 patients (80%). Lysis of autologous CMV-infected target cells was significantly greater than lysis of the other 3 targets at 1 month (P = .04), 2 months (P = .04), and 3 months (P = .005) after transplantation (Fig 1). The preferential lysis of autologous CMV-infected fibroblast targets over HLA-mismatched infected targets at all time points before and after transplantation indicates that the culture method used generated classical HLA class I-restricted CD8+ CTL. This was further supported by the effect of the anti-class I monoclonal antibody W6/32 on specific cytotoxic activity. Preincubation of autologous CMV-infected fibroblast targets with this monoclonal antibody reduced lysis by an average of 62% (P < .0001; data not shown).
The magnitude of CMV-specific CD8+ CTL activity in the study patients before transplantation was compared with that of a historical group of 20 CMV seropositive healthy individuals who had been investigated with identical CTL culture conditions and assessment of cytotoxicity in an earlier study.26 Median specific lysis of autologous CMV-infected targets among pretransplant autograft recipients was 10% compared with 32% among the healthy individuals of that earlier study (P < .0001). The CMV-specific CD8+ CTL response at 1, 2, and 3 months after autologous transplantation was quantitatively similar to the CTL response measured in autograft recipients before transplantation (Fig 1).
Of the 4 autograft recipients without detectable CMV-specific CD8+ CTL activity before transplantation, 2 developed a specific CTL response during the first 3 months after transplantation and 2 did not.
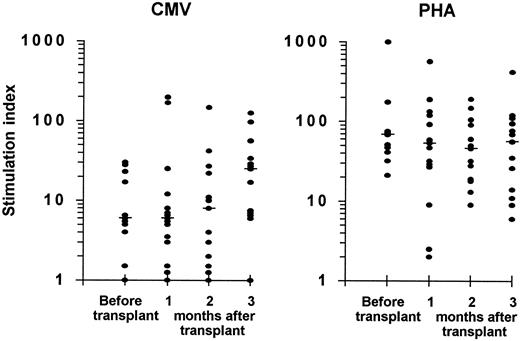
Lymphoproliferative response to CMV and to PHA.Eleven patients were evaluated before transplantation; a lymphoproliferation to CMV antigen, which reflects the specific CD4+ Th response, was demonstrable in 9 (82%) and a proliferative response to PHA was present in all 11 (Fig 2).
Lymphoproliferative responses to CMV antigen (left panel) and to PHA (right panel) in CMV seropositive autograft recipients. Eleven patients were evaluated before transplantation and 15 patients were assessed during the first 3 months after transplantation. The stimulation index was calculated as described in the Patients and Methods. A stimulation index ≥4.0 was considered positive. The median is indicated by the horizontal bar.
Lymphoproliferative responses to CMV antigen (left panel) and to PHA (right panel) in CMV seropositive autograft recipients. Eleven patients were evaluated before transplantation and 15 patients were assessed during the first 3 months after transplantation. The stimulation index was calculated as described in the Patients and Methods. A stimulation index ≥4.0 was considered positive. The median is indicated by the horizontal bar.
In the first 3 months after autologous PBSCT or BMT, a lymphoproliferative response to CMV antigen was detectable in 14 of the 15 study patients (93%), and lymphoproliferation to PHA was present in all patients. One patient who showed a proliferative response to CMV before transplantation (stimulation index of 5) remained consistently negative during the posttransplant period and also had no detectable CMV-specific CTL activity. This patient developed CMV infection on day 58 after transplantation (repeatedly positive urinary cultures).
There were no statistically significant differences between the magnitude of lymphoproliferative responses to CMV or to PHA determined at 1, 2, or 3 months after transplantation and the corresponding values measured in patients before transplantation (Fig 2).
All patients who had a demonstrable CD8+ CTL activity specific for CMV during the posttransplant course had a positive lymphoproliferative response to CMV antigen by the time of first CTL detection. There was a significant correlation between simultaneous presence or absence of these two immune functions (P = .001).
CMV infection and CMV-specific CD8+ CTL and CD4+ T-helper cell responses.CMV infection occurred in 6 of the 15 (40%) autograft recipients within the first 3 months after transplantation. Median onset of infection was on day 29 (range, 22 to 58) posttransplantation. No patient developed CMV disease.
Of the 12 patients with a demonstrable CMV-specific CD8+ CTL activity in the posttransplant study period, 9 showed no evidence of CMV infection, whereas 3 were diagnosed with CMV infection; by contrast, the 3 patients without detectable specific CTL response all developed CMV infection. When the association of CTL response and CMV infection was analyzed independently from the sequence of detection of these events in the posttransplant course, there was a significant inverse correlation between the two variables (P = .04).
Because CMV-specific CD8+ CTL are thought to have a protective effect against CMV infection, a time-dependent analysis was also performed in which a CTL response was only considered if it was detected before the onset of CMV infection or if it was present in patients who did not develop CMV infection after transplantation (Table 2). In this analysis, the inverse correlation between CTL response and CMV infection was even stronger (P = .002).
Similar analyses were performed for the association of CMV-specific CD4+ Th response and CMV infection during the first 3 months after transplantation. Among the 14 patients with detectable Th response to CMV in this period, CMV infection occurred in 5 and was absent in 9; CMV infection also developed in the single patient without specific Th response posttransplant. No correlation was found between the presence of a CMV-specific CD4+ Th response and the occurrence of CMV infection after transplantation (P = .4). There was also no statistically significant association between these two variables when the specific Th response was only considered if detected before the onset of CMV infection (Table 3).
DISCUSSION
This study characterizes the CMV-specific T-cell immunity in CMV seropositive patients before and after autologous PBSCT or BMT. The presence of a CMV-specific HLA class I-restricted CD8+ CTL response was demonstrable in 67% of patients evaluated before transplant and was detectable in 80% of patients within the first 3 months after transplantation. Most importantly, the presence of a CD8+ CTL activity specific for CMV in the posttranplantation study period was associated with protection from CMV infection.
Among patients after allogeneic BMT who were studied with identical methods of CMV-specific CTL generation and by using the same definition of a positive CTL response as in the present investigation, the recovery of a HLA class I-restricted CD8+ CTL response specific for CMV in the first 3 months after transplantation was demonstrable in 50% of patients and was associated with protection from CMV pneumonia.26 However, there was no correlation between detection of specific CTL and occurrence of CMV infection in that study. Thus, CMV-specific CD8+ CTL in allograft recipients do not appear to prevent virus reactivation, but are able to control the development of CMV disease, presumably by limiting the systemic viral load. In the present study of autograft recipients, a CD8+ CTL activity specific for CMV was detectable in 80% of patients during the posttransplant course and was associated with prevention of CMV infection. Moreover, no patient developed CMV disease. The unequal levels of protection from CMV infection and disease associated with CTL immunity in autograft and allograft recipients could be related in part to the different proportions of patients in these two groups who develop a CMV-specific CD8+ CTL response in the first 3 months after transplantation.
The proportion of patients with detectable CMV-specific CD8+ CTL activity posttransplant can be influenced by the administration of ganciclovir prophylaxis, which was shown to delay the recovery of T-cell immunity to CMV after BMT.18 However, none of the autograft or allograft recipients of our two studies discussed above received prophylactic ganciclovir. A probably decisive factor for differences in the CMV-specific CTL response between the two groups of transplant recipients is the degree of posttransplantation immunosuppression. In contrast to allograft recipients, patients after autologous PBSCT or BMT are not exposed to the immunosuppression induced by the prevention or therapy of acute graft-versus-host disease (GVHD) or by GVHD itself, which is a major risk factor for CMV reactivation and CMV disease1 3 and which could impair the generation and function of CTL specific for CMV after allogeneic BMT.
The CMV-specific CD8+ CTL activity among our autograft recipients before transplantation was significantly weaker than in a group of CMV seropositive healthy individuals investigated with identical methods in an earlier study.26 This CTL activity was furthermore demonstrable in only two thirds of our autograft recipients pretransplant, whereas it is readily detectable in immunocompetent CMV seropositive subjects who show no evidence of viral reactivation.26,30-33,39 40 Patients undergoing autologous PBSCT or BMT have usually been pretreated with several courses of cytotoxic chemotherapy, and some patients could still have residual malignant disease, which are factors that might contribute to the deficient immunity to CMV observed in our patients before transplant. Moreover, the impaired CMV-specific CD8+ CTL response in our autograft recipients before transplantation may account for the fact that the magnitude of this immune response up to 3 months after transplantation remained in the range of the pretransplantation values.
In the present study, the proportion of patients with a lymphoproliferative response to CMV antigen, which is an index of specific HLA class II-restricted CD4+ Th cell function,18,35 was 82% before and 93% after transplantation. In addition, all patients who developed a CMV-specific CTL activity in the posttransplant course had a lymphoproliferation to CMV by the time of first CTL detection. However, no correlation was discernible between proliferative response to CMV and occurrence of CMV infection during the posttransplant period. Thus, CMV-specific CD4+ Th cells appear to be an important requirement for the generation of a specific CD8+ CTL response in autograft recipients, but do not exert a direct protective effect against CMV infection in these patients, which is consistent with similar observations in allogeneic BMT recipients.26 28
In conclusion, CMV seropositive patients undergoing autologous PBSCT or BMT have an impaired T-cell immunity to CMV before transplant, which might explain the weak CMV-specific HLA class I-restricted CD8+ CTL response during the first 3 months after transplantation. However, this CTL response is present in a large proportion of patients after transplantation, which may account for the protection from CMV infection associated with these effector cells during the posttransplant period. CMV seropositive autograft recipients who have no detectable CD8+ CTL response to CMV after transplantation could be potential candidates for the adoptive transfer of CMV-specific CD8+ CTL clones if this new treatment strategy is shown to be effective in preventing CMV infection and disease in immunocompromised hosts.
ACKNOWLEDGMENT
We thank M. Marti for preparation of the manuscript.
P.R. was supported by Grant No. 32-31314.91 from the Swiss National Research Foundation.
Address reprint requests to Pierre Reusser, MD, Department of Medicine, University Hospital, CH-4031 Basel, Switzerland.