Abstract
The glycoprotein (GP) Ib-IX-V complex contains a high-affinity binding site for thrombin on the platelet surface with a poorly defined role in platelet activation by this agonist. Four polypeptides comprise the complex: GP Ibα, GP Ibβ, GP IX, and GP V. The site within the complex that binds thrombin has been localized to a 45-kD region at the amino terminus of GP Ibα, which also contains the site through which the complex interacts with von Willebrand factor. A GP Ib-IX complex that lacks GP V can be efficiently expressed on the surface of transfected cells. We examined the ability of L cells expressing the GP Ib-IX complex (L2H cells) to bind thrombin at high affinity, and found no increase over the level of thrombin binding to control L cells. Because it is one of the few substrates for thrombin on the platelet surface, GP V has also been implicated as possibly participating in thrombin's actions on the platelet. To examine the role of GP V in forming the high-affinity thrombin-binding site, we compared the binding of thrombin to L2H cells versus cells that express the entire GP Ib-IX-V complex (L2H/V cells). Surface expression of GP Ibα was equivalent in these two stable cell lines. Thrombin binding to L2H/V cells was detectable at 0.25 nmol/L thrombin and reached a plateau at 1 nmol/L. No binding to L2H cells was detectable at these concentrations. Comparable results were obtained when thrombin binding to L2H cells transiently expressing GP V was compared with its binding to sham-transfected L2H cells. Again, only cells transiently expressing GP V bound thrombin specifically. As with the platelet polypeptide, thrombin cleaved GP V from the surface of L2H/V cells. To test whether GP V cleavage was required for enhancing thrombin binding to the complex, we tested the binding of enzymatically inactive D-phenylalanyl-Lprolyl-L-arginine chloromethylketone (PPACK)-thrombin to L2H and L2H/V cells. Like native thrombin, PPACK-thrombin at 1 nmol/L bound only to L2H/V cells, indicating that GP V cleavage is not a prerequisite for the formation of the high-affinity thrombin receptor. These data provide the first indication of a physiologic function for GP V, and suggest that formation of the high-affinity thrombin receptor on the platelet surface has complex allosteric requirements.
THROMBIN IS A major mediator of inflammatory, hemostatic, and thrombotic processes. The protease influences these processes at a number of levels, cleaving soluble proteins that are components of protease cascades or cleaving the surface membrane proteins of cells to activate these cells or to regulate their activity. Of the cellular elements of the blood, platelets are the most involved in thrombin's actions. Thrombin binding and activation of platelets is complex, involving at least three molecular interactions. Thrombin binding to protease nexins is of very high affinity and may be a mechanism for regulating the protease, as the nexin will bind and inactivate thrombin, preventing it from passing on a signal for platelet activation.1 This mechanism has been proposed as a means of preventing very low thrombin concentrations from indiscriminately activating platelets.1
Thrombin activation of platelets involves two membrane components: a seven-transmembrane–domain receptor that is cleaved by thrombin and is capable of transmitting activation signals,2 and the glycoprotein (GP)1Ib-IX-V complex, a multisubunit complex that also plays a vital role in platelet adhesion by binding von Willebrand factor.3 The precise roles of each of these membrane components in thrombin activation of platelets are still not well defined, but both are necessary for optimal response to the protease.4-6
Thrombin binds to a hirudin-like sequence in the seven-transmembrane-domain receptor and cleaves its amino terminus, generating a new amino terminus that functions as an internal ligand that interacts with another region of the receptor.2 This interaction transmits a signal across the plasma membrane that coordinates a number of intracellular processes that result in platelet aggregation and secretion. This receptor has been shown to be completely capable of signal transduction in a Xenopus oocyte system and in transfected cells,2,7 and its importance as a requisite receptor for thrombin activation of human platelets is strongly supported by experiments showing that blocking the receptor with antibodies also blocks platelet activation.4
The role of the GP Ib-IX-V complex in thrombin's action on the platelet is much more poorly defined. This complex binds thrombin with very high affinity, variously estimated at between 0.1 and 2 nmol/L.8-11 Its involvement in platelet activation has been suggested by three types of studies: studies of platelets from patients with Bernard-Soulier syndrome, in whom the complex is congenitally missing or dysfunctional,5,12-14 studies of platelets treated with proteases that remove the thrombin-binding region,6,15-22 and studies of platelets treated with monoclonal antibodies that block thrombin binding to the complex.14,23-25 From these studies, it is clear that the complex is necessary for platelet activation at low thrombin concentrations. More recent studies by Greco et al26 indicate that thrombin's interaction with the complex induces calcium influx in platelets in the absence of any contribution from the seven-transmembrane-domain receptor. In addition, several groups have shown recently that the characteristics of the calcium influx induced by tethered-ligand peptides based on the seven-transmembrane-domain receptor are different from those of α-thrombin22,27 28 and resemble the calcium influx induced by thrombin in platelets missing the high-affinity site.
Four polypeptides comprise the GP Ib-IX-V complex, GP Ibα, GP Ibβ, GP IX, and GP V, two of which — GP Ibα and GP V — directly interact with thrombin.3 GP Ibα contains at least one and possibly two binding sites for thrombin in a region in the N-terminus that also binds the adhesive ligand von Willebrand factor.10,11,29 One of these regions contains a cluster of acidic amino acid residues that include three tyrosine residues that become fully sulfated during complex assembly.30 Sulfation of these tyrosines is required for GP Ibα to interact optimally with von Willebrand factor30-32 and thrombin.31,32 The high-affinity binding site for thrombin resides on GP Ibα, as it is effectively removed by proteases that cleave its extracellular region and is blocked by antibodies that bind within this region.18,23 24
GP V is also involved in the interaction with thrombin.5 Although this polypeptide does not contain a hirudin-like sequence,33,34 it is cleaved by thrombin to generate a 69-kD soluble fragment.35,36 Several studies have shown that there is little correlation between the rate and extent of GP V hydrolysis and platelet activation or secretion.37-39 Moreover, Bienz et al40 demonstrated that inhibition of thrombin hydrolysis of GP V with antibodies failed to inhibit platelet activation. Nevertheless, because cleavage of the seven-transmembrane-domain receptor was not accounted for in these studies, whether GP V has a role in thrombin-induced platelet activation is still very much an open question.
Because platelets contain several thrombin-binding proteins and are easily activated by thrombin, we chose to study the high-affinity site in a nonplatelet system: transfected mouse L cells. This system allows the individual components of the GP Ib-IX-V complex to be studied in the absence of other platelet proteins or proteins that are activated by thrombin. In the current study, we report that GP V expression on the cell surface is necessary for expression of the high-affinity binding site, even though that site resides on GP Ibα.
MATERIALS AND METHODS
Reagents.Thrombin was a gift from Dr Mark Wardell (University of Cambridge, Cambridge, UK). D-Phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK)-thrombin was a gift from Dr Paula Tracy of the University of Vermont. Mocarhagin, a protease purified from the venom of the Mozambican spitting cobra, Naja mocambique mocambique,41 was provided by Drs Michael Berndt and Robert Andrews (Baker Medical Research Institute, Prahran, Victoria, Australia).
Cell lines and transfections.The L2H cell line is a clone of thymidine kinase-deficient L cells that stably expresses the GP Ib-IX complex (GP Ibα, GP Ibβ, and GP IX) and the herpes simplex virus thymidine kinase gene. These cells have been described previously.42 To obtain a cell line that stably expresses the four polypeptides of the GP Ib-IX-V complex, we cotransfected L2H cells with the gene for GP V34 (a kind gift from Dr François Lanza, Centre Régional de Transfusion Sanguine, Institute National de la Santé et de la Recherche Médicale, Unité 67085, Strasbourg, France) cloned into the mammalian expression vector ZEM229R and the selection plasmid pREP4 (Invitrogen, San Diego, CA) as previously described.43 All cell lines were cultured in a mixture of Dulbecco's modified Eagle's medium and F12 medium (1:1; Life Technologies Inc, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum. The medium for L2H cells contained 1 mmol/L sodium hypoxanthine, 4 μmol/L aminopterin, and 160 μmol/L thymidine (HAT; Life Technologies Inc); in addition to these drugs, the medium for L2H/V cells was supplemented with 500 μg/mL hygromycin. The cells were maintained in an atmosphere of 5% CO2 and 99% humidity.
L2H cells transiently expressing GP V were also used in these studies. L2H cells were transiently transfected either with a vector alone (control) or with the ZEM229R/GPV vector, which contains the gene for GP V. Lipofection was used to deliver the DNA by a standard technique as described previously30,43; the cells were tested 48 to 72 hours after transfection.
Flow cytometry.Cell surface levels of both GP Ibα and GP V were detected using flow cytometry. The cells were first labeled with a primary monoclonal antibody, either AN51 (DAKO, Carpinteria, CA) for GP Ibα or SW16 (Accurate Chemical and Scientific, Westbury, NY) for GP V. The bound antibody was then detected with fluorescein isothiocyanate (FITC)-conjugated rabbit antimouse IgG. An argon ion laser was used to excite the fluorophor at 488 nm; a 520-nm band-pass filter was used to detect emitted light. Fluorescence in test cells was compared with that in untransfected L cells for the stable transfectants and in sham-transfected cells for the transient transfectants.
Thrombin binding.Thrombin binding was also measured using a flow cytometry-based assay. Cells were first detached from the culture dishes with 0.54 mmol/L EDTA and washed twice before resuspension in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and a specified concentration of thrombin. The cells were incubated for 10 minutes (which we found to be adequate for thrombin binding to reach equilibrium), and excess thrombin was washed off. Bound thrombin was then detected with the anti-thrombin monoclonal antibody EST-7 (American Diagnostics, Greenwich, CT; 1 μg/mL, 1 hour at room temperature) followed by FITC-conjugated antimouse IgG (30 minutes at room temperature) and flow cytometry was performed as described above for detecting surface GP Ibα and GP V.
For comparing thrombin binding of the L2H and L2H/V stable cell lines, a double-staining technique was developed to eliminate the potential effects on thrombin binding of different surface levels of GP Ibα in the two cell lines. After treatment with a specified concentration of thrombin, the cells were stained first with 1.5 μg/mL polyclonal thrombin antibody (American Diagnostic) and 1 μg/mL monoclonal GP Ibα antibody (WM23) for 1 hour at room temperature. The cells were then incubated with 0.5 μg/mL FITC-conjugated goat antirabbit IgG (Zymed, South San Francisco, CA) and 0.5 μg/mL Texas Red-conjugated goat antimouse IgG (Zymed) for 30 minutes at room temperature. At the end of incubation, unbound antibodies were removed by washing the cells twice in PBS. For flow cytometric analysis, the cells were first gated using the Texas Red window (excitation at 596 nm and emission at >620 nm) to obtain cells expressing GP Ibα. Ten thousand cells from the GP Ibα-positive gate were analyzed for thrombin binding as described earlier. All thrombin-binding studies using stable cell lines were performed on gated populations.
Two methods were used to control for nonspecific binding. First, thrombin binding to cells was compared in cells expressing different components of the GP Ib-IX-V complex versus parental L cells, which served as negative controls. As a second control, thrombin was omitted and the cells were incubated only with the primary and secondary antibodies. This is particularly important when comparing thrombin binding in stably expressing cells, which were not entirely comparable (L2H/V cells were grown in hygromycin and L2H cells were not).
GP V hydrolysis.We used two methods to determine the effect of thrombin or PPACK-thrombin on surface levels of GP V, one that measures residual GP V remaining on the cells after thrombin treatment, and the other that measures the amount of soluble GP V released into the cell supernatant. Both methods were used to follow the rate of GP V hydrolysis.
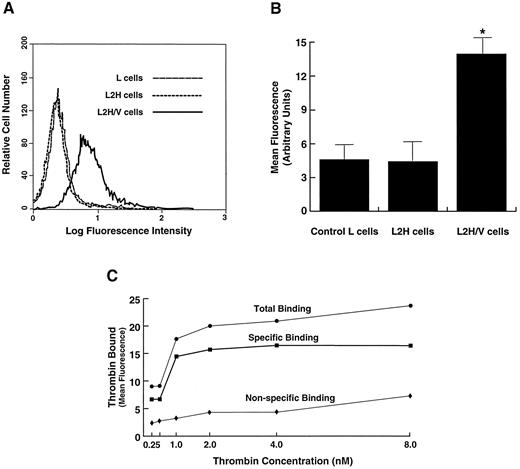
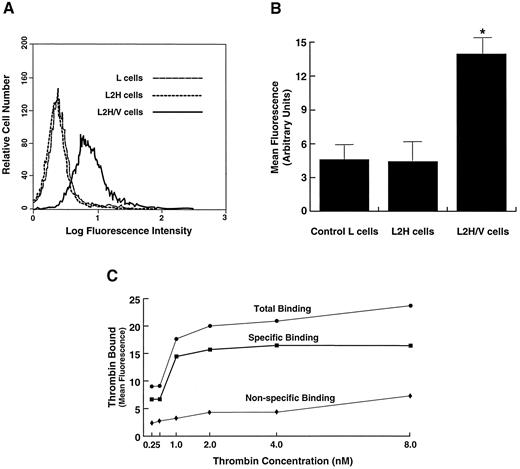
Comparison of thrombin binding in cells expressing the full GP Ib-IX-V complex versus cells expressing only GP Ib-IX. (A) Cells were incubated with thrombin at a concentration of 1 nmol/L, and bound thrombin was detected by flow cytometry after labeling with the FITC-conjugated monoclonal antibody EST-7. Shown is a representative flow cytometry histogram for comparison of thrombin binding at 1 nmol/L in L2H cells (expressing GP Ib-IX), L2H/V cells (expressing the full GP Ib-IX-V complex), and control untransfected parental L cells. (B) Mean fluorescence intensity of the whole cell population from 5 experiments like the 1 depicted in A. *Differences in thrombin binding between L2H/V cells and the other 2 cell lines were statistically significant (Student's t test: t = 10.795, n = 7, P < .001). (C) Thrombin binding to L2H/V cells was evaluated at thrombin concentrations of 0.25 to 8 nmol/L. Specific binding was determined by subtracting the amount of thrombin bound to parental L cells (nonspecific binding) from that bound to L2H/V cells (total binding).
Comparison of thrombin binding in cells expressing the full GP Ib-IX-V complex versus cells expressing only GP Ib-IX. (A) Cells were incubated with thrombin at a concentration of 1 nmol/L, and bound thrombin was detected by flow cytometry after labeling with the FITC-conjugated monoclonal antibody EST-7. Shown is a representative flow cytometry histogram for comparison of thrombin binding at 1 nmol/L in L2H cells (expressing GP Ib-IX), L2H/V cells (expressing the full GP Ib-IX-V complex), and control untransfected parental L cells. (B) Mean fluorescence intensity of the whole cell population from 5 experiments like the 1 depicted in A. *Differences in thrombin binding between L2H/V cells and the other 2 cell lines were statistically significant (Student's t test: t = 10.795, n = 7, P < .001). (C) Thrombin binding to L2H/V cells was evaluated at thrombin concentrations of 0.25 to 8 nmol/L. Specific binding was determined by subtracting the amount of thrombin bound to parental L cells (nonspecific binding) from that bound to L2H/V cells (total binding).
To determine the residual amount of GP V left on cells after thrombin treatment, we first treated the cells for different times with thrombin or PPACK-thrombin (1 nmol/L). At a specified time, the thrombin was inactivated by adding hirudin to a final concentration of 10 μmol/L, and the cells were washed twice. The level of GP V remaining on the cell surface was then determined using SW16, as described earlier.
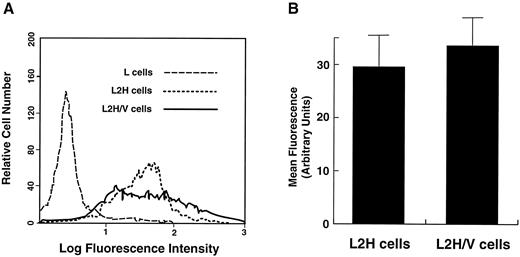
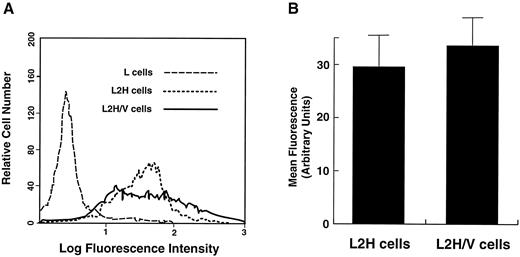
Comparison of GP Ibα surface levels in L2H and L2H/V cells. The cells were labeled with FITC-conjugated anti-GP Ibα monoclonal antibody AN51. (A) Representative flow cytometry histograms of GP Ibα surface levels. (B) Mean fluorescence intensity from 3 experiments. Differences in antibody binding to the 2 cell lines were not statistically significant (paired Student's t test: n = 3, t = 2.275, P = .18).
Comparison of GP Ibα surface levels in L2H and L2H/V cells. The cells were labeled with FITC-conjugated anti-GP Ibα monoclonal antibody AN51. (A) Representative flow cytometry histograms of GP Ibα surface levels. (B) Mean fluorescence intensity from 3 experiments. Differences in antibody binding to the 2 cell lines were not statistically significant (paired Student's t test: n = 3, t = 2.275, P = .18).
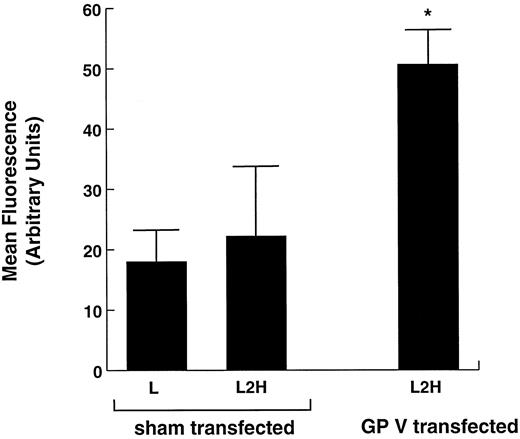
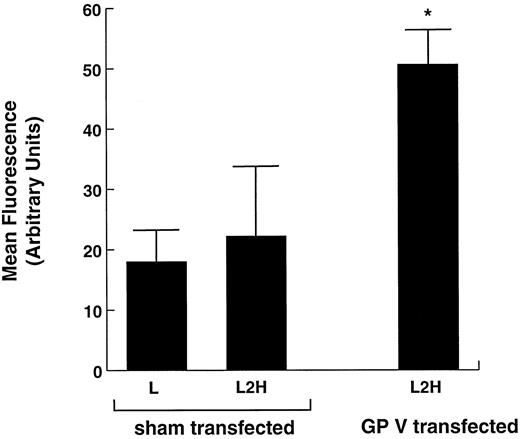
Thrombin binding to L2H cells transiently expressing GP V. L2H cells were either sham-transfected with an empty plasmid or transfected with a plasmid containing the gene for GP V. Seventy-two hours after transfection, the thrombin-binding capacity of the cells was compared as in Fig 1. *Binding to this cell line was significantly greater than to the other 2 cell lines (Student's t test: n = 5, t = 13.263, P < .001).
Thrombin binding to L2H cells transiently expressing GP V. L2H cells were either sham-transfected with an empty plasmid or transfected with a plasmid containing the gene for GP V. Seventy-two hours after transfection, the thrombin-binding capacity of the cells was compared as in Fig 1. *Binding to this cell line was significantly greater than to the other 2 cell lines (Student's t test: n = 5, t = 13.263, P < .001).
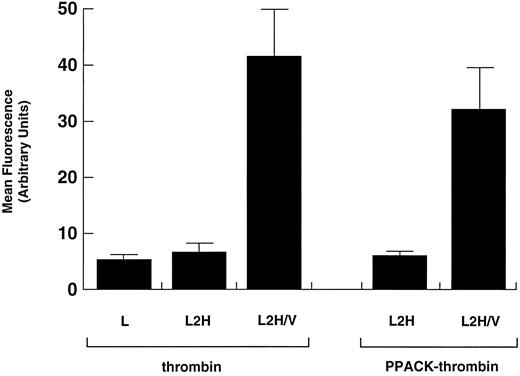
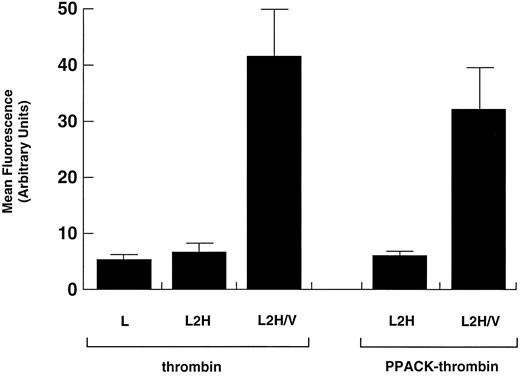
Binding of PPACK-thrombin. Binding of native α-thrombin was compared with that of proteolytically inactivated thrombin (PPACK-thrombin) in L2H/V cells. Data for binding of α-thrombin to L and L2H cells and of PPACK-thrombin to L2H cells are shown for comparison. Binding was evaluated as in Fig 1. Binding of native thrombin or PPACK-thrombin to L2H/V cells was significantly greater than to the other cell lines (Student's t test: n = 6, t = 11.240, P < .001). The difference in binding to L2H/V cells between native thrombin and PPACK-thrombin did not reach statistical significance (P = .223).
Binding of PPACK-thrombin. Binding of native α-thrombin was compared with that of proteolytically inactivated thrombin (PPACK-thrombin) in L2H/V cells. Data for binding of α-thrombin to L and L2H cells and of PPACK-thrombin to L2H cells are shown for comparison. Binding was evaluated as in Fig 1. Binding of native thrombin or PPACK-thrombin to L2H/V cells was significantly greater than to the other cell lines (Student's t test: n = 6, t = 11.240, P < .001). The difference in binding to L2H/V cells between native thrombin and PPACK-thrombin did not reach statistical significance (P = .223).
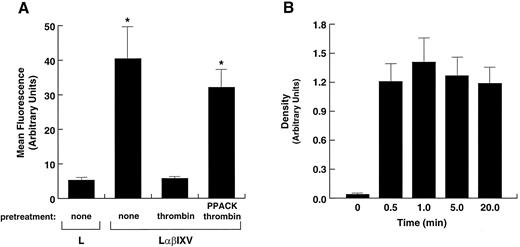
Thrombin proteolysis of GP V from the surfaces of L2H/V cells. (A) Residual binding of the anti-GP V monoclonal antibody SW16 to L2H/V cells incubated with either buffer alone (none) or 1 nmol/L α-thrombin or PPACK-thrombin for 20 minutes. Untreated parental L cells represent background binding. After the incubation period, hirudin was added to inactivate thrombin. *Significantly different v pretreatment of cells with native thrombin (Student's t test: n = 4, t = 12.415, P < .001). (B) Analysis of GP V released into the supernatant of thrombin-treated cells. Cells were incubated with thrombin for the designated times before addition of hirudin. Aliquots of the supernatant were vacuumed onto nitrocellulose paper using a dot-blot apparatus, and the retained GP V was detected using SW16 and a chemiluminescence detection system. Five similar experiments were performed; the mean ± SD of the values obtained by densitometry of the x-ray film are shown.
Thrombin proteolysis of GP V from the surfaces of L2H/V cells. (A) Residual binding of the anti-GP V monoclonal antibody SW16 to L2H/V cells incubated with either buffer alone (none) or 1 nmol/L α-thrombin or PPACK-thrombin for 20 minutes. Untreated parental L cells represent background binding. After the incubation period, hirudin was added to inactivate thrombin. *Significantly different v pretreatment of cells with native thrombin (Student's t test: n = 4, t = 12.415, P < .001). (B) Analysis of GP V released into the supernatant of thrombin-treated cells. Cells were incubated with thrombin for the designated times before addition of hirudin. Aliquots of the supernatant were vacuumed onto nitrocellulose paper using a dot-blot apparatus, and the retained GP V was detected using SW16 and a chemiluminescence detection system. Five similar experiments were performed; the mean ± SD of the values obtained by densitometry of the x-ray film are shown.
To measure the amount of soluble GP V fragment released into the supernatant, flasks of confluent cells were incubated with 1 nmol/L thrombin, and aliquots of the medium were removed at specified intervals. The aliquots of medium were vacuumed through a nitrocellulose membrane using a dot-blot apparatus (Bio-Rad, Hercules, CA). Nonspecific binding sites on the membrane were then blocked by incubation in 5% nonfat milk in Tris-buffered saline ([TBS] 20 mmol/L Tris hydrochloride and 500 mmol/L NaCl, pH 7.5) for 1 hour at room temperature. After washing, the membrane was incubated in TBS containing 1% milk/0.1% Tween-20 (Sigma Chemical Co, St Louis, MO) and 2 μg/mL SW16 for 1 hour, followed by biotinylated donkey antimouse IgG (Amersham, Arlington Heights, IL). A horseradish peroxidase-conjugated tertiary antibody was then applied for 30 minutes at room temperature. The bound antibody was then detected with a chemiluminescence detection kit (Amersham). Densitometry of the exposed film was performed using an LKB 2400 Ultrascan XL densitometer (Bromma, Sweden).
Protease treatment of cells.To cleave the N-terminus of GP Ibα from the cells, 1 × 106 cells were treated with 10 μg mocarhagin in PBS containing 1% BSA buffer for 10 minutes and then washed twice. The residual GP Ibα was detected on the cell surface with AN51. Essentially all of the GP Ibα was removed within 10 minutes.
RESULTS
No high-affinity thrombin binding is detected on cells expressing the GP Ib-IX complex.Numerous studies on platelets have demonstrated that the high-affinity binding site for thrombin on platelets resides within the N-terminus of GP Ibα. In our own studies, we found that efficient GP Ibα expression requires GP Ibβ and GP IX42 and therefore decided to examine the ability of L2H cells (a cloned cell line with high-level GP Ib-IX complex surface expression) to bind thrombin with high affinity. Because the highest-affinity site for thrombin on the platelet surface has been estimated to have a kd of between 0.1 and 2 nmol/L,8-11 we incubated the cells in thrombin at a concentration of 1 nmol/L. The assay we used involved first incubating the cells in the thrombin-containing buffer and then detecting the surface-bound thrombin with an antithrombin monoclonal antibody. With this assay, we could not detect a difference in thrombin binding between L2H cells and control L cells (Fig 1A).
GP V is required for high-affinity thrombin binding.When the GP V gene became available for expression studies, we decided to reevaluate thrombin binding, this time using cells expressing the full GP Ib-IX-V complex. We constructed a cell line, L2H/V, with stable expression of the four GP Ib-IX-V complex polypeptides.43 Thrombin binding to these cells was markedly higher than to L2H or control cells (Fig 1A). This increase in high-affinity thrombin binding in the presence of GP V was consistently reproducible (Fig 1B).
To determine if 1 nmol/L was the appropriate thrombin concentration for these studies, we examined binding at thrombin concentrations between 0.25 and 8 nmol/L. Binding reached a plateau at 1 nmol/L (Fig 1C).
GP V does not increase thrombin binding by increasing surface expression of GP Ibα.Because L2H cells express a significant quantity of GP Ibα on the cell surface and display no increase in thrombin binding over that observed in control L cells, we considered it unlikely that GP V augments thrombin binding by increasing GP Ibα surface expression. Nevertheless, because other investigators have reported an increase in GP Ibα expression concomitant with GP V expression44,45 (which we have not43 ), we examined whether the increase in high-affinity thrombin binding seen in the cells that express the full GP Ib-IX-V complex might have been secondary to an increase in surface levels of GP Ibα induced by GP V. In several of the thrombin-binding experiments, we simultaneously evaluated surface expression of GP Ibα. We found no significant differences in surface GP Ibα levels when comparing L2H with L2H/V cells (Fig 2).
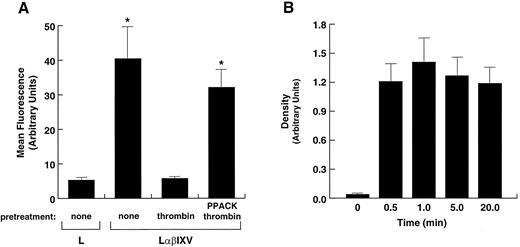
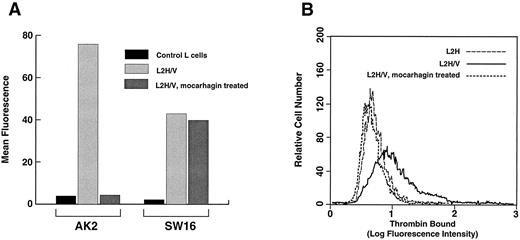
Removal of the high-affinity thrombin-binding site by protease treatment. L2H/V cells were treated with mocarhagin, a protease that specifically removes the GP Ibα N-terminus by cleaving between residues 282 and 283. (A) Surface levels of GP Ibα and GP V were compared before and after treatment. GP Ibα was detected with AK2, a monoclonal antibody that binds the GP Ibα N-terminus, and GP V was detected with SW16. Mocarhagin treatment removed essentially all of the GP Ibα N-terminus from the cell surface, but had no effect on GP V levels. (B) Binding of thrombin at 1 nmol/L was evaluated and compared in mocarhagin-treated L2H/V cells v untreated cells and L2H cells. Removal of the GP Ibα N-terminus abolished high-affinity thrombin binding.
Removal of the high-affinity thrombin-binding site by protease treatment. L2H/V cells were treated with mocarhagin, a protease that specifically removes the GP Ibα N-terminus by cleaving between residues 282 and 283. (A) Surface levels of GP Ibα and GP V were compared before and after treatment. GP Ibα was detected with AK2, a monoclonal antibody that binds the GP Ibα N-terminus, and GP V was detected with SW16. Mocarhagin treatment removed essentially all of the GP Ibα N-terminus from the cell surface, but had no effect on GP V levels. (B) Binding of thrombin at 1 nmol/L was evaluated and compared in mocarhagin-treated L2H/V cells v untreated cells and L2H cells. Removal of the GP Ibα N-terminus abolished high-affinity thrombin binding.
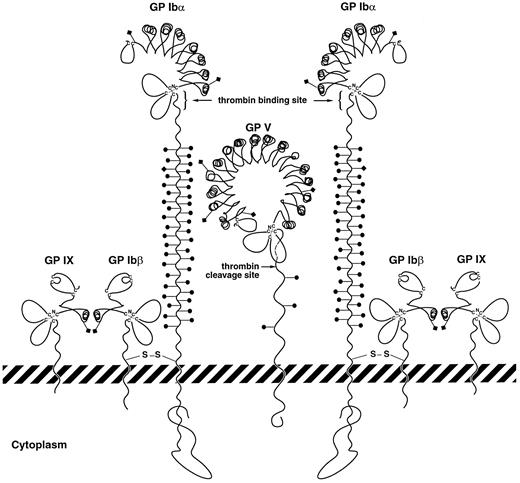
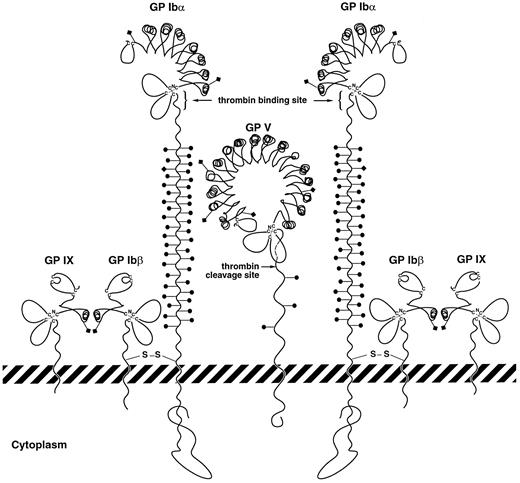
Schematic of potential interactions between GP V and GP Ibα in forming a high-affinity site for thrombin. GP V is depicted between 2 GP Ib-IX complexes, associating with these complexes directly through GP Ibα. In the process of this association, GP Ibα regions important for thrombin binding on 2 nearby polypeptides may become juxtaposed or be influenced allosterically by GP V. Structures of the leucine-rich repeats of the polypeptides are drawn based on the crystal structure of the porcine ribonuclease inhibitor determined by Kobe and Diesenhofer.51
Schematic of potential interactions between GP V and GP Ibα in forming a high-affinity site for thrombin. GP V is depicted between 2 GP Ib-IX complexes, associating with these complexes directly through GP Ibα. In the process of this association, GP Ibα regions important for thrombin binding on 2 nearby polypeptides may become juxtaposed or be influenced allosterically by GP V. Structures of the leucine-rich repeats of the polypeptides are drawn based on the crystal structure of the porcine ribonuclease inhibitor determined by Kobe and Diesenhofer.51
High-affinity thrombin binding in L2H cells transiently expressing GP V.In addition to possible differences in surface levels of GP Ibα, other variables unrelated to the presence or absence of GP V could affect thrombin binding in stable cell lines, such as growth of the cell lines in different antibiotic combinations and potential phenotypic drifts between individual cloned cell lines while they are maintained in culture. For this reason, we also examined high-affinity thrombin binding in L2H cells transiently expressing GP V. For these studies, L2H cells were transfected with either a control plasmid or the plasmid that contains the GP V gene. Thrombin binding in these cells was examined 48 to 72 hours after transfection. Sham-transfected parental L cells were also used as controls (Fig 3). The results of these experiments were consistent with those obtained with the stable cell lines, ie, only when expressing GP V were L2H cells capable of high-affinity thrombin binding. As in the stable cell lines, our previous studies have demonstrated that transient GP V expression in L2H cells does not increase GP Ibα levels on the cell surface.43
Thrombin's proteolytic activity is not required for high-affinity binding.Numerous studies have demonstrated that proteolytic activity is not a requirement for binding to the high-affinity site on platelets. To determine if the same is true of the recombinant complex, we examined the binding of PPACK-thrombin, which is rendered inactive by covalent modification of its active site by PPACK. Binding of PPACK-thrombin was compared with that of unmodified thrombin. Like native thrombin, PPACK-thrombin bound to L2H/V cells but not to L2H cells, although the level of binding to L2H/V cells was less than for native thrombin (Fig 4). This difference may reflect interbatch variability between PPACK-thrombin and native thrombin, which were obtained from different sources.
Thrombin remains bound to the high-affinity site after GP V has been removed by proteolysis.We evaluated the extent to which GP V is proteolyzed by analyzing the residual amount of this polypeptide remaining on the cell surface after treating the cells for increasing periods with thrombin. After the specified time, thrombin was inactivated with hirudin and the cells were washed and incubated with the monoclonal antibody SW16. We also examined the amount of soluble GP V released into the cell supernatant by removing aliquots of the supernatant at different time points after adding thrombin and quantifying the released GP V using dot-blot analysis. Little residual GP V was detected on the cell surface after 20 minutes of incubation with thrombin (Fig 5A). Analysis of the amount of GP V released into the supernatant indicated that thrombin proteolysis is rapid: no significant difference was noted in the amount of GP V found in the supernatants of cells treated for 30 seconds to 20 minutes (Fig 5B).
The high-affinity site for thrombin resides on GP Ibα.The rapid removal by thrombin of GP V from the cell surface indicates that GP V is not the high-affinity thrombin-binding site. To confirm that this site resides on GP Ibα, we proteolytically removed the GP Ibα N-terminus with mocarhagin, a metalloprotease purified from the venom of the Mozambican spitting cobra, Naja mocambique mocambique. Ward et al32 have demonstrated that this protease selectively cleaves GP Ibα from platelets, removing its N-terminus by cleaving between residues 282 and 283, a region that has been identified as important for thrombin binding.10 We confirmed that mocarhagin selectively cleaves GP Ibα and not GP V from the cells by examining the levels of both polypeptides on the surfaces of L2H/V cells after treatment with the protease. Mocarhagin treatment removed essentially all of the GP Ibα N-terminus from the cell surface, but had no effect on GP V levels (Fig 6A). This treatment also completely abolished high-affinity thrombin binding, as expected (Fig 6B). In addition, pretreatment with mocarhagin seemed to have little effect on the amount of GP V hydrolyzed after 10 minutes of thrombin treatment (data not shown).
DISCUSSION
Thrombin's action on platelets at low thrombin concentrations, which may be the most important physiologically in regions of rapid blood flow, requires the presence of a high-affinity receptor on the platelet surface. Although numerous lines of evidence have demonstrated that the high-affinity binding site resides on the N-terminus of GP Ibα,10,23,24,46 two observations have been difficult to reconcile with this finding: the number of binding sites on the platelet surface and the estimated molecular mass of the high-affinity receptor. Only between 300 and 4,000 sites have been identified on the platelet that are capable of binding thrombin with subnanomolar affinity,11,47,48 whereas the number of GP Ibα molecules on the cell surface has been estimated at about 25,000 per platelet.49 Similarly, radiation inactivation studies have indicated that the molecular mass of the high-affinity receptor is 900,000 daltons,9 whereas the combined molecular mass of one copy each of GP Ibα, GP Ibβ, GP IX, and GP V is only about 264 kD (based on a molecular mass of 135 kD for GP Ibα, 25 kD for GP Ibβ, 21 kD for GP IX, and 83 kD for GP V).
The data presented in the current report provide information that may help to reconcile these disparities. We demonstrate that although it resides on the GP Ibα N-terminus, the high-affinity site within the complex requires GP V for expression. At thrombin concentrations at which only binding to the high-affinity site is expected, no binding was observed in cells that express the GP Ib-IX complex without GP V. However, when GP V was expressed as part of the larger complex either stably or transiently, the complex gained the capacity for high-affinity thrombin binding.
Thus, the high-affinity site requires more than merely the presence of GP Ibα on the cell surface. The observation that GP V is needed indicates either that the high-affinity site is partly composed of sequences from GP V or that the site (or sites) on GP Ibα is allosterically influenced by the presence of GP V such that it can bind thrombin more tightly. GP V might also bring together two sites from adjacent GP Ibα chains to form a site of higher affinity than either site alone. Our recent demonstration that GP V associates with the GP Ib-IX complex directly through an interaction with GP Ibα supports either of these possibilities.43
If the first of these possibilities is correct, then the region contributed by GP V must be available both before and after thrombin cleavage, because thrombin remains bound to the cell surface even after a significant portion of the GP V extracellular region has been removed (Figs 1 and 5). Such an arrangement of binding sites would be limited by the number of GP V molecules complexed to GP Ibα. Because the number of GP V molecules has been estimated at approximately 11,000 per platelet,50 the necessity for this polypeptide would partially account for the disparity between GP Ibα molecules and high-affinity sites.
This disparity could also be explained by the second model, if one GP V molecule interacts with more than one molecule of GP Ibα (Fig 7), a model that we have previously proposed as a representation of the actual functional GP Ib-IX-V complex on the platelet surface.3,43 If the actual functional complex contains four GP Ibα molecules and two GP V molecules, the number of high-affinity sites predicted by this model (∼6,000) is much closer to that determined experimentally.11,47,48 A high-affinity site of this arrangement would also fit well with the molecular mass for the high-affinity thrombin site predicted by the radiation inactivation studies reported by Harmon and Jamieson.9 Although this model is more attractive because it better explains the experimental observations, no direct verification for either one has yet been provided.
In summary, we have discovered an important new role for GP V as a component of the platelet high-affinity thrombin receptor. This GP is also cleaved by thrombin, but whether this cleavage influences platelet activation by low concentrations of the agonist is still unknown and a current topic of investigation. Of particular importance, these studies must be performed in a system in which the cleavage of GP V can be manipulated and the end point of thrombin's action on this polypeptide is not obscured by the presence of the seven-transmembrane-domain receptor. Such studies could uncover an important function for GP V, a polypeptide that until now has been an “orphan,” a thrombin substrate with no apparent function.
ACKNOWLEDGMENT
We thank Drs Andrew I. Schafer and Perumal Thiagarajan for critically reading the manuscript and for helpful suggestions.
Supported by National Institutes of Health Grants No. HL02463 and HL46416.
Address reprint requests to José A. López, MD, Veterans Affairs Medical Center, Hematology/Oncology (111H), 2002 Holcombe Blvd, Houston, TX 77030.