Abstract
An important risk factor for thrombosis is the polymorphism R506Q in factor V that causes resistance of factor Va to proteolytic inactivation by activated protein C (APC). To study the potential influence of the carbohydrate moieties of factor Va on its inactivation by APC, factor V was subjected to mild deglycosylation (neuraminidase plus N-glycanase) under nondenaturing conditions. The APC resistance ratio values (ratio of activated partial thromboplastin time [APTT] clotting times with and without APC) of the treated factor V were increased (2.4 to 3.4) as measured in APTT assays. O-glycanase treatment of factor V did not change the APC resistance ratio. The procoagulant activity of factor V as well as its activation by thrombin was not affected by mild deglycosylation. Treatment of factor V with neuraminidase and N-glycanase mainly altered the electrophoretic mobility of the factor Va heavy chain, whereas treatment with O-glycanase changed the mobility of the connecting region. This suggests that the removal of the N-linked carbohydrates from the heavy chain of factor Va, which is the substrate for APC, is responsible for the increase in susceptibility to inactivation by APC. Thus, variability in carbohydrate could account for some of the known variability in APC resistance ratios, including the presence of borderline or low APC resistance ratios among patients who lack the R506Q mutation.
FACTOR V IS A single-chain glycoprotein of 330 kD that plays a critical role in hemostasis as a cofactor for the factor Xa-dependent proteolytic conversion of prothrombin to thrombin.1 The complete primary sequence of the 2,196 amino acids in human factor V has been determined.2 The polypeptide chain of factor V undergoes extensive posttranslational modifications.3 Carbohydrates, including large amounts of sialic acid4 and multiple N-linked and O-linked oligosaccharides,2,4,5 account for approximately 13% to 25% of the mass of human factor V. The heavy chain of human factor V contains 9 potential glycosylation sites, the light chain has 3 potential sites, and the intermediate region has 25 potential sites.2,6 Membrane-bound factor Va is inactivated by activated protein C (APC) after cleavage at Arg506, Arg306, and perhaps Arg679.7,8 Membrane-bound factor V is cleaved at Arg306 and contains an additional APC-cleavage site at Lys994, and no factor Va activity could be observed after thrombin treatment of APC-cleaved factor V.7 Recently, the polymorphism of R506Q in factor V has been identified as an important risk factor for thrombosis because Gln506-factor Va is resistant to APC cleavage.9-19 However, DNA analysis has shown that some individuals with APC resistance, based on coagulation assays, lack this factor V mutation.10,11,17 20 Thus, the inactivation of factor Va by APC in plasma may be influenced by factors other than the R506Q polymorphism.
A high percentage of the proteins that transit the secretory pathway of eucaryotic cells, including plasma coagulation factors, are glycosylated by enzymes of the endoplasmic reticulum. In humans, a minimum of 250 to 300 enzymes and an estimated 2% to 4% of the active human genome are involved in the glycosylation process.21 One consistent observation about the structure of the oligosaccharides present on glycoproteins is that considerable heterogeneity exists in the sugar chains attached at each glycosylation site.22 In biologic systems, the heterogeneity and branching of carbohydrate side chains allow glycoconjugates to display further levels of structural and functional diversity, compared either with linear polypeptides and nucleic acids or with lipids.22 23 In this report, we identify the protective role that the carbohydrate moiety of human factor V/Va plays in its inactivation by APC and describe the importance of N-linked carbohydrate side chains in the heavy chain of factor Va.
MATERIALS AND METHODS
Proteins.Human factor V was purified as described.24 The factor V preparation had a specific activity of 93 U/mg as measured in a prothrombin time (PT) clotting assay and could be activated approximately 37-fold by human α-thrombin. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of the purified factor V showed a single band at approximately 330 kD. Bovine factor V and human α-thrombin were obtained from Enzyme Research Laboratories (South Bend, IN); factor V-deficient plasma from George King (Overland Park, KS); Platelin LS from Organon Teknika (Durham, NC); and Innovin from Baxter Diagnostics, Inc (Deerfield, IL). Recombinant N-glycosidase F (N-glycanase) at 20 U/100 μL and highly purified neuraminidase from Arthrobacter aureofaciens at 1 U/100 μL were obtained from Boehringer Mannheim (Indianapolis, IN). Endo-α-N-acetyl-galactosaminidase (O-glycanase) at 30 mU/100 μL from Streptococcus pneumoniae was obtained from Oxford Glycosystems (Rosedale, NY). All other reagents were of the best quality available.
Factor Va inactivation assay.Forty microliters of 100 μg/mL human factor V or bovine factor V were incubated at 37°C with different glycanases, neuraminidase at 0.0125 U/mL, N-glycanase at 0.1 U/mL, O-glycanase at 0.033 U/mL (final concentrations), or with a mixture of two or more of these enzymes in 50 mmol/L Tris, 100 mmol/L NaCl, 0.02% NaN3 , pH 7.4 (TBS), with 5 mmol/L CaCl2 . Aliquots were taken at various times from the incubation mixtures, diluted to 10 μg/mL factor V in TBS containing 5 mmol/L CaCl2 and 0.1% bovine serum albumin (BSA), and then assayed in an activated partial thromboplastin time (APTT) clotting assay. Fifty microliters of factor V-deficient plasma, 50 μL of APTT reagent, and 5 μL of diluted deglycosylation incubation mixture containing factor V were incubated for 3 minutes at 37°C. Clotting was initiated by the addition of 50 μL of 30 mmol/L CaCl2 in 10 mmol/L Tris, 50 mmol/L NaCl, pH 7.4, 0.1% BSA in the presence or absence of 1 μg/mL of human APC. The ratio of clotting times with and without APC was calculated to give the APC resistance ratio.
Factor V activity assay.Factor V coagulant activity was determined using a PT clotting assay. The diluted factor V-deglycosylation incubation mixtures (5 μL) described above were incubated with 50 μL factor V-deficient plasma for 60 seconds at 37°C before adding 50 μL of thromboplastin reagent. One unit of factor V was defined as the activity present in 1 mL of normal pooled plasma.
Activation of factor V by thrombin.Factor V (100 μg/mL) treated with neuraminidase and N-glycanase as indicated above was activated with human α-thrombin (33 ng/mL, final concentration) in TBS in the presence of 5 mmol/L CaCl2 . After 0, 3, 6, 9, 15, 20, 30, 40, 50, and 60 minutes, aliquots were taken, diluted 1:100 to 1:500 in TBS containing 2% BSA and 5 mmol/L CaCl2 , and assayed for procoagulant factor V(a) activity using factor V-deficient plasma as described above. Factor V incubated in the absence of thrombin was used as a control.
SDS-PAGE.SDS-PAGE analyses were made according to Laemmli.25 Samples containing 2 μg of factor V, with or without treatment with the indicated glycosidases, were run on 5% SDS-polyacrylamide gels and stained with Coomassie Blue G-250. In addition, samples (3 μg in 30 μL) of glycosidase-treated factor V were activated with 5 ng human thrombin for 1 hour at 37°C and then 500 ng of the deglycosylated factor Va was analyzed on 5% SDS-polyacrylamide gels using silver-staining.
Immunoblotting analysis.Single-chain human factor V (50 μL of 50 μg/mL) was incubated for 1 hour at 37°C in the presence or absence of neuraminidase (0.015 U/mL) and N-glycanase (0.1 U/mL), as described above. Human α-thrombin (57 ng) was added and incubated for 30 minutes to activate normal and deglycosylated factor V. After the addition of APC to a concentration of 14 ng/mL APC, 10-μL aliquots were sampled into SDS-buffer containing 10 mmol/L EDTA to quench the reaction at 0, 5, 10, 15, and 20 minutes. In parallel experiments, phospholipid vesicles (40 μmol/L final concentration) containing 10% phosphatidyl serine/90% phosphatidyl choline were added before initiating the inactivation of factor Va by APC. After the samples were run on 9% SDS polyacrylamide gels under nonreducing conditions, they were transferred to nitrocellulose membranes and blocked with TBS/1% casein. A murine monoclonal antibody against the heavy chain of factor Va (4 μg/mL) was incubated with the membranes for 2 hours at room temperature. After washing the membranes, biotinylated antimouse IgG (1 μg/mL) was added for 1 hour at room temperature, followed by Streptavidin alkaline phosphatase for 30 minutes, and 5-bromo-4-chloro 3′-indolyl phosphate/nitro blue tetrazolium substrate for color development.
RESULTS
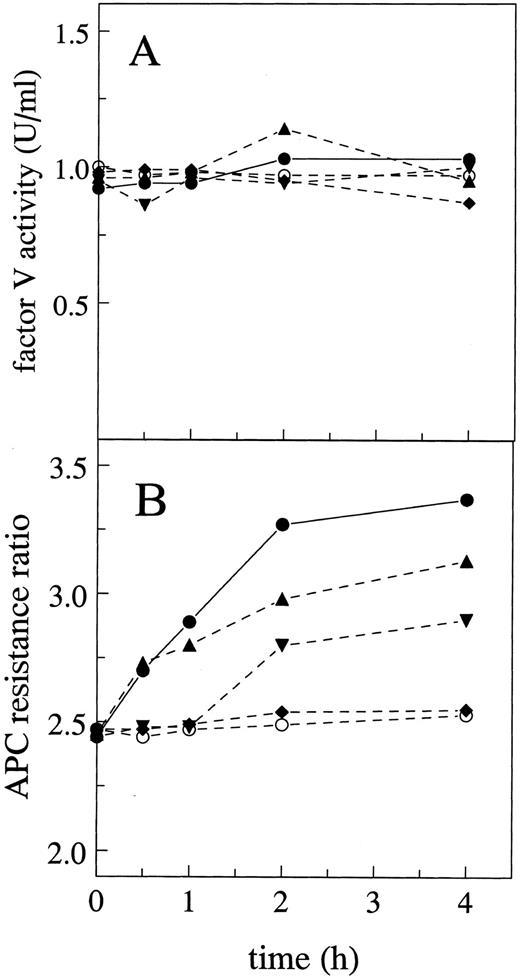
Effect of glycosidase treatment on factor V activity.The influence of highly purified glycosidases on the procoagulant activity of factor V was tested. None of the treatments with neuraminidase, N-glycanase, O-glycanase, or a combination of neuraminidase and N-glycanase affected the activity of factor V in a PT coagulation assay and the activity of nontreated factor V was constant during the 4 hours of incubation at 37°C (Fig 1A). To eliminate the effect of protease contamination of the glycosidase reagents, a pretreatment of glycosidases with phenylmethylsulfonyl fluoride (PMSF ) was made before incubation with factor V. All samples were analyzed on SDS-PAGE and no proteolysis was detectable on the gels after treatment with glycosidases (see below). In controls to assess the influence of glycanases on the clotting assays, after the addition of the different glycanases and factor V, the samples were mixed and immediately assayed for factor V activity using factor V-deficient plasma. There were no effects of the various glycanases on the PT or APTT clotting times or on the APC resistance ratio values, showing that these enzymes themselves did not affect any clotting tests.
Effects of glycosidase treatment on factor V procoagulant activity and inactivation by APC. (A) Procoagulant activity of purified factor V was determined using a PT clotting assay with factor V-deficient plasma after preincubation of factor V with neuraminidase (▴), N-glycanase (▾), O-glycanase (♦), or buffer (○) or with a mixture of neuraminidase and N-glycanase (•) for the indicated time periods. (B) APC resistance tests were performed according to the Materials and Methods using the glycosidase-treated purified factor V samples as described in (A).
Effects of glycosidase treatment on factor V procoagulant activity and inactivation by APC. (A) Procoagulant activity of purified factor V was determined using a PT clotting assay with factor V-deficient plasma after preincubation of factor V with neuraminidase (▴), N-glycanase (▾), O-glycanase (♦), or buffer (○) or with a mixture of neuraminidase and N-glycanase (•) for the indicated time periods. (B) APC resistance tests were performed according to the Materials and Methods using the glycosidase-treated purified factor V samples as described in (A).
Effects of glycosidase treatment on factor V inactivation by APC.The effectiveness of the inactivation of factor V(a) by APC was measured as the APC resistance ratio using an APTT assay as previously described,9 with minor modifications.13 20 Briefly, glycosidase-treated or control factor V was added to factor V-deficient plasma and APTT assays were performed in the presence or absence of APC (6 nmol/L final concentration). The APC resistance ratio for all samples was determined as the ratio of APTT values. When factor V was preincubated with neuraminidase for 4 hours, the ratio increased from 2.45 ± 0.1 to 3.13 ± 0.23 (Fig 1B). With N-glycanase treatment of factor V, the APC ratio increased from 2.44 ± 0.09 to 2.90 ± 0.21 after 4 hours of preincubation. A mixture of neuraminidase and N-glycanase produced a large upward effect on the APC ratio, from 2.44 ± 0.07 to 3.37 ± 0.33. O-glycanase treatment of factor V had no effect on the APC ratio. For untreated control factor V, no significant variation in the ratio was observed during 4 hours of incubation at 37°C (2.46 ± 0.07 to 2.56 ± 0.13; Fig 1B).
Influence of factor V levels on the ratio of APC inactivation.To determine if the amount of factor V present during the APTT clotting assay influenced the APC resistance ratio, varying amounts of purified factor V (0 to 10 μg/mL) or normal plasma as a factor V source (0% to 50%) were added to factor V-deficient plasma, and the APC resistance ratios were obtained. Although at lower factor V concentrations the clotting times with and without APC were prolonged, the APC resistance ratio did not change over a wide range of factor V concentrations (data not shown).
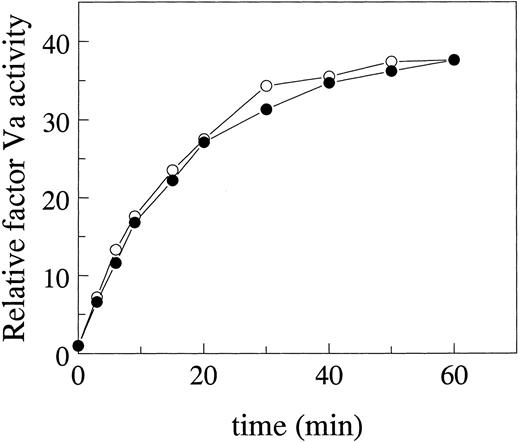
Factor V activation.To investigate the possibility that the increased APC resistance ratio of glycosidase-treated factor V was due to an altered activation of factor V, factor V (100 μg/mL) with or without glycosidase pretreatment was subjected to thrombin activation. Aliquots were collected after various times of thrombin activation and the factor Va procoagulant activity was determined with a PT assay using factor V-deficient plasma. The time courses and maximal extent for activation of deglycosylated factor V and normal factor V were identical, as both deglycosylated and normal factor V were activated similarly by thrombin, approximately 37-fold compared with untreated factor V (Fig 2).
Effect of glycosidase treatment of factor V on its activation by thrombin. Factor V was preincubated with neuraminidase plus N-glycanase according to the Materials and Methods and then activated by thrombin. The time course of activation of factor V treated with glycosidases was followed (○) and compared with the control nontreated factor V (•). Factor Va procoagulant activity was determined in PT assays using factor V-deficient plasma and expressed relative to the activity of single-chain factor V that was not activated by thrombin.
Effect of glycosidase treatment of factor V on its activation by thrombin. Factor V was preincubated with neuraminidase plus N-glycanase according to the Materials and Methods and then activated by thrombin. The time course of activation of factor V treated with glycosidases was followed (○) and compared with the control nontreated factor V (•). Factor Va procoagulant activity was determined in PT assays using factor V-deficient plasma and expressed relative to the activity of single-chain factor V that was not activated by thrombin.
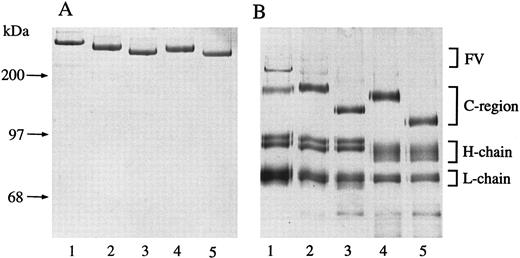
SDS-polyacrylamide electrophoresis.To characterize qualitatively the deglycosylation reactions, factor V was treated with different glycosidases and the products were analyzed using PAGE. The mobility of human factor V treated with glycosidases increased moderately compared with native factor V, but no proteolytic cleavage was observed in any of the preparations using Coomassie blue staining (Fig 3A, lanes 1 through 5). When the glycosidase-treated forms of factor V were subjected to thrombin activation, the heavy chain of factor Va showed increased mobility due to neuraminidase and N-glycanase treatment (Fig 3B, lane 4). O-glycanase treatment caused increased mobility of the connecting region of factor V, but there was no obvious change in the mobility of the heavy chain of factor Va (Fig 3B, lane 3). None of the treatments affected the mobility of the light chain of factor Va significantly. These results are related to previously reported data5 and suggest that the heavy chain of factor Va contained most of the N-linked carbohydrate side chains, whereas the connecting region of factor V contained most of the O-linked carbohydrate side chains.
Electrophoretic mobility of glycosidase-treated factor V. (A) Purified factor V on 5% SDS-PAGE stained with Coomassie blue G-250. Native factor V (lane 1); neuraminidase-treated factor V (lane 2); neuraminidase, O-glycanase–treated factor V (lane 3); neuraminidase, N-glycanase–treated factor V (lane 4); and neuraminidase, O-glycanase, N-glycanase–treated factor V (lane 5). (B) Silver-stained 5% SDS-PAGE analysis of thrombin-activated factor V samples described in (A).
Electrophoretic mobility of glycosidase-treated factor V. (A) Purified factor V on 5% SDS-PAGE stained with Coomassie blue G-250. Native factor V (lane 1); neuraminidase-treated factor V (lane 2); neuraminidase, O-glycanase–treated factor V (lane 3); neuraminidase, N-glycanase–treated factor V (lane 4); and neuraminidase, O-glycanase, N-glycanase–treated factor V (lane 5). (B) Silver-stained 5% SDS-PAGE analysis of thrombin-activated factor V samples described in (A).
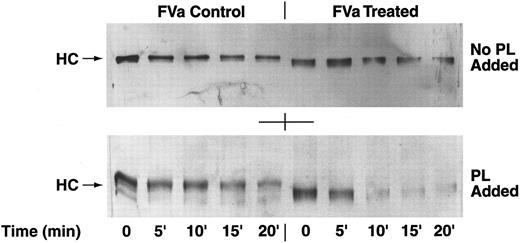
APC cleavage of the heavy chain of human deglycosylated factor Va.Immunoblotting studies using specific monoclonal anti-heavy chain factor Va antibodies were performed to analyze the rate of APC cleavage of the heavy chain of glycosidase-treated and untreated human factor Va. After the treatment of factor V with glycosidases and activation by human α-thrombin, APC was added and aliquots were taken at different time points. The cleavage of the factor Va heavy chain by APC was studied in the presence and absence of anionic phospholipid vesicles (Fig 4). Based on an estimate of the intensity of the 94,000 molecular weight factor Va heavy chain bands on the immunoblots in the absence of phospholipids, we observed a slight but similar decrease in intensities of factor Va heavy chains for glycosidase-treated and untreated factor Va (Fig 4, top panel). However, in the presence of phospholipid vesicles, the cleavage of the factor Va heavy chain was more pronounced and twofold to fourfold faster for the deglycosylated factor Va compared with nontreated factor Va, because the heavy chain of the control factor Va was still present at 20 minutes, whereas the heavy chain of the deglycosylated factor Va disappeared after 5 minutes (Fig 4, bottom panel). Because of the inability of the monoclonal antibody to recognize APC-derived fragments of the heavy chain of factor Va, only the intact factor Va heavy chains are seen (Fig 4).
APC cleavage of the heavy chain of factor Va. (Top Panel) Purified factor V was treated with glycosidases, activated with thrombin, and incubated with APC for 0, 5, 10, 15, and 20 minutes without the addition of phospholipids under the conditions described in the Materials and Methods. Disappearance of the factor Va heavy chain indicates cleavage by APC. The samples were run on SDS-PAGE and blotted with specific anti-heavy chain antibodies. (Bottom Panel) Reaction conditions were the same for the samples seen in the top panel, except for the presence of phospholipid vesicles (phosphatidyl serine:phosphatidyl choline = 1:9). In both panels, the position of migration of the heavy chains (HC) is indicated by the arrows. Note that the deglycosylated heavy chain migrated further than the untreated control heavy chain.
APC cleavage of the heavy chain of factor Va. (Top Panel) Purified factor V was treated with glycosidases, activated with thrombin, and incubated with APC for 0, 5, 10, 15, and 20 minutes without the addition of phospholipids under the conditions described in the Materials and Methods. Disappearance of the factor Va heavy chain indicates cleavage by APC. The samples were run on SDS-PAGE and blotted with specific anti-heavy chain antibodies. (Bottom Panel) Reaction conditions were the same for the samples seen in the top panel, except for the presence of phospholipid vesicles (phosphatidyl serine:phosphatidyl choline = 1:9). In both panels, the position of migration of the heavy chains (HC) is indicated by the arrows. Note that the deglycosylated heavy chain migrated further than the untreated control heavy chain.
Bovine factor V deglycosylation and APC resistance.To gain insight in the possible involvement of specific carbohydrate attachment sites in the heavy chain of human factor Va in the protection against APC, bovine factor V was used in a comparative study. Bovine factor Va contains 7 potential N-linked glycosylation sites in its heavy chain,26 of which 5 are in common with the 9 potential sites in the human factor Va heavy chain (Asn 211, 269, 354, 432, and 526; human factor V numbering). Bovine factor V and human factor V were treated with a neuraminidase and N-glycanase mixture to compare the effects of the glycosidase treatment on the susceptibility of human and bovine factor V/Va to inactivation by APC as reflected in the APC resistance ratio. When human factor V was incubated with the neuraminidase plus N-glycanase mixture, the ratio increased from 2.10 ± 0.09 to 3.49 ± 0.11 after 3 hours of treatment (Fig 5), similar to other results seen in Fig 1. In contrast, the APC resistance ratio of bovine factor V increased only slightly after 3 hours of incubation with the neuraminidase plus N-glycanase mixture (2.06 ± 0.06 to 2.34 ± 0.07; Fig 5). The mobility of the heavy chain of bovine factor Va also increased after glycosidase treatment (results not shown), as was observed for human factor Va (Fig 3B). For the control nontreated human and bovine factor V, no significant increase of the APC resistance ratio was observed during the 3 hours of incubation.
Comparison of APC resistance ratios for deglycosylated human and bovine factor V. Purified human (○, •) and bovine (▵, ▴) factor V were treated with neuraminidase and N-glycanase under conditions as in Fig 1 and then APC resistance ratios were determined after diluting factor V ([•, ▴] glycosidase-treated; [○, ▵] untreated controls) in factor V-deficient plasma using APTT assays.
Comparison of APC resistance ratios for deglycosylated human and bovine factor V. Purified human (○, •) and bovine (▵, ▴) factor V were treated with neuraminidase and N-glycanase under conditions as in Fig 1 and then APC resistance ratios were determined after diluting factor V ([•, ▴] glycosidase-treated; [○, ▵] untreated controls) in factor V-deficient plasma using APTT assays.
DISCUSSION
The factor V polymorphism R506Q is a prothrombotic risk factor and results in resistance to APC inactivation.9-19 Purified Gln506-factor Va exhibits subnormal susceptibility to proteolytic inactivation by APC because a normal APC cleavage site at Arg 506 is altered although inactivation cleavage at Arg 306 in factor Va can occur.7,8,13,18,19 The R506Q polymorphism is found in the majority of APC-resistant thrombotic patients; however, there is a subgroup of APC-resistant patients who lack the factor V mutation.10,11,17 20 Multiple causes of APC resistance, genetic or acquired, may potentially contribute to this phenomenon. In this study, we investigated the influence of the carbohydrates of factor V on its inactivation by APC using the APC resistance assay.
Among glycoproteins, genetic intraspecies polymorphisms are commonly found that may affect oligosaccharide structures. Factor V's carbohydrate side chains were suggested to play an important role in its functional activity.27 Different glycosidases were used at low concentrations under nondenaturing conditions to deglycosylate factor V. The resulting deglycosylated factor V was identical to native factor V in its procoagulant activity and in its rate of activation by α-thrombin. A time-dependent increase of the APC resistance ratio was observed when factor V was incubated with neuraminidase at 37°C. This effect was enhanced by additional cotreatment with N-glycanase. In contrast, incubation of O-glycanase alone with factor V had no effect on the APC resistance ratio.
Thus, we hypothesize that removal of N-linked carbohydrates from human factor V by the combination of neuraminidase and N-glycanase increases markedly its susceptibility to inactivation by APC without altering its procoagulant activity. SDS-PAGE studies show that removal of N-linked carbohydrates from factor V alters mainly the heavy chain of factor Va, whereas treatment of factor V with neuraminidase and O-glycanase largely affects the mobility of the connecting region. These effects of glycosidases on the mobility of factor Va polypeptide chains agree with previous reports.3 Because the heavy chain contains Arg 306 and 506, the primary targets for proteolytic inactivation by APC,7 it is reasonable that removal of heavy chain N-linked sugars could affect APC inactivation. The reason that removal of the O-linked carbohydrate moiety from factor V did not affect the APC resistance ratio is that the majority of O-linked carbohydrates are in the connecting region that is not present in factor Va.
Although others have reported that neuraminidase treatment resulted in an approximately twofold increase of factor V procoagulant activity,27,28 we did not observe such increase when factor V was incubated with neuraminidase. Treatment of factor IX or factor IXa with neuraminidase neither decreased the rate of factor IX activation nor the proteolytic properties of human factor IXa. Factor X showed no detectable loss of clotting activity in the PT assay after desialation.29 Impairment of factor V activation is reportedly caused by complete deglycosylation,27 probably due to the harsh experimental conditions used for removal of sugars. Thus, the mild conditions used in our study including physiologic pH and ionic strength under nondenaturing conditions together with low amounts of high-purity glycosidases that were pretreated with PMSF likely resulted in full preservation of procoagulant activity and thrombin activation of the deglycosylated factor V compared with normal factor V.
A decrease in factor V levels or specific activity by glycosidase treatment cannot explain the observed effects on the APC resistance ratio because the coagulant activity of factor V was not affected by deglycosylation and because the APC resistance ratio is independent of factor V levels, similar to a previous report.30 Moreover, no proteolysis of factor V on SDS-PAGE was observed (Fig 3A), ruling out effects due to proteases contaminating the glycosidases.
Deglycosylation of the heavy chain of human factor Va increased approximately twofold to fourfold its susceptibility to cleavage by APC based on an estimate of the time-dependent disappearance of the heavy chain of factor Va in immunoblot experiments. The increased rate of the heavy chain cleavage was observed in the presence but not in the absence of anionic phospholipids. Because Arg 306 cleavage in the heavy chain of factor Va is phospholipid-dependent,7 this suggests that the cleavage of Arg 306 by APC is involved in the observed phenomenon. It appears that removal of one or more N-linked carbohydrate side chains results in better access of APC to the region of Arg 306 located in the sequence connecting the A1 and A2 domains of the heavy chain. Because we have not characterized the APC cleavage products of deglycosylated factor Va in the present study, we cannot exclude the possibility that deglycosylation exposes cryptic APC cleavage sites in the heavy chain that contribute to the inactivation of the cofactor.
Bovine factor V has some different potential carbohydrate attachment sites compared with human factor V.2,3,5,6 26 To help identify possible specific carbohydrate attachment sites in human factor V that might affect APC inactivation, bovine factor V was subjected to glycosidase treatment under the same conditions as human factor V. Interestingly, glycosidase treatment of bovine factor V did not result in an increase of susceptibility towards inactivation by APC as seen for human factor V. Bovine factor V is known to have two potential carbohydrate attachments less than human factor V in the heavy chain.
Comparison of the sequence of human and bovine factor V show that both species have 5 identical potential carbohydrate attachment sites (Asn 211, 269, 354, 432, and 526), whereas human factor V has 4 additional potential sites (Asn 23, 27, 440, and 639) and bovine factor V has two additional attachment sites (Asn 197 and 560).1,2,6 26 Although other explanations are possible, it is suggested that N-linked carbohydrates at Asn 23, 27, 440, and/or 639 in human factor V may impair APC inactivation. Future site-directed mutagenesis studies using point mutations of each of the 4 specific attachment sites (Asn 23, 27, 440, and 639) not present in bovine factor V could clarify whether the carbohydrate chains at one or more of these sites give rise to protection against inactivation by APC.
Different glycoforms of factor V have been identified with distinct carbohydrate content28,31 and heterogeneity in the amount of sialic acid present.4 Because the content and composition of the carbohydrate side chains of glycoproteins21-23 may be different among different individuals,31 heterogeneity in sialic acid or carbohydrate content might cause different susceptibilities of factor Va to APC. Sialic acid is known to protect some glycoproteins from cleavage by proteases.32
In summary, this study suggests that variability in N-linked carbohydrate attached to the heavy chain region of factor V could account for some of the known variability in APC resistance ratios, including low values among subjects who lack the R506Q factor V polymorphism.10-12,20 33 Moreover, excessive or abnormal carbohydrate attachment to factor V, or perhaps factor VIII, might contribute to acquired APC resistance, whereas subnormal levels of carbohydrate might enhance sensitivity to APC.
Supported in part by grants from the National Institutes of Health (R37HL52246 and RO1HL21544) and the Netherlands Organization for Scientific Research (NWO: S94-140) to T.M.H.
Address reprint requests to John H. Griffin, PhD, Department of Molecular and Experimental Medicine, SBR-5, The Scripps Research Institute, 10550 N Torrey Pines Rd, La Jolla, CA 92037.

![Fig. 5. Comparison of APC resistance ratios for deglycosylated human and bovine factor V. Purified human (○, •) and bovine (▵, ▴) factor V were treated with neuraminidase and N-glycanase under conditions as in Fig 1 and then APC resistance ratios were determined after diluting factor V ([•, ▴] glycosidase-treated; [○, ▵] untreated controls) in factor V-deficient plasma using APTT assays.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/12/10.1182_blood.v89.12.4348/3/m_bl_0038f5.jpeg?Expires=1767696332&Signature=SgVJiimm1SWZqhA4EJVoj3kdDXZEL-EE1IERpBaYi8p797VxnTEROtEVj0bTZwWuImTxVAEQkhT6FxGxj9MSzuF8614tPWjDSh9LYk2szkvUgHWRRC0w0DYbfRZkNc5k6AZntLfblVhlWqPsoaMa2F79A4eECdj5oVrG3122YQAI77XJsUbF4jmgyiuAYx5J~EIoTR5wvk5hrcucOBMyb-WYW8msOMYBJzDEQpvMH1J2A83yP~CeyfxccrDAxiEorBphdRBI3Gl5qMv5K0XJD-l3~KiqVPcM33pG5Qc7P5wjK901fgHpZRAFuNz-sIUWyta1JdqybveaecYSr0YQvw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal