Abstract
The OX-40 molecule is expressed on the surface of recently activated T lymphocytes. The presence of OX-40 on CD4+ T cells was analyzed in a rat haplo-identical (parental → F1) bone marrow transplant model of acute graft-versus-host disease (aGVHD). Increased numbers of activated CD4+ T cells that expressed the OX-40 antigen were detected in peripheral blood soon after transplantation before the earliest sign of disease. The peak of OX-40 expression occurred 12 days posttransplantation with a range of 18% to 36% of circulating T cells and remained 10-fold above background, never returning to baseline. A slight increase in OX-40 expression (range, 1% to 6%) was also detected on peripheral blood lymphocytes from control syngeneic F1 → F1 recipients. OX-40+ T cells were isolated from spleen, skin, lymph node, and liver tissue of rats undergoing aGVHD, but not in syngeneic transplants. OX-40+ T cells isolated from these tissues were of donor origin and were shown to be allo-reactive. These data raise the possibility of using the OX-40 antibody to detect and deplete selectively the T cells that cause aGVHD.
GRAFT-VERSUS-HOST disease (GVHD) is a major and often lethal consequence of allogeneic bone marrow transplantation (BMT) therapy. The disease occurs when there are major or minor1-4 histocompatibility antigen differences between the BM donor and the recipient of the transplant. GVHD is caused by mature T cells present in the transplanted marrow, as well as other minor cell populations.5,6 Prophylaxis of GVHD is achieved by pan-T–cell immunosuppressive agents such as cyclosporine, corticosteroids, or methotrexate; these agents are associated with significant morbidity. Current clinical approaches to decrease GVHD disrupt normal T-cell activation or proliferation and include the administration of cytokines,7-10 immunotoxin to the interleukin-2 (IL-2) receptor,11-13 and CTLA4:Ig.14-16 These approaches are based on the premise that the targeting of T cells actively involved with allorecognition could potentially lead to novel therapeutic interventions in GVHD without inhibiting the entire T-cell repertoire.
The OX-40 molecule is a 50-kD glycoprotein that is expressed on recently activated CD4+ T cells. The OX-40 receptor was originally identified by the monoclonal antibody, OX-40, and was shown to be expressed on T cells activated during a mixed lymphocyte reaction (MLR), the in vitro correlate of GVHD.17 Through DNA sequence analysis, OX-40 was shown to be a member of the tumor necrosis factor (TNF) receptor family18 that includes TNFR, Fas, CD27, CD30, CD40, 4-1BB, and other gene products.19 The members of this family play a critical role in either activation/mitogenesis or programmed cell death of lymphocytes. The ligand for OX-40 was found on activated antigen presenting cells20 and the interaction of OX-40 with its ligand was shown to deliver a potent costimulatory signal to the CD4+ T cell.21 22
Recent studies showed that the OX-40 molecule could be found on activated T lymphocytes involved in recognizing autoantigen at the site of inflammation in autoimmunity.23-25 In an experimental autoimmune encephalitis (EAE) model, autoreactive T cells isolated from the target organ and the spinal cord expressed OX-40, whereas those autoreactive T cells from a non-target tissue were OX-40 negative.23 Furthermore, the disease course was ameliorated by treating animals with an OX-40–specific immunotoxin.24 These results suggested that OX40 might be a particularly useful surface marker to assess T-cell activation in GVHD. Therefore, an analysis of OX-40 expression on T cells in rats undergoing haplo-identical BMT was performed to determine whether allospecific T cells could be detected in vivo through the use of this marker. We show here that OX-40 was highly expressed on donor T cells isolated from peripheral blood lymphocytes (PBL), spleen, and other target organs during acute GVHD (aGVHD) and that these T cells were alloreactive.
MATERIALS AND METHODS
Rats.Female Buffalo or (Lewis X Buffalo)F1 rats 8 to 12 weeks of age were used throughout the study. Rats were originally purchased from Harlan Sprague-Dawley, Inc (Indianapolis, IN) and those used here were obtained from the VA breeding colony. DA animals were provided by Dr David Hinrichs (Portland VA Medical Center [VAMC], Portland, OR). All rats were housed at the VAMC veterinary medicine unit (VMU; Portland, OR) and were cared for according to institutional guidelines with free access to food and water.
Induction of GVHD.Recipients (3 per group) were prepared for transplant with 600 rads γ irradiation using a Mark 168A Irradiator (J.L. Sheperd and Associates, San Fernando, CA). BM cells were obtained from the femurs and tibias. Lymph node (LN) cells were obtained from the inguinal, superficial cervical, and superior mesenteric nodes. Buffalo or (Lewis X Buffalo)F1 BM (2 × 107) and LN cells (5 × 107) were injected intravenously into (Lewis X Buffalo)F1 recipients (P → F1 or F1 → F1, respectively). All animals were monitored during the disease and euthanized when identified in the near moribund state, as determined by the onset of diarrhea, according to VMU protocol. All recipients of allogeneic lymphoid cells (24/24) used in these experiments developed severe GVHD and did not survive beyond day 29 posttransplantation. Average weight loss in this model on day 15 posttransplantation was 15% ± 3% for allografted rats and 2.7% ± 1% for syngeneic rats.
Fluorescence-activated cell sorting (FACS) analysis.All staining was performed in RPMI 1640 (GIBCO BRL, Gaithersburg, MD) containing 2% fetal bovine serum (Tissue Culture Biologicals, Tulare, CA) at room temperature. Anti-CD4:phycoerythrin (PE) and anti-OX40: fluorescein isothiocyanate (FITC) were from PharMingen (La Jolla, CA). The OX-40 antibody (IgG2b ) was from murine hybridoma clone OX-40, as originally described by Paterson et al.17 The degree of chimerism, ie, the proportion of donor cells, was determined by using the allotype-specific rat antirat antibody, RT7.1, that recognizes allelic differences in the leukocyte common antigen (CD45) on Lewis and F1 lymphocytes. Thus, only donor (Buffalo) cells are negative for this antigen. Unconjugated RT7.1 monoclonal rat antibodies (Bioproducts, Indianapolis, IN) were detected on the cell surface of the rat lymphocytes with a goat antirat-Ig:FITC (Cappel, Durham, NC). The stained cells were analyzed by FACScan (Becton Dickinson, San Jose, CA).
Cell sorting.All of the OX-40–dependent separations were performed with MACS columns (Miltenyi Biotech, Sunnyvale, CA). The lymphocytes isolated from rats with GVHD were stained with an OX-40:FITC antibody, washed, and incubated with anti-IgG2a+b microbeads (Miltenyi Biotech). The cells were then passed through a magnetized column. The bound cells were eluted by removal of the column from the magnet and analyzed on the FACScan for purity.
T-cell lines.Sorted T cells were maintained by culturing in RPMI containing 10% (vol/vol) fetal calf serum, 10 mmol/L HEPES, 2 mmol/L L-glutamine, 5 × 105 mol/L 2-mercaptoethanol, penicillin (100 U/mL), and streptomycin (100 μg/mL). Cells were initially cultured in the presence of 20 U human recombinant IL-2 per milliliter.
CD4+ T-cell proliferation assays.For the allospecific proliferation assays described for the T-cell lines, 2 × 104 T cells were incubated with medium alone (control) or with 106 irradiated splenocytes from Buffalo, DA, or F1 at 37°C in 7% CO2 . The cells were cultured in the presence of 10% fetal calf serum or 1% Buffalo serum to eliminate the possibility of antigenic stimulation by foreign proteins. The cultures were incubated for 72 hours, with the last 18 hours being in the presence of 0.5 μCi 3H-TdR (6.7 Ci/mmol). The cells were harvested onto glass fiber filters and TdR uptake was assessed by liquid scintillation. The data are reported as the mean counts per minute (cpm) from triplicate wells.
Tissue preparation.PBL were obtained from the lateral tail vein and freed of red blood cells with TRIS-buffered ammonium chloride before use. Spleens were obtained and single-cell suspensions prepared by passage through a wire screen. Liver was similarly processed with large clumps and debris being removed by 1 g sedimentation. Skin-derived T cells were obtained by digesting ear tissue with an enzymatic mixture of collagenase, hyaluronidase, and DNAse in Hank's Balanced Salt Solution.26
Statistical analyses.Data from these studies were analyzed using the STATVIEW 512+ program (Brainpower Inc, Calabasas, CA) on a Power Macintosh 7200/75 computer (Apple Computer Inc, Cupertino, CA); comparisons included analysis of variance and the Student's t-test.
RESULTS
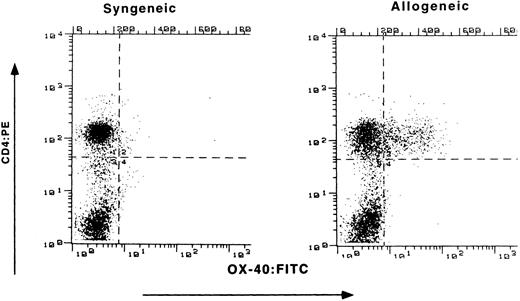
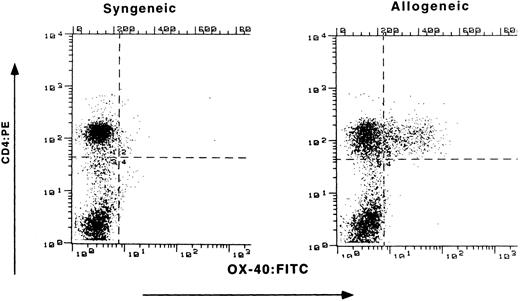
Expression of OX-40 on PBL of rats with GVHD.Buffalo parental BM and LN cells were transplanted into (Lewis X Buffalo)F1 rats to induce GVHD (P → F1), whereas syngeneic transfer served as a control (F1 → F1). Preliminary experiments determined the optimal numbers of LN cells to transfer for the induction of aGVHD. Recipients received 600 rad of whole body irradiation and underwent transplantation in groups of three throughout these studies. Initial flow cytometry analysis for OX-40 expression on circulating PBL was conducted at the first sign of disease (Fig 1). Both control and allografted animals had similar percentages of CD4+ T cells (52% and 59%, respectively), as seen on the ordinate. However, differences were observed for OX-40 expression (abscissa). The T cells isolated from the peripheral blood of allografted rats were 29% OX-40+/CD4+, whereas only 2% of the peripheral blood T cells from control (F1 → F1) rats expressed OX-40.
Expression of OX-40 on PBL during GVHD. (Lewis × Buffalo)F1 rats were injected with either syngeneic F1 (left panel) or allogeneic Buffalo (right panel) BM and LN cells, as described in the text. The percentage of PBL staining with OX-40:FITC (absissa) and CD4:PE (ordinate) was determined 10 days posttransplantation as described in the Materials and Methods.
Expression of OX-40 on PBL during GVHD. (Lewis × Buffalo)F1 rats were injected with either syngeneic F1 (left panel) or allogeneic Buffalo (right panel) BM and LN cells, as described in the text. The percentage of PBL staining with OX-40:FITC (absissa) and CD4:PE (ordinate) was determined 10 days posttransplantation as described in the Materials and Methods.
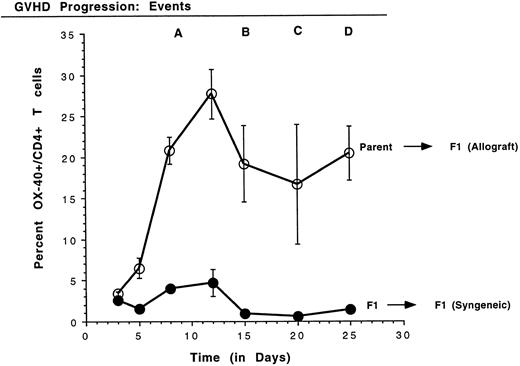
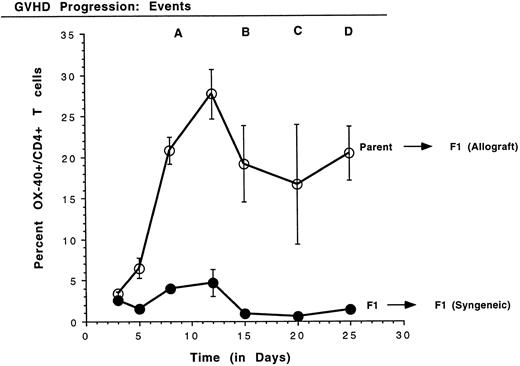
Kinetics of the expression of OX-40 on rat PBL in GVHD.Because high levels of OX-40+ T cells were detected in PBL of rats with aGVHD, the kinetics of T-cell activation, as manifest by OX-40 expression, were analyzed. Small volumes of peripheral blood were obtained periodically and PBL were stained with antibodies to OX-40:FITC and to CD4:PE. Four time-course experiments were conducted in which similar results were obtained and data from a representative experiment are shown in Fig 2. The mean and standard deviation of the percentage of CD4 cells that coexpress OX-40 from each group are shown. A statistically significant difference between T cells expressing OX-40 in the allografted and control transplant groups was observed as early as day 5 posttransplantation (P ≤ .05). The OX-40+ T cells peaked around day 12 for both allografted (28%) and control (5%) rats (P ≤ .005) and then declined. In contrast to the control rats, in which OX-40+ T-cell numbers returned to background (1.0 ± 0.1; n = 16), the number of OX-40+ T cells in allografted animals still remained elevated better than 10-fold over background. The first signs of GVHD in these animals appeared on day 10 in a near uniform pattern (±1 day), as manifest by periocular edema and erythroderma. Other late characteristic clinical events were reproducibly identified, as noted (Fig 2). Mortality for this group of animals (event D) was on days 25, 27, and 28. Thus, OX-40 was expressed on a high percentage of peripheral T lymphocytes in rats undergoing GVHD, but only transiently on a low percentage of T cells in syngeneic control rats.
Kinetics of OX40 expression on rat PBL. The percentage of CD4+ T cells expressing OX40 was determined on PBL by FACScan analysis using specific monoclonal antibodies on days 3, 5, 8, 12, 15, 20, and 25 posttransplantation. PBL were obtained from three rats per group and the data are presented as the mean ± standard deviation. Quadrants were set using nontransplanted Buffalo PBL as a negative control for OX-40 expression. GVHD was first detected on day 10 (event A) as erythroderma around the eyes. As the disease progressed, blistering of the feet and ear swelling (day 15, event B), hair loss (day 20, event C), and diarrhea (day 25, event D) were also observed.
Kinetics of OX40 expression on rat PBL. The percentage of CD4+ T cells expressing OX40 was determined on PBL by FACScan analysis using specific monoclonal antibodies on days 3, 5, 8, 12, 15, 20, and 25 posttransplantation. PBL were obtained from three rats per group and the data are presented as the mean ± standard deviation. Quadrants were set using nontransplanted Buffalo PBL as a negative control for OX-40 expression. GVHD was first detected on day 10 (event A) as erythroderma around the eyes. As the disease progressed, blistering of the feet and ear swelling (day 15, event B), hair loss (day 20, event C), and diarrhea (day 25, event D) were also observed.
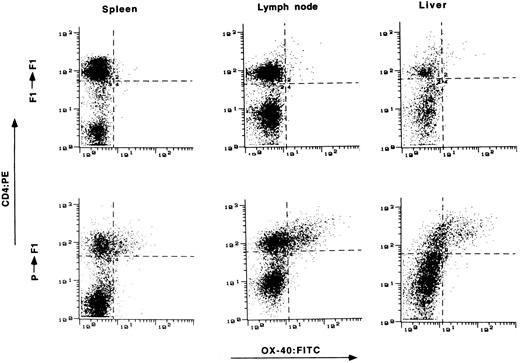
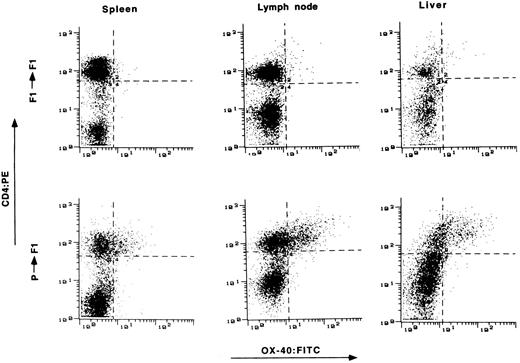
Expression of OX-40 on T cells from target tissue of GVHD.The presence of OX-40 on T cells in the target organs of GVHD was assessed. Tissue samples were obtained from animals 2 to 3 weeks posttransplantation and single-cell suspensions were prepared. The cells were stained with OX-40:FITC and CD4:PE and analyzed by FACS. Tissue from syngeneic transplanted rats served as a control. Typical results from spleen, lymph node, and liver are shown in Fig 3. OX-40 expression was found on increased numbers of T cells and at high surface expression levels in all three tissues of allotransplanted rats, but not in control syngeneic transplanted rats. Typically 20% to 50% of CD4+ T cells from these tissues were OX-40+ between day 15 and day 25 posttransplantation, showing that activated T cells were present in the tissues of allografted versus syngeneic transplanted animals.
The presence of OX-40+ T cells in tissues from rats with aGVHD. Splenocytes, liver, and lymph node cells were obtained on day 15 posttransplantation and stained with antibodies to CD4 (ordinate) and OX-40 (absissa). The top row represents tissue obtained from control syngeneic transplanted rats. The bottom row represents tissue obtained from allogeneic transplanted rats with aGVHD. Tissue from control rats had 3%, 4%, and 3% double-positive cells in spleen, lymph node, and liver, respectively. Tissue from rats with GVHD had 22%, 33%, and 42% double-positive T cells from spleen, lymph node, and liver, respectively.
The presence of OX-40+ T cells in tissues from rats with aGVHD. Splenocytes, liver, and lymph node cells were obtained on day 15 posttransplantation and stained with antibodies to CD4 (ordinate) and OX-40 (absissa). The top row represents tissue obtained from control syngeneic transplanted rats. The bottom row represents tissue obtained from allogeneic transplanted rats with aGVHD. Tissue from control rats had 3%, 4%, and 3% double-positive cells in spleen, lymph node, and liver, respectively. Tissue from rats with GVHD had 22%, 33%, and 42% double-positive T cells from spleen, lymph node, and liver, respectively.
Recent studies have shown OX-40 expression on activated CD8+ T cells.22 It can be reasoned, therefore, that alloreactive CD8+ T cells could be detected in our system as a CD4−/OX-40+ population in the lower right quadrant. The cytograms from PBL (Fig 1), spleen, lymph node, and liver (Fig 3) show only a minor percentage of cells with that phenotype in these tissues. This result was confirmed by direct assessment using CD8b:FITC and OX-40:PE reagents (data not shown).
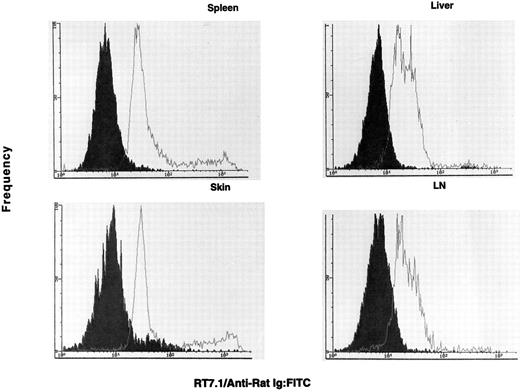
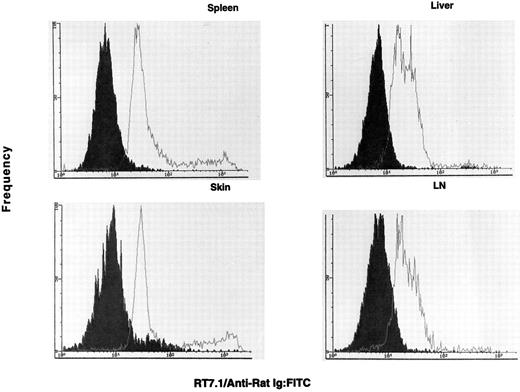
OX-40 is predominantly expressed on donor T cells in rats undergoing aGVHD.If the OX-40+ T cells observed in the allografted animals were involved in GVHD, they should be donor derived. To determine the origin of the activated T cells in allografted rats, lymphocytes were analyzed for the presence of the RT7.1 allotype and OX-40. The RT7.1− population identifies the donor cells, whereas an RT7.1+ population identifies recipient cells. Greater than 80% of the OX-40+ cells isolated from PBL (33% of total CD4+ cells) were of donor origin. Therefore, OX-40+ T cells were sorted from each of the target tissues mentioned above and cultured in low-dose IL-2. The sorted cells initially ranged between 70% and 90% OX-40+ and, after a few days of culture in low-dose IL-2, were ≥95% OX-40+. The OX-40+ cells from spleen, lymph node, skin, and liver were then analyzed for host or donor origin. PBL from syngeneic (F1 → F1) transplanted rats served as positive controls for RT7.1 staining. These data are shown in Fig 4. The majority of the OX-40+ sorted cells from each of these tissues were of donor origin (>90%), ie, they were RT7.1 negative. Skin was the only tissue to show an appreciable recipient component in the sorted T-cell populations (∼10%). Thus, the OX40+ T cells in the allografted rats were of donor origin.
The OX40+ cells are negative for RT7.1 and are therefore of donor allotype. The OX40+ cells (solid histogram) are negative for RT7.1, which indicates they possess the donor allele. The control F1 lymphocytes (open histogram) were positive for RT7.1, indicative of Lewis or (Lewis × Buffalo)F1 cells. The cell lines were stained on day 3 (spleen), day 10 (skin), day 14 (lymph node), and day 14 (liver) postsorting.
The OX40+ cells are negative for RT7.1 and are therefore of donor allotype. The OX40+ cells (solid histogram) are negative for RT7.1, which indicates they possess the donor allele. The control F1 lymphocytes (open histogram) were positive for RT7.1, indicative of Lewis or (Lewis × Buffalo)F1 cells. The cell lines were stained on day 3 (spleen), day 10 (skin), day 14 (lymph node), and day 14 (liver) postsorting.
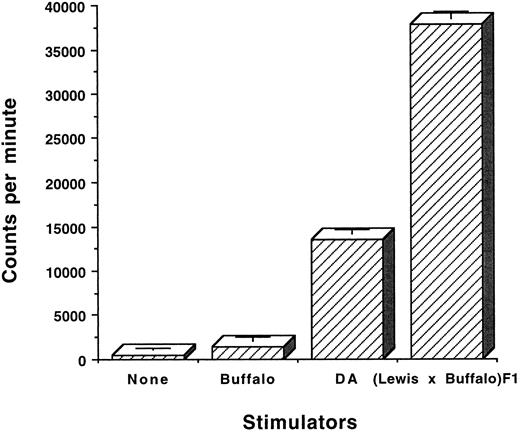
The OX-40+ T cells from rats with aGVHD are alloreactive.Two studies were performed to determine whether OX-40+ T cells were alloreactive. Sorted OX-40+ T cells were cultured in IL-2 and were then stimulated in vitro in an MLR and proliferation was assessed by the incorporation of 3H-thymidine. OX-40+ T cells (>95%) from the above-mentioned tissues were cultured in medium alone or in the presence of self (Buffalo), third party (DA), or allogeneic (F1) splenocytes. After 2 days in culture, 3H-thymidine was added and the cells were harvested 18 hours later. The results of one such experiment with OX-40+ T cells sorted from the liver are shown in Fig 5. The OX40 sorted donor T cells responded to F1 (37, 732 ± 858 cpm) and to a lesser extent to third party DA (13,494 ± 444 cpm) but not to self (Buffalo; 1,446 ± 437 cpm). Background (464 ± 236 cpm) was not subtracted. The difference between Buffalo versus F1 stimulation was statistically significant (P ≤ .0001). The difference between Buffalo responses to F1 and DA were also significantly different (P < .001). Primary MLR responses of Buffalo to F1 and DA were equivalent and not statistically different.
OX-40+ sorted cells are alloreactive. OX40 sorted cells from liver were cultured in low-dose IL-2 and allowed to lose expression of the OX-40 antigen. The cells (day 12 postsort) were then cultured at 2 × 104 cells/well in the presence of 105 stimulator cells from the rats strains listed. After 2 days, 3H-thymidine was added and the cultures were incubated for an additional 18 hours. The cells were harvested and 3H incorporation was measured by liquid scintillation. The data are presented as the mean ± standard deviation of triplicate samples.
OX-40+ sorted cells are alloreactive. OX40 sorted cells from liver were cultured in low-dose IL-2 and allowed to lose expression of the OX-40 antigen. The cells (day 12 postsort) were then cultured at 2 × 104 cells/well in the presence of 105 stimulator cells from the rats strains listed. After 2 days, 3H-thymidine was added and the cultures were incubated for an additional 18 hours. The cells were harvested and 3H incorporation was measured by liquid scintillation. The data are presented as the mean ± standard deviation of triplicate samples.
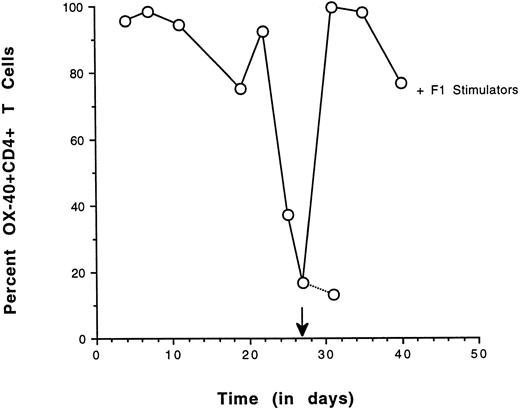
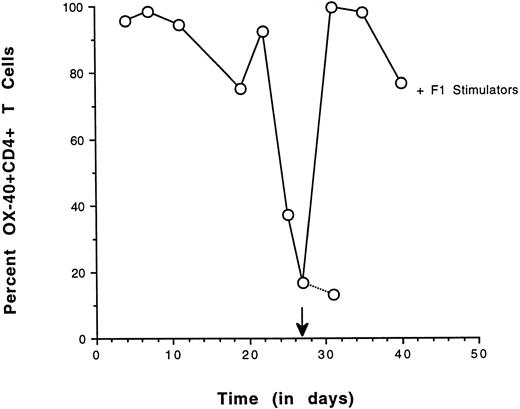
As a second approach, the ability of OX-40+ sorted T cells to re-express the OX-40 activation antigen after antigenic challenge was determined. OX-40 expression was followed over time in a sorted population and high levels could be maintained if IL-2 was supplemented in the medium. In cultures in which there was no IL-2, OX-40 surface expression decayed. If these sorted cells were alloreactive they should re-express the OX-40 activation antigen when cultured in the presence of F1 stimulator cells (alloantigen). Sorted splenic OX-40+ T cells were cultured until the surface expression of OX-40 was diminished. The culture was then restimulated with irradiated F1 splenocytes and OX-40 expression was reassessed 4 days later (Fig 6). The level of OX-40 expression had decayed to 16% and the cells were rechallenged with a 1:1 ratio of stimulator cells. Four days after stimulation, greater than 95% of the cells had re-expressed OX-40, indicative of alloreactivity. By day 13 poststimulation, greater than 75% of the cells were still OX-40+ (not shown). The re-expression of OX40 on the population of the previously OX40+ sorted cells after allostimulation was nearly uniform. Control studies in which cultures were challenged with autologous stimulators resulted in a modest (3%) increase in OX-40 expression (not shown). The results from these re-expression experiments were congruent with the MLR data and suggested that the donor-derived OX-40–expressing T cells were alloreactive.
Re-expression of OX40 on sorted CD4+ T cells by allospecific stimulation. OX40 sorted splenic T cells were cultured in vitro with low-dose IL-2 and assessed for OX-40 expression on the days indicated. After the addition of IL-2 on day 20, the population increased to greater than 95% positive in 3 days and OX40 expression decreased to 16% by day 27, at which time they were stimulated with F1 stimulator cells. After 4 days, greater than 95% of the cells were again OX40+. Control cultures had 13% positive cells.
Re-expression of OX40 on sorted CD4+ T cells by allospecific stimulation. OX40 sorted splenic T cells were cultured in vitro with low-dose IL-2 and assessed for OX-40 expression on the days indicated. After the addition of IL-2 on day 20, the population increased to greater than 95% positive in 3 days and OX40 expression decreased to 16% by day 27, at which time they were stimulated with F1 stimulator cells. After 4 days, greater than 95% of the cells were again OX40+. Control cultures had 13% positive cells.
DISCUSSION
This study shows the expression of the T-cell activation antigen, OX-40, on CD4+ T lymphocytes in rats destined to develop aGVHD. Activated CD4+/OX-40+ T cells were found in the peripheral blood by 5 days after transplantation in a preclinical stage and their numbers increased dramatically over time and remained high until the experiment was terminated. Activated OX-40+ T cells were found in skin, liver, and hematolymphoid organs, which are all target tissues of GVHD.27 Preliminary studies show that the final target organ of GVHD, the gut, also contains high levels of OX-40+ T cells (Tittle et al, unpublished data). The OX-40+ T cells isolated from animals with aGVHD were alloreactive. This was shown in a standard allospecific proliferation assay and by the induced re-expression of the activation marker by F1 stimulator cells. A minor third party response was noted consistent with the response of uncloned alloreactive T-cell populations.28 Whether the OX-40− fraction also retained F1 or third party reactivity has not been assessed. The re-expression of OX-40 on the entire population of sorted cells suggests that the precursor frequency for alloreactivity in that population is near unity. This possibility is currently being explored by limiting dilution analysis of sorted and unsorted T cells. A small increase in OX-40 expression was observed in rats receiving transplants of syngeneic BM and LN. Whether this transient expression was due to bacterial contamination or the effects of radiation is not known.
The majority of the OX-40+ T cells were shown to be donor in origin. A small (10%) population of OX-40+/RT7.1+ was observed (see skin, Fig 4), which may be indicative of a population of activated host T cells. Perhaps during the cytokine storm associated with aGVHD,1 the recipient's T cells can participate in the disease. Consistent with this hypothesis, host T cells were found in immunohistochemical studies of cutaneous GVHD induced by intradermal injection of cloned alloreactive T cells in the mouse,29 but their activation state was not studied. Further characterization will be required to determine whether these T cells are truly of the host allotype and what role they play in the induction or maintenance of the disease.
Laboratory models have shown that both CD4+ T cells in the case of single class II disparity and CD8+ T cells in the case of single class I disparity are capable of causing aGVHD. Berger et al30 have presented evidence that both CD4+ and CD4-dependent CD8+ T cells are involved in lethal GVHD against immunodominant minor histocompatibility antigens. Although the original description of the MRC OX-40 antibody showed expression limited to CD4+ T cells,17 Baum et al22 more recently found OX-40 on purified CD8+ T cells stimulated with anti-TCR antibodies, as detected by soluble OX-40 ligand. It is interesting that, in our in vivo model, only a small CD4−/OX40+ population was identified that may represent an activated CD8+ alloreactive T-cell subset. It remains to be determined whether this small fraction of cells is clinically significant.
This study was undertaken to assess the general applicability of findings obtained in an EAE model in which OX-40 was shown to be expressed selectively on the pathogenic T cells and further that an OX-40 immunotoxin administered in vivo could abrogate disease. In the case of EAE, OX-40 expression on encephalitogenic T cells isolated from the inflammatory compartment was shown to be transient.23 That model represents a self-limited disease and is associated with spontaneous recovery of the rats. In the GVHD model presented here, high numbers of OX-40+ T cells persist throughout clinical signs of disease (10-fold over background). Even after isolation and purification of these cells from endogenous antigen presenting cells (APC's) and other cells in situ, OX-40 was detected in vitro for over 20 days, provided that low-dose IL-2 was continuously supplied. It is not clear whether the continued high numbers of T cells expressing OX-40 in vivo is related to the nature of the stimulation signal, the persistence of the stimulation signal, or whether new alloreactive T-cell clones are being induced over time. It is also possible that the expression of the OX-40 ligand on antigen-presenting cells differs in the two models. To this end, a soluble form of OX-40 has been prepared to study and to identify surface expression of the OX-40 ligand on the antigen-presenting cells in aGVHD (Tittle et al, unpublished data). Nevertheless, the expression of OX-40 does not appear to be transient in these animals with aGVHD, nor after in vitro allo-stimulation. A slight decline in the numbers of T cells expressing OX-40 was noted after day 12 in every experiment to date. A significant decrease in the total number of circulating CD4+ T cells was noted in some of our experiments, whereas the number of circulating T cells in autografted rats remained stable (data not shown). This suggested that activated cells were trafficking to other target organs. Collectively, these data extend our findings in autoimmune disease23-25 and suggest that the OX40 antibody might have further utility for the diagnosis and treatment of GVHD as well as other posttransplantation diseases.
There are two different forms of GVHD, aGVHD and chronic GVHD (cGVHD). aGVHD is associated with a cascade of cytokines, including IL-1, IL-2, tumor necrosis factor β, and interferon γ.1 cGVHD is associated with an entirely different set of cytokines, predominantly IL-4, IL-5, and IL-10.31 We have identified the cytokines associated with the OX-40+ subset of CD4+ T cells as Th1 type, consistent with their role in aGVHD (data not shown). A recent report shows that mice with cGVHD could be switched to aGVHD by the delivery of costimulatory anti–OX-40 antibody. This suggests that OX-40+ T cells may play a role in cGVHD.32 Those data further suggest that the expression of the OX-40 ligand may contribute to the selection of either the acute or chronic pathway of disease.
Weinberg et al23 first determined that T cells that express the OX40 receptor were a reliable indicator of pathogenic T cells. This was demonstrated in the EAE adoptive transfer model in which OX40 expression was exclusively found in the target tissues on donor T cells. Subsequent analysis identified the OX-40+ T cells in actively immunized rats with EAE and demonstrated that these T cells were enriched for T cells expressing the T-cell receptor (Vβ8.2), which is specific for autoantigen.25 Although these activated T cells also expressed IL-2 receptors (IL-2R), the IL-2R was also expressed on T cells at other nontarget sites. Thus, the IL-2R was less able to distinguish autoimmune T cells. Perhaps this is due to the fact that IL-2R is upregulated by IL-2,33 which is known to be expressed in EAE.23 Alternatively, this could be due to the reported high background expression of the IL-2R (10% to 20% of normal T cells).13 In light of the limited efficacy of anti–IL-2R treatment for aGVHD,11-13 other selective T-cell depletion methods will be needed for the treatment of GVHD. Based on our findings in EAE23-25 and aGVHD, the OX-40 receptor might represent a more selective target molecule for depletion studies in aGVHD.
These findings may have significant implications for the diagnosis and possible treatment of GVHD. We have recently initiated an analysis of OX-40 expression on PBL from human recipients of allogeneic BMT. The OX-40 receptor was expressed on high numbers of circulating CD4+ T cells before the first symptoms of aGVHD as well as during aGVHD (Tittle et al, manuscript in preparation). In addition, a decrease in the numbers of OX-40+ T cells appeared to parallel a response of aGVHD to steroid treatment. Thus, a rapid staining procedure for OX-40 on PBL could be diagnostic for allogeneic BMT patients at risk of developing aGVHD. Whether targeted therapy to the OX-40 receptor could also be used for a selective treatment that would eliminate only the cells causing GVHD while allowing T cells with other nonself specificities to be unharmed is under investigation in our rat model.
ACKNOWLEDGMENT
The authors gratefully acknowledge Drs David Hinrichs and David Parker for critical review of this manuscript.
Equal contributions to this study were provided by T.V.T. and A.D.W.
Supported by an OHSU Foundation Grant and a VA Merit Award to R.T.M. A.D.W. was supported by funds from CANTAB (Cambridge, UK). C.N.S. was supported by an AOA Scholar Award.
Address reprint requests to Richard T. Maziarz, MD Division of Hematology and Medical Oncology, OHSU L580, 3181 SW Sam Jackson Park Rd, Portland, OR 97201.