Abstract
The receptors for erythropoietin (Epo) and interleukin-3 (IL-3) both induce the ligand-dependent activation of the Jak2 tyrosine kinase. Activated Jak2 then phosphorylates these receptors and thereby recruits various signaling molecules containing the Src homology (SH)-2 domain, including Stat5, to the tyrosine phosphorylated receptors. In the present study, we demonstrate that Epo stimulation induces unidirectional cross-phosphorylation of the IL-3 receptor β subunit (βIL3) on tyrosines and its rapid and transient association with Stat5 in murine IL-3–dependent cell lines engineered to express the Epo receptor (EpoR). Using cell lines expressing various EpoR mutants, it was demonstrated that the Epo-induced tyrosine phosphorylation of βIL3 is dependent on the membrane-proximal EpoR cytoplasmic region involved in the activation of Jak2, but not on the extracellular and transmembrane regions or on the carboxy-terminal 145 amino acid region containing all the intracellular tyrosine residues. It was also shown that IL-3 induces rapid and dose-dependent association of Jak2 with βIL3. However, Epo failed to induce any detectable association of βIL3 with Jak2 or the EpoR. The present study also demonstrates that in IL-3–stimulated cells, an ovine Stat5 mutant harboring a substitution of Tyr694 to Phe, which abolishes the tyrosine phosphorylation required for activation, fails to dimerize with endogenous Stat5, shows sustained binding with tyrosine-phosphorylated βIL3, and inhibits the tyrosine phosphorylation of endogenous Stat5. These results suggest that βIL3 may have Stat5 docking sites, similar to those found in the EpoR, that facilitate the activation of Stat5 by Jak2 and raise the possibility that Epo may cross-activate or transmodulate the IL-3 receptor signaling pathways.
HEMATOPOIESIS IS regulated through the interaction of various hematopoietic cytokines with their cognate surface receptors. Among these cytokines, erythropoietin (Epo) regulates the growth and differentiation of the erythroid progenitor cells,1 while interleukin-3 (IL-3) stimulates the proliferation and differentiation of various hematopoietic cell lineages and activates the functions of mature macrophages, eosinophils, and mast cells.2 Most of the hematopoietic growth factor receptors belong to the cytokine receptor superfamily, which is characterized by four conserved cysteine residues and the WSXWS motif in the extracellular domain.3,4 Although lacking intrinsic tyrosine kinase activity, all members of the cytokine receptor superfamily have been shown to activate the Jak family of tyrosine kinases, which physically associate with the membrane proximal domain of the receptors containing the conserved Box 1 and Box 2 regions.5 The activation of Jak kinases leads to tyrosine phosphorylation of the cytokine receptors themselves and activation of a class of latent cytoplasmic transcriptional factors known as signal transducers and activators of transcription (Stats).6 Six members of the Stat family, Stat1 through Stat6, are currently known.
The receptor for Epo (EpoR) consists of a single chain, which is hypothesized to be activated by forming a homodimer upon Epo binding, although cross-linking studies with 125I-Epo have suggested a possibility for the second subunit of EpoR.7-9 On the other hand, the receptor for IL-3 (IL-3R) consists of the α and β subunits, both of which belong to the cytokine receptor superfamily.10 The mouse has two highly homologous β subunits, βc and βIL3, while the human has only one type of β subunit, βc. Both mouse and human βc, which do not bind any cytokine by themselves, are shared with the receptors for granulocyte-macrophage colony-stimulating factor (GM-CSF ) and IL-5. In contrast, βIL3 binds IL-3 with a low-affinity and forms a high-affinity receptor only with the IL-3R α subunit.10 When expressed in IL-3–dependent cell lines, the EpoR transduces a mitogenic signal and thus abrogates the IL-3 dependency of the cells.11,12 In these cells, the activation of the EpoR or the IL-3R induces quite similar downstream signaling events. We and others have shown that both of the receptors specifically couple with Jak2 and predominantly activate Stat5.13-18 We also demonstrated that the binding of Jak2 with the EpoR depends on stimulation with Epo and correlates with mitogenic abilities of various mutant EpoRs.19 Other signaling events shared with the EpoR and the IL-3R include (1) tyrosine phosphorylation of Shc, Syp, and Vav, (2) activation of the Ras/mitogen activated protein (MAP) kinase pathway, (3) physical association of hematopoietic cell phosphatase (HCP) and the 85-kD subunit of phosphatidylinositol 3′-kinase, and (4) induction of the pim-1 and cis genes.5 We previously showed that the Epo-induced tyrosine phosphorylation of Vav and expression of the pim-1 gene correlated with the Epo-induced activation of Jak2, but not with the EpoR tyrosine phosphorylation.20 However, most of the other downstream signaling events from the EpoR and the IL-3R are dependent not only on the activation of Jak2, but also on the tyrosine phosphorylation of the receptors, which recruit various signaling molecules through interaction with the Src homology 2 (SH2) domain, a conserved modular domain that binds to phosphotyrosine-containing sequences.21 Therefore, the ligand-induced receptor tyrosine phosphorylation should play significant roles in signaling through these receptors.
Initially purified and cloned as a mediator of prolactin-induced transcription of the β-casein gene,22 Stat5 has been shown to be activated by a variety of other cytokines, including IL-3, Epo, growth hormone, GM-CSF, IL-5, IL-2, and thrombopoietin.6 Two murine homologues, Stat5A and Stat5B, sharing 96% amino acid sequence identity, have been cloned, although no functional differences have yet been observed between these two molecules.15 Very recently, we have demonstrated that Stat5 is recruited, very rapidly and transiently, to the tyrosine-phosphorylated receptors for Epo and IL-3.23 The recruitment of Stat5 to the EpoR was shown to be mediated through the interaction between the Stat5 SH2 domain and specific phosphorylated tyrosine residues, including Tyr343, in the EpoR cytoplasmic domain.23 Furthermore, it was demonstrated that the recruitment of Stat5 plays a significant role in the specific activation of Stat5 by the EpoR, although the significance of Stat5 activation on the EpoR-mediated growth signaling has remained controversial.23-27 On the other hand, the mechanism and significance of the IL-3–induced binding of Stat5 to βIL3 remain unknown.23
To address the possibility that the receptors for Epo and IL-3 may cross-activate each other, we examined whether Epo and IL-3 may induce cross-phosphorylation of their receptors. The present study demonstrates that Epo induces unidirectional cross-phosphorylation of βIL3, which is dependent on the membrane-proximal EpoR cytoplasmic region involved in the activation of Jak2. In addition, Epo induced a very rapid and transient binding of Stat5 to tyrosine-phosphorylated βIL3. We also examined the mechanisms of binding of Stat5 to βIL3 by introducing an ovine Stat5 mutant, Stat5-Y694F, with a substitution of Tyr694 with Phe, which abolishes the phosphorylation site required for activation of Stat5,28 into an IL-3–dependent cell line. Stat5-Y694F failed to dimerize with endogenous Stat5, stably associated with tyrosine phosphorylated βIL3, and inhibited the tyrosine phosphorylation of endogenous Stat5. This is consistent with the hypothesis that βIL3 has Stat5 docking sites that facilitate the activation of Stat5.
MATERIALS AND METHODS
Cells and reagents.IL-3–dependent 32D cell clones expressing the wild-type and mutant EpoRs were previously described.19 29 An IL-3–dependent murine cell line, BaF3, was obtained through the Riken Gene Bank (Ibaraki, Japan). These cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 10% WEHI-3 conditioned medium as a source of IL-3. Recombinant human Epo was kindly provided by Chugai Pharmaceutical Co Ltd (Tokyo, Japan). Recombinant murine IL-3 was purchased from PeproTech Inc. (Rocky Hill, NJ).
A rabbit antiserum against the EpoR cytoplasmic domain was previously described.30 A rabbit antiserum against Stat5A25 was kindly provided by Dr J.N. Ihle (St. Jude Children's Research Hospital, Memphis, TN). An anti-Jak2 antibody raised against a synthetic peptide corresponding to amino acids 1110-1129 mapping at the carboxy terminus of murine Jak2 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit antibodies against Stat5B and βIL3 (K-19) were also from Santa Cruz Biotechnology. Monoclonal antibodies against phosphotyrosine, 4G10, and against the epidermal growth factor (EGF ) receptor (EGFR), LA22, were purchased from Upstate Biotechnology, Inc (Lake Placid, NY). A monoclonal antibody, 12CA5, raised against the influenza virus hemagglutinin (HA) epitope was from Boehringer Mannheim (Indianapolis, IN). A glucocorticoid-inducible expression vector, pMAM2-BSD,31 conferring resistance to blasticidin S, was purchased from Funakoshi (Tokyo, Japan).
Construction of expression plasmids and transfection into BaF3 cells.To construct an expression plasmid for a chimeric receptor containing the EpoR intracellular domain linked to the extracellular and transmembrane regions of the EGFR, a portion of the EGFR cDNA coding for amino acids −24 to 647 was amplified by the polymerase chain reaction (PCR) and subcloned into the expression plasmid pcDNA3, as described previously.32 A portion of the murine EpoR cDNA containing the region coding for amino acid 252 to the stop codon and the 3′ noncoding region was amplified by the PCR. The 5′ primer used was 5′-CCGATATCACTCTGCAGCAGAAGATCT-3′, designed to add the EcoRV recognition sequence at the 5′-end of the amplified fragment, while the 3′ primer was derived from a sequence in the pXM vector. The PCR fragment was digested with both EcoRV and Avr II, whose recognition site is in the 3′-noncoding region, and subcloned in frame into the EcoRV-Xba I site of the pcDNA3 clone containing the 5′ portion of EGFR cDNA to create pcD/EGF-EpoR. The structure of pcD/EGF-EpoR was confirmed by digestion with multiple restriction enzymes. The chimeric receptor encoded by pcD/EGF-EpoR contains the signal peptide, extracellular domain, and transmembrane region of the EGFR followed by an Asp residue resulting from the artificially added EcoRV recognition sequence and the EpoR cytoplasmic region lacking the membrane proximal 4 amino acid residues.
BaF3 clones expressing the wild-type EpoR were isolated as described previously.12 Transfection of pcD/EGF-EpoR into BaF3 cells and isolation of clones expressing the EGFR/EpoR chimeric receptor were also performed essentially as described previously.12 In brief, BaF3 cells were transfected with 10 μg of pcD/EGF-EpoR by electroporation and selected in medium containing G418. Four clones were isolated by limiting dilution and examined for the growth response to EGF. All the clones proliferated in response to EGF and one clone was arbitrarily chosen for subsequent studies.
To construct inducible expression plasmids for wild-type ovine Stat5 and its mutant with a substitution of Tyr694 with Phe, the Sal I-Not I fragment coding for wild-type or mutant Stat5 tagged with the HA epitope at the amino-terminus28 was subcloned between the Sal I and Not I sites within the multiple cloning region of the eukaryotic expression vector pMAM2-BSD to create pMB/Stat5-Wt or pMB/Stat5-Y694F, respectively. The inserted Stat5 cDNAs were expressed under the control of the mouse mammary tumor virus long-terminal repeat promoter, which is inducible by dexamethazone. These plasmids were electroporated into BaF3 cells followed by selection in medium containing blasticidin S. The expression of transfected Stat5 was induced by incubating selected clones in medium containing 1 μmol/L dexamethazone for 2 days and evaluated by immunoblotting of the cell lysates with anti-HA. The clone expressing the highest level of the wild-type or mutant Stat5 was selected for the subsequent studies.
Immunoprecipitation and immunoblotting.Cells were washed free of IL-3, cultured overnight, and left unstimulated as a negative control or stimulated with Epo, EGF, or IL-3 at indicated concentrations. Cells were solubilized at 4 × 107 cells/mL with a lysis buffer composed of 1% Triton X-100, 20 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L sodium orthovanadate, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF ), 10 μg/mL aprotinin, and 10 μg/mL leupeptin. For detection of the association of Jak2 with the cytokine receptors, Triton X-100 in the lysis buffer was replaced with 1% digitonin. Cell lysates were subjected to immunoprecipitation as described previously. Immunoprecipitates were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and electrotransferred to Immobilon P membranes (Millipore, Bedford, MA). The membranes were probed with a relevant antibody followed by detection using enhanced chemiluminescence Western blotting detection system (Amersham, Buckinghamshire, UK). For reprobing of the membranes, they were treated with stripping buffer composed of 100 mmol/L 2-mercaptoethanol, 2% SDS, and 62.5 mmol/L Tris-HCl (pH 6.7) at 50°C for 30 minutes and subsequently probed with a different antibody.
RESULTS
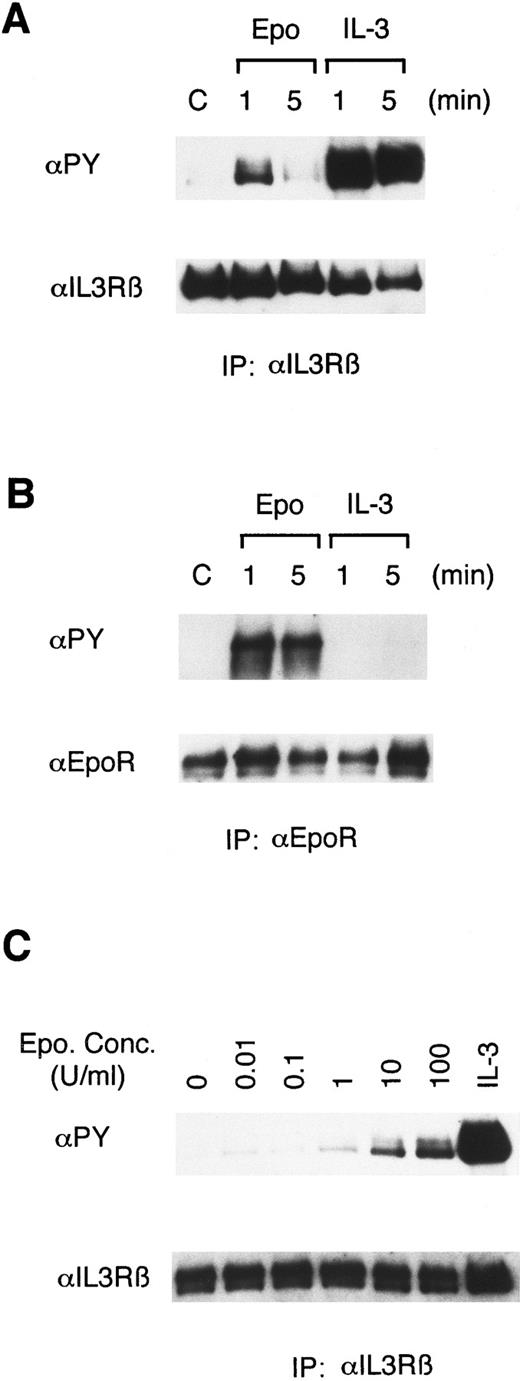
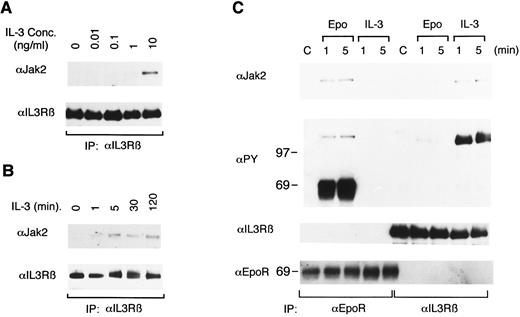
Epo rapidly induces tyrosine phosphorylation of βIL3.To address the possibility that the receptors for Epo and IL-3 may cross-activate each other, we stimulated 32D/EpoR-Wt, a clone of IL-3–dependent 32D cells expressing the transfected murine EpoR, with Epo or IL-3 for 1 or 5 minutes and examined the tyrosine phosphorylation status of these receptors. As shown in Fig 1A, antiphosphotyrosine blotting of anti-βIL3 immunoprecipitates showed that Epo stimulation induced tyrosine phosphorylation of βIL3, albeit to a lesser extent than IL-3 stimulation. The Epo-induced tyrosine phosphorylation of βIL3 occurred rapidly and transiently, as it peaked at 1 minute after stimulation and significantly decreased at 5 minutes. As shown in Fig 1C, the tyrosine phosphorylation of βIL3 was faintly induced when stimulated at as low as 0.01 U/mL of Epo and gradually increased in intensity as the concentration of Epo increased. In contrast to the observation that Epo induced the tyrosine phosphorylation of βIL3, IL-3 stimulation did not induce any detectable tyrosine phosphorylation of the EpoR (Fig 1B).
Epo induces tyrosine phosphorylation of βIL3 in 32D/EpoR-Wt cells. In (A) and (B), 32D/EpoR-Wt cells, a clone of IL-3–dependent 32D cell line expressing the transfected wild-type murine EpoR cDNA, were washed out of IL-3 for 12 hours and left unstimulated (C) or stimulated with 100 U/mL of Epo or 25 ng/mL of IL-3 for 1 or 5 minutes as indicated, at 37°C before solubilization. In (C), 32D/EpoR-Wt cells were stimulated with the indicated concentrations of Epo or 25 ng/mL of IL-3, as indicated, for 1 minute. The cell lysates were immunoprecipitated with anti-βIL3 (A, C) or anti-EpoR (B). Immunoprecipitates were resolved by 6% SDS-PAGE and subjected to immunoblotting with an antiphosphotyrosine monoclonal antibody, 4G10 (αPY). The membranes were then stripped and reprobed with the antibody used for immunoprecipitation, as indicated.
Epo induces tyrosine phosphorylation of βIL3 in 32D/EpoR-Wt cells. In (A) and (B), 32D/EpoR-Wt cells, a clone of IL-3–dependent 32D cell line expressing the transfected wild-type murine EpoR cDNA, were washed out of IL-3 for 12 hours and left unstimulated (C) or stimulated with 100 U/mL of Epo or 25 ng/mL of IL-3 for 1 or 5 minutes as indicated, at 37°C before solubilization. In (C), 32D/EpoR-Wt cells were stimulated with the indicated concentrations of Epo or 25 ng/mL of IL-3, as indicated, for 1 minute. The cell lysates were immunoprecipitated with anti-βIL3 (A, C) or anti-EpoR (B). Immunoprecipitates were resolved by 6% SDS-PAGE and subjected to immunoblotting with an antiphosphotyrosine monoclonal antibody, 4G10 (αPY). The membranes were then stripped and reprobed with the antibody used for immunoprecipitation, as indicated.
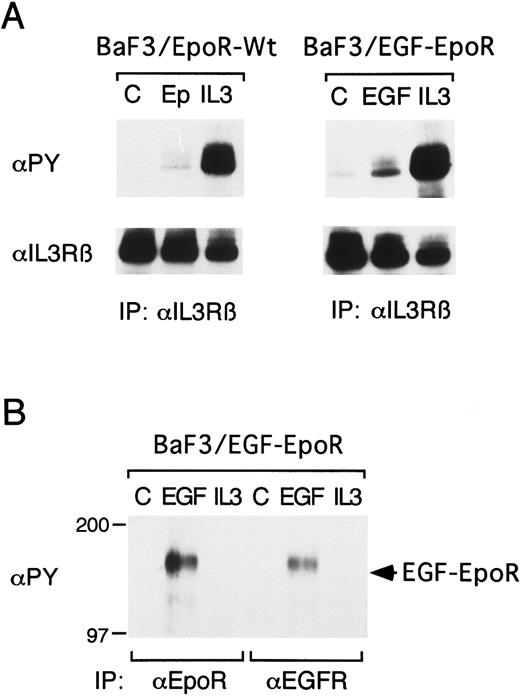
Epo-induced tyrosine phosphorylation of βIL3 depends on the EpoR membrane-proximal domain involved in activation of Jak2.To investigate the mechanisms by which the EpoR mediates the Epo-induced tyrosine phosphorylation of βIL3, IL-3–dependent clones expressing various EpoR mutants shown in Fig 2 were examined. We first examined BaF3/EGF-EpoR cells expressing a chimeric receptor, EGF-EpoR, in which the extracellular and transmembrane regions of the EpoR were replaced by those of the EGFR and compared these cells with BaF3/EpoR cells expressing the wild-type EpoR. As shown in Fig 3A, Epo and EGF similarly induced tyrosine phosphorylation of βIL3 in BaF3/EpoR-Wt and BaF3/EGF-EpoR cells, respectively. In BaF3/EGF-EpoR cells, EGF also induced tyrosine phosphorylation of the 160-kD chimeric receptor, which was reactive with antibodies against the EGFR extracellular domain and the EpoR cytoplasmic domain (Fig 3B). On the other hand, IL-3 failed to induce tyrosine phosphorylation of the chimeric receptor in BaF3/EGF-EpoR cells or the EpoR in BaF3/EpoR cells (Fig 3B and data not shown). These results agree with those obtained with 32D/EpoR-Wt cells and further indicate that only the intracellular domain, but not the extracellular and transmembrane domains, of the EpoR is required for the induction of tyrosine phosphorylation of βIL3.
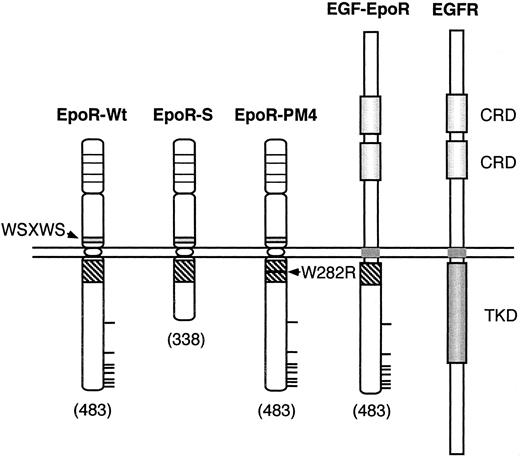
Schematic representation of mutant EpoRs. Mutant EpoRs employed are schematically shown along with the wild-type EpoR and EGFR. The membrane-proximal cytoplasmic domain of EpoR containing the Box 1 and Box 2 sequences is represented by a striped box. Thick horizontal lines represent tyrosine residues in the EpoR cytoplasmic region. The four conserved cysteine residues in the EpoR extracellular domain are represented by thin horizontal lines. Numbers in parentheses denote the carboxy-terminal amino-acid numbers. Abbreviations used are: WSXWS, the WSXWS motif; W282R, the mutation of Trp282 to Arg; CRD, the cysteine-rich domain; TKD, the tyrosine kinase domain.
Schematic representation of mutant EpoRs. Mutant EpoRs employed are schematically shown along with the wild-type EpoR and EGFR. The membrane-proximal cytoplasmic domain of EpoR containing the Box 1 and Box 2 sequences is represented by a striped box. Thick horizontal lines represent tyrosine residues in the EpoR cytoplasmic region. The four conserved cysteine residues in the EpoR extracellular domain are represented by thin horizontal lines. Numbers in parentheses denote the carboxy-terminal amino-acid numbers. Abbreviations used are: WSXWS, the WSXWS motif; W282R, the mutation of Trp282 to Arg; CRD, the cysteine-rich domain; TKD, the tyrosine kinase domain.
The EpoR extracellular and transmembrane domains are not required for the induction of βIL3 tyrosine phosphorylation. A clone of BaF3 cells expressing the wild-type EpoR (BaF3/EpoR-Wt) or a chimeric receptor in which the extracellular and transmembrane domains of the EpoR were replaced with those of the EGFR (BaF3/EGF-EpoR) was starved for 12 hours and left unstimulated (C) or stimulated with 100 U/mL of Epo, 25 ng/mL of IL-3, or 100 ng/mL of EGF, as indicated, for 1 minute. The cells were lysed and subjected to immunoprecipitation with anti-βIL3 , anti-EpoR, or anti-EGFR, as indicated, followed by antiphosphotyrosine immunoblotting (αPY). In (A), the membranes were stripped and reprobed with anti-βIL3 , as indicated. The size markers are indicated and given in kD.
The EpoR extracellular and transmembrane domains are not required for the induction of βIL3 tyrosine phosphorylation. A clone of BaF3 cells expressing the wild-type EpoR (BaF3/EpoR-Wt) or a chimeric receptor in which the extracellular and transmembrane domains of the EpoR were replaced with those of the EGFR (BaF3/EGF-EpoR) was starved for 12 hours and left unstimulated (C) or stimulated with 100 U/mL of Epo, 25 ng/mL of IL-3, or 100 ng/mL of EGF, as indicated, for 1 minute. The cells were lysed and subjected to immunoprecipitation with anti-βIL3 , anti-EpoR, or anti-EGFR, as indicated, followed by antiphosphotyrosine immunoblotting (αPY). In (A), the membranes were stripped and reprobed with anti-βIL3 , as indicated. The size markers are indicated and given in kD.
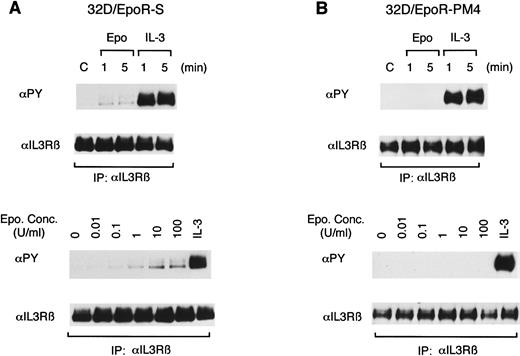
To further define the EpoR cytoplasmic region involved in induction of the tyrosine phosphorylation of βIL3, we next examined previously characterized two clones of 32D cells expressing mutant EpoRs shown in Fig 2. 32D/EpoR-S expresses the S-mutant EpoR in which the carboxy-terminal 145 amino acids, including all the cytoplasm tyrosines, were deleted by carboxy-terminal truncation,12 and 32D/EpoR-PM4 expresses the PM4-mutant EpoR in which a mutation, Trp282 to Arg, in the membrane-proximal cytoplasmic region has abolished the abilities of EpoR to associate with Jak2 and to activate its kinase activity.19 29 In 32D/EpoR-S cells, Epo induced the tyrosine phosphorylation of βIL3, which was observed when cells were stimulated with as low as 0.01 U/mL of Epo (Fig 4A). In contrast, Epo failed to induce any detectable tyrosine phosphorylation of βIL3 in 32D/EpoR-PM4 cells (Fig 4B). These results indicate that the Epo-induced tyrosine phosphorylation of βIL3 depends on the membrane-proximal cytoplasmic region of the EpoR, which is critical for coupling with Jak2, but not on the carboxy-terminal 145 amino-acid region containing all the tyrosine phosphorylation sites.
Induction of tyrosine phosphorylation of βIL3 is dependent on the membrane-proximal EpoR cytoplasmic region involved in the activation of Jak2. A clone of 32D cells expressing the S-mutant EpoR with carboxy-terminal deletion of 145 amino acids (A) or the PM4-mutant EpoR, which harbors a mutation, Trp282 to Arg, in the membrane-proximal cytoplasmic region that abolishes the ability to couple with Jak2 (B) were examined as described in the legend to Fig 1.
Induction of tyrosine phosphorylation of βIL3 is dependent on the membrane-proximal EpoR cytoplasmic region involved in the activation of Jak2. A clone of 32D cells expressing the S-mutant EpoR with carboxy-terminal deletion of 145 amino acids (A) or the PM4-mutant EpoR, which harbors a mutation, Trp282 to Arg, in the membrane-proximal cytoplasmic region that abolishes the ability to couple with Jak2 (B) were examined as described in the legend to Fig 1.
IL-3 induces physical association of Jak2 with βIL3.To further investigate the mechanisms of Epo-induced tyrosine phosphorylation of βIL3, we first examined the physical association of βIL3 with Jak2, the tyrosine kinase implicated in tyrosine phosphorylation of the EpoR, as well as βIL3.13,19 33 For this purpose, 32D/EpoR-Wt cells were stimulated with various concentrations of IL-3 and solubilized with a lysis buffer containing digitonin instead of Triton X-100. The cell lysates were subjected to immunoprecipitation with anti-βIL3 followed by immunoblotting with anti-Jak2. As shown in Fig 5A, the association of Jak2 with βIL3 was readily observed when cells were stimulated with IL-3 at 1 ng/mL and increased remarkably at 10 ng/mL, although low background levels of Jak2 association with βIL3 were faintly visible in cells stimulated with lower concentrations of IL-3 or even in unstimulated cells. To examine the time course of Jak2 association with βIL3, 32D/EpoR-Wt cells were then stimulated with 10 ng/mL of IL-3 for various times and examined in the same way. The IL-3–induced association of Jak2 with βIL3 was observed as early as 1 minute after IL-3 simulation, increased to a plateau level at 5 minutes, and was stably observed for as long as 2 hours after stimulation (Fig 5B).
IL-3, but not Epo induces the physical association of Jak2 with βIL3. After starvation, 32D/EpoR-Wt cells were stimulated with various concentrations of IL-3 for 1 minute (A) or for the indicated times with 10 ng/mL IL-3 (B). Cells were lysed in a lysis buffer containing 1% digitonin instead of 1% Triton X-100, immunoprecipitated with anti-βIL3 , and subjected to anti-Jak2 immunoblotting. The membranes were then reprobed with anti-βIL3 (C) 32D/EpoR-Wt cells were treated as described in the legend to Fig 1, except that the cells were lysed in the digitonin lysis buffer. The cell lysates were immunoprecipitated with anti-EpoR or anti-βIL3 , as indicated, and subjected to anti-Jak2 immunoblotting. The membrane was reprobed sequentially with antiphosphotyrosine (αPY), anti-βIL3 , and anti-EpoR, as indicated. The size markers are indicated and given in kD.
IL-3, but not Epo induces the physical association of Jak2 with βIL3. After starvation, 32D/EpoR-Wt cells were stimulated with various concentrations of IL-3 for 1 minute (A) or for the indicated times with 10 ng/mL IL-3 (B). Cells were lysed in a lysis buffer containing 1% digitonin instead of 1% Triton X-100, immunoprecipitated with anti-βIL3 , and subjected to anti-Jak2 immunoblotting. The membranes were then reprobed with anti-βIL3 (C) 32D/EpoR-Wt cells were treated as described in the legend to Fig 1, except that the cells were lysed in the digitonin lysis buffer. The cell lysates were immunoprecipitated with anti-EpoR or anti-βIL3 , as indicated, and subjected to anti-Jak2 immunoblotting. The membrane was reprobed sequentially with antiphosphotyrosine (αPY), anti-βIL3 , and anti-EpoR, as indicated. The size markers are indicated and given in kD.
We next examined whether Epo induces the association of Jak2 or the EpoR with βIL3. As shown in Fig 5C, Epo and IL-3 rapidly induced the association of Jak2 with their cognate receptors. However, neither Epo nor IL-3 induced any significant increase in binding of Jak2 to βIL3 nor the EpoR, respectively. Reprobing of the membrane with antiphosphotyrosine confirmed that tyrosine-phosphorylated 130-kD Jak2 associated with the EpoR in Epo-stimulated cells, although the presence of Jak2 in the anti-βIL3 immunoprecipitates from IL-3–stimulated cells could not be confirmed because of its similar size with tyrosine-phosphorylated βIL3 (Fig 5C). Further reprobing of the membrane with anti-EpoR and anti-βIL3 failed to show any physical association between the EpoR and βIL3, as shown in Fig 5C.
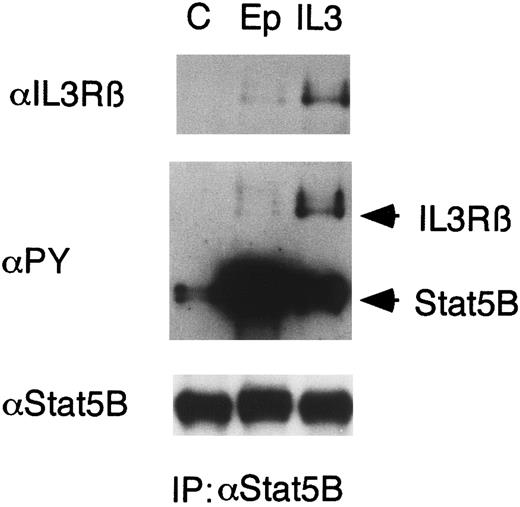
Epo induces binding of Stat5 to βIL3.IL-3, as well as Epo, induces tyrosine phosphorylation and activation of Stat5. We previously demonstrated that Stat5 transiently binds to the tyrosine phosphorylated receptors for Epo or IL-3 in cells stimulated with Epo or IL-3, respectively.23 To address the possibility that the Epo-induced tyrosine phosphorylation of βIL3 may have effects on the IL-3R–mediated downstream signaling, we examined a possible binding of Stat5 to tyrosine-phosphorylated βIL3 in Epo-stimulated cells. As shown in Fig 6, anti-βIL3 immunoblotting of anti-Stat5B immunoprecipitates from 32D/EpoR-Wt cells showed that Epo induced, albeit to a lesser extent than IL-3, binding of Stat5 to βIL3. Reprobing with antiphosphotyrosine also demonstrated the association of Stat5 with a tyrosine phosphorylated protein that corresponds in size to βIL3 in Epo-stimulated cells (Fig 6).
Epo induces binding of Stat5 to βIL3 . 32D/EpoR-Wt cells were starved for 12 hours and left unstimulated (C) or stimulated with 100 U/mL of Epo or 10 ng/mL of IL-3 for 1 minute. The cells were lysed and immunoprecipitated with anti-Stat5B. The immunoprecipitates were subjected to immunoblotting with anti-βIL3 followed by reprobing with antiphosphotyrosine (αPY) and anti-Stat5B, as indicated.
Epo induces binding of Stat5 to βIL3 . 32D/EpoR-Wt cells were starved for 12 hours and left unstimulated (C) or stimulated with 100 U/mL of Epo or 10 ng/mL of IL-3 for 1 minute. The cells were lysed and immunoprecipitated with anti-Stat5B. The immunoprecipitates were subjected to immunoblotting with anti-βIL3 followed by reprobing with antiphosphotyrosine (αPY) and anti-Stat5B, as indicated.
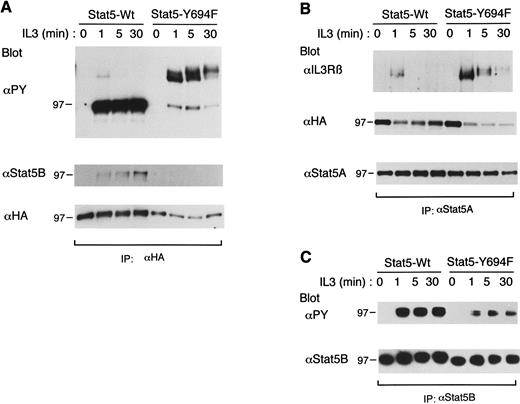
Stat5-Y694F stably binds to tyrosine-phosphorylated βIL3 in IL-3–stimulated cells and inhibits the tyrosine phosphorylation of endogenous Stat5.We previously demonstrated that the recruitment of Stat5 to the EpoR is mediated through the interaction between the Stat5 SH2 domain and the phosphotyrosyl docking sites in the EpoR, which facilitate the activation of Stat5 by receptor-associated Jak2.23 The binding of Stat5 to βIL3 was also found to be induced by IL-3 stimulation, but was not studied in detail.23 Thus, to explore the mechanisms of Stat5 binding to βIL3, we expressed an ovine Stat5 mutant, designated Stat5-Y694F, in which Tyr694 was changed to Phe. The phosphorylation of Tyr694 has been shown to be required for prolactin-dependent activation of Stat5 DNA binding and induction of transcription of the β-casein promoter.28 A clone of BaF3 cells transfected with the dexamethasone-inducible expression plasmid for wild-type ovine Stat5 (Stat5-Wt) or Stat5-Y694F was incubated for 24 hours in the presence of 1 μmol/L dexamethasone, deprived of IL-3 for 12 hours, and left unstimulated as a control or stimulated with a saturating concentration of IL-3 for the indicated times (Fig 7). Stat5-Wt and Stat5-Y694F, both tagged with the HA epitope, were then immunoprecipitated with anti-HA and subjected to immunoblotting with antiphosphotyrosine. As shown in Fig 7A, IL-3 induced tyrosine phosphorylation of Stat5-Wt and its rapid and transient association with a tyrosine-phosphorylated protein of 140-kD, pp140, corresponding in size to tyrosine-phosphorylated βIL3. This is consistent with our previous findings with murine Stat5. On the other hand, Stat5-Y694F was only faintly tyrosine phosphorylated, but its association with pp140 was much more conspicuous and prolonged as compared with that of Stat5-Wt. Reprobing with anti-Stat5B, which does not cross-react with ovine Stat5 (data not shown), further showed that Stat5-Wt, but not Stat5-Y694F, dimerized with Stat5B in IL-3–stimulated cells (Fig 7A, middle panel). Further reprobing with anti-HA showed that the expression level of Stat5-Wt was moderately higher than that of Stat5-Y694F. Because βIL3 was tyrosine phosphorylated to equivalent levels in these two clones (data not shown), these data suggest that the binding of Stat5-Y694 to tyrosine phosphorylated βIL3 was sustained in contrast to the transient binding of Stat5-Wt. Anti-βIL3 blotting of the anti-HA immunoprecipitates, however, failed to demonstrate unambiguously that pp140 corresponded to tyrosine phosphorylated βIL3 because of a technical difficulty due to high backgrounds. We thus immunoprecipitated Stat5-Wt and Stat5-Y694F with anti-Stat5A, which cross-reacts with ovine Stat5 (data not shown), and examined their physical association with βIL3. As shown in Fig 7B, anti-βIL3 blotting of the anti-Stat5A immunoprecipitates obtained from the BaF3 clone expressing Stat5-Y694F confirmed the sustained binding of Stat5-Y694F to βIL3. These data indicate that the phosphorylation of Tyr694 of Stat5 is critical for the formation of Stat5 dimers, as well as for the dissociation of Stat5 from tyrosine-phosphorylated βIL3. These data thus strongly support a hypothetical model of Stat5 activation in which Stat5 is recruited to tyrosine-phosphorylated docking sites in βIL3 and, after phosphorylated by Jak2, rapidly dissociate from βIL3 to form a homodimer through the stable reciprocal interaction between the phosphorylated Tyr694 and the SH2 domain.
Sustained binding of Stat5-Y694 to tyrosine-phosphorylated βIL3 and its inhibitory effect on tyrosine phosphorylation of endogenous Stat5. A clone of BaF3 cells expressing wild-type ovine Stat5 (Stat5-Wt) or mutant ovine Stat5 in which Tyr694 was changed to Phe (Stat5-Y694F ) was stimulated with 25 ng/mL of IL-3 for the indicated times. Cells were then lysed and subjected to immunoprecipitation with anti-HA (A), anti-Stat5A (B), or anti-Stat5B (C). Immunoprecipitates were subjected to immunoblotting with the antibody indicated (upper panels) and then reprobed with the other antibodies indicated (middle and lower panels).
Sustained binding of Stat5-Y694 to tyrosine-phosphorylated βIL3 and its inhibitory effect on tyrosine phosphorylation of endogenous Stat5. A clone of BaF3 cells expressing wild-type ovine Stat5 (Stat5-Wt) or mutant ovine Stat5 in which Tyr694 was changed to Phe (Stat5-Y694F ) was stimulated with 25 ng/mL of IL-3 for the indicated times. Cells were then lysed and subjected to immunoprecipitation with anti-HA (A), anti-Stat5A (B), or anti-Stat5B (C). Immunoprecipitates were subjected to immunoblotting with the antibody indicated (upper panels) and then reprobed with the other antibodies indicated (middle and lower panels).
To examine the effect of sustained binding of Stat5-Y694F to βIL3 on the IL-3R–mediated activation of Stat5, we next selectively immunoprecipitated endogenous Stat5B with anti-Stat5B and analyzed its phosphorylation status by antiphosphotyrosine immunoblotting. As shown in Fig 7C, the IL-3–induced tyrosine phosphorylation of Stat5B was significantly diminished in the BaF3 clone expressing Stat5-Y694F as compared with the clone expressing Stat5-Wt, although Jak2 and βIL3 were tyrosine-phosphorylated comparably in these clones stimulated with IL-3 (data not shown). These data suggest that Stat5-Y694F inhibited the IL-3R–mediated activation of Stat5 by stably binding to the Stat5 docking sites in βIL3 and thereby preventing endogenous Stat5 from binding to these sites to be tyrosine-phosphorylated efficiently by Jak2. These results thus strongly support the hypothesis that βIL3 may have Stat5 docking sites that facilitate the activation of Stat5.
DISCUSSION
In the present study, we have demonstrated that Epo induces rapid and transient unidirectional cross-phosphorylation of βIL3 on tyrosine(s) and its transient association with Stat5. Although we could not demonstrate any detectable Epo-induced binding of βIL3 with Jak2 or the EpoR, the Epo-induced tyrosine phosphorylation of βIL3 was dependent on the membrane-proximal EpoR cytoplasmic region involved in the activation of Jak2. It was also demonstrated that a Stat5 mutant, Stat5-Y694F, shows a sustained binding to tyrosine-phosphorylated βIL3 and inhibits the IL-3–induced activation of endogenous Stat5. The results of the present study support a model for IL-3R–mediated Stat5 activation in which the Stat5-docking site(s) in βIL3 transiently recruits Stat5 to facilitate its tyrosine phosphorylation and raise a possibility that Epo may cross-activate the IL-3R–signaling pathways.
The present study has also shown that IL-3 induces the association of Jak2 with βIL3 in a dose- and time-dependent manner in IL-3–dependent hematopoietic cells. Consistent with this, Cattaneo et al34 have very recently reported that Jak2 physically associates with βIL3 in a ligand-dependent manner in a central nervous system progenitor cell line, ST14A, transfected with both βIL3 and the α chain of the murine IL-3R. These observations are, however, inconsistent with a previous report by Quelle et al,33 which showed that Jak2 constitutively associated with human βc when both were expressed in insect cells using the baculovirus expression vector. It is thus possible that the interaction of Jak2 with human βc may be different from that with murine βIL3. However, in contrast to observations in hematopoietic cells,12-14,19 Jak2 was constitutively activated when expressed in insect cells, and the EpoR was constitutively tyrosine phosphorylated and associated with Jak2 in these cells.33 It is thus more likely, as suggested by Quelle et al33 that the very high concentrations that were achieved in insect cells precluded the detection of subtle changes in affinities that would affect associations in mammalian cells. In agreement with this idea, Brizzi et al35 have recently reported that Jak2 physically associates with βc only upon GM-CSF stimulation in human polymorphonuclear leukocytes, thus suggesting that, under physiological conditions, the association between Jak2 and human βc may also be ligand-dependent. Although the exact mechanisms of how IL-3 and GM-CSF induce the Jak2 binding to the β subunit of their receptors remain unknown, it is speculated that the ligand-dependent heterodimerization of the β subunit with the α subunit or homodimerization of the β subunits may be involved in increasing the affinity of Jak2 binding to βc or βIL3.
The induction of tyrosine phosphorylation of βIL3 by Epo suggests that Jak2 activated by the EpoR may physically interact with βIL3 to cause its tyrosine phosphorylation upon Epo stimulation. However, in coimmunoprecipitation experiments, neither Jak2 nor the EpoR was demonstrated to associate with βIL3 upon Epo stimulation of cells. This could be due to a low stoichiometry of the association. It is also possible that the association is very transient or unstable to be detected by the method employed. Consistent with the idea that Jak2 activated by the EpoR may phosphorylate βIL3, the ability of the EpoR to induce the tyrosine phosphorylation of βIL3 was abrogated by a mutation in the membrane-proximal cytoplasmic region of the EpoR, W282R, that abolishes the ability of the receptor to activate Jak2.12,19 The extracellular and transmembrane regions of the EpoR, on the other hand, was not required for the induction of βIL3 phosphorylation, thus indicating that the EpoR does not interact with βIL3 through these regions. This finding is also against the possibility that a hypothetical second subunit that has been shown to bind Epo in 125I-Epo binding studies36-38 may be involved in induction of the tyrosine phosphorylation of βIL3. The carboxy-terminal 145 amino acid region of the EpoR containing all the intracellular tyrosines was also dispensable for the induction of βIL3 phosphorylation. Therefore, neither the phosphorylated tyrosines in the EpoR nor the various signaling molecules recruited to the phosphotyrosines are involved in the possible interaction of the EpoR with βIL3.
Unidirectional cross-phosphorylation of the β subunits of the IL-3/GM-CSF receptor by Epo39 or G-CSF40 stimulation has previously been reported, although the mechanisms for the cross-phosphorylation or the possible effects on downstream signaling events from the IL-3R was not examined in these studies. Hanazono et al39 recently reported that Epo induces the tyrosine phosphorylation of human βc in a human leukemic cell line, UT-7. We could not, however, demonstrate Epo-induced tyrosine phosphorylation of murine βc in the 32D and BaF3 transfectants nor human βc in human leukemic cell lines, TF-1 and F36E (data not shown). This discrepancy might be due to differences in the cell lines and reagents used in the two studies or due to other differences in experimental design. Previously, Pan et al40 reported that granulocyte colony-stimulating-factor (G-CSF ) induces unidirectional cross-phosphorylation of both βIL3 and βc on tyrosines in 32D cells expressing the transfected murine G-CSF receptor. Unlike the Epo-induced phosphorylation in the present study, G-CSF induced the tyrosine phosphorylation of βIL3 to the degree comparable with that induced by IL-3. This difference may reflect the difference in the numbers of the receptors expressed on the cell surface; whereas the G-CSF receptor was expressed at around 10,000 molecules/cell,40 the EpoR is inefficiently transported to the cell surface and expressed at around a 10-fold lower level, as compared with the G-CSF receptor, in transfected IL-3–dependent cell lines.12,19,29,41 42
In a previous report, we demonstrated that Stat5 binds very rapidly and transiently to βIL3 after IL-3 stimulation.23 The time course of this binding was very similar to that of the Epo-dependent Stat5 binding to the EpoR, which was shown to have specific phosphotyrosyl Stat5 docking sites that facilitate the activation of Stat5. Therefore, we speculated that βIL3 may also have Stat5 docking sites similar to those demonstrated in the EpoR.23 In the present study, it was demonstrated that Stat5-Y694F failed to dimerize with endogenous Stat5, showed a sustained binding to tyrosine phosphorylated βIL3, and inhibited the activation of endogenous Stat5 in cells stimulated with IL-3. These results suggest that Stat5-Y694F may inhibit the activation of Stat5 by the IL-3R in a dominant-negative manner by stably binding to tyrosine-phosphorylated βIL3 and thereby inhibiting the recruitment of endogenous Stat5. These findings thus agree with the idea that βIL3 has the Stat5 docking sties that facilitate its activation and strongly support a hypothetical model for Stat activation by cytokine receptors in which Stats are recruited, through their SH2 domains, to phosphotyrosyl docking sites in receptors and, after tyrosine phosphorylated by receptor-associated Jaks, rapidly dissociate from receptors to form homo-or heterodimers through reciprocal SH2 domain-phosphotyrosine interactions.6 Notably, the intracellular domain of murine βIL343 has 5 tyrosine residues conforming to a YXXL motif that has been proposed as a putative motif for Stat5 docking sites in the EpoR.27 Further studies are, however, required to identify the Stat5 docking sites in βIL3 and to determine its significance on activation of Stat5 by the IL-3R.
The present study has raised the possibility that Epo may cross-activate the IL-3R to induce activation of Stat5, because Stat5 was shown to be recruited to the possible Stat5-docking sites in βIL3 upon Epo stimulation. A possible recruitment of other SH2-containing signaling molecules, such as those involved in activation of the Ras/MAP kinase pathway, to tyrosine-phosphorylated βIL3 upon Epo stimulation also needs to be examined. In this regard, it should be noted that, unlike the carboxy-terminal truncation of βc,44 the truncation of the EpoR cytoplasmic domain severely impairs, but dose not completely abrogate the ability of EpoR to activate the Ras/MAP kinase pathway in IL-3–dependent cells.45 It is thus possible that the truncated mutant EpoR may activate the Ras/MAP kinase pathway at a very low level by cross-activating the IL-3R. Alternatively, Epo may downregulate the IL-3R–mediated signaling, because the tyrosine phosphorylation of βIL3 causes an intrinsic change, which greatly increases its susceptibility to proteolysis both in vitro and in vivo.46 The present study, however, could not determine the functional significance of the Epo-induced tyrosine phosphorylation of βIL3, because the EpoR and the IL-3R activate strikingly similar signaling events and cellular responses in the hematopoietic cell lines examined. These two receptors, however, elicit different biological responses when expressed on hematopoietic progenitor cells, including burst-forming units erythroid (BFU-E). Further studies using purified erythroid progenitor cells will be required to elucidate the functional significance of Epo-induced tyrosine-phosphorylation of βIL3 on the regulation of the hematopoiesis.
ACKNOWLEDGMENT
We are grateful to Dr James N. Ihle for encouragement and for the generous gift of the anti-Stat5A antibody. We thank Drs Yoji Ikawa, Masataka Nakamura, and Koh Yamamoto for helpful discussions, and Kaori Okada for excellent technical assistance.
Supported by grants from the Ministry of Education, Science and Culture of Japan.
Address reprint requests to Osamu Miura, MD, First Department of Internal Medicine, Tokyo Medical and Dental University, 1-5-45 Yushima, Bunkyoku, Tokyo 113, Japan.