Abstract
Recombinant human erythropoietin (rHuEPO) variants have been constructed to identify amino acid residues important for biological activity. Immunoassays were used to determine the effect of each mutation on rHuEPO folding. With this strategy, we could distinguish between mutations that affected bioactivity directly and those that affected bioactivity because the mutation altered rHuEPO conformation. Four regions were found to be important for bioactivity: amino acids 11 to 15, 44 to 51, 100 to 108, and 147 to 151. EPO variants could be divided into two groups according to the differential effects on EPO receptor binding activity and in vitro biologic activity. This suggests that rHuEPO has two separate receptor binding sites. Mutations in basic residues reduced the biologic activity, whereas mutations in acidic residues did not. This suggests that electrostatic interactions between rHuEPO and the human EPO receptor may involve positive charges on rHuEPO.
ERYTHROPOIETIN (EPO) is a hormone produced primarily by the kidney. It is involved in the growth and maturation of erythroid cells from precursors. Decreased production of EPO due to kidney failure results in anemia. The human EPO gene has been cloned,1,2 and the hormone has been produced by recombinant DNA techniques (recombinant human EPO [rHuEPO]). The human gene encodes a 165–amino acid mature protein. The secreted protein contains approximately 40% carbohydrate and has an approximate molecular weight of 30 kD.3 The carbohydrate has been shown to have an effect on protein stability and solubility, but it is not required for in vitro bioactivity.4 5 This indicates that the active site is retained in the protein portion of the molecule.
The mechanism by which EPO stimulates erythropoiesis is partly understood. EPO binds to specific cell-surface EPO receptors. Activation of the EPO receptor triggers intracellular signaling events including phosphorylation of the receptor, followed by activation of the JAK-STAT, RAS, and PI3 kinase pathways.6-11 These signaling pathways trigger cells to undergo proliferation and differentiation and to block apoptosis. Cloning of the murine and human EPO receptors12,13 and later identification of a constitutively active receptor mutant with an Arg129 to Cys mutation14,15 led to the proposal that dimerization of the receptor is responsible for its activation. This proposal was supported by the observation that bivalent monoclonal antibodies but not monovalent Fab fragments could activate the EPO receptor in the absence of EPO.16
If activation of the EPO receptor is indeed due to homodimerization, then EPO must be capable of simultaneously binding two receptors. The fact that rHuEPO exists in solution as a monomer suggests that it must have two receptor binding sites much like that of growth hormone. In this case, a growth hormone monomer activates its receptor by cross-linking two growth hormone receptors.17-19 However, for EPO, the location of the two binding sites has not been definitively identified.
Several strategies have been used to try to identify the active site of EPO. These studies included mapping of neutralizing antibody epitopes20-26 and analysis of the effect of deletions27,28 or point mutations21,29-32 on bioactivity. These studies implicate several regions that are important for bioactivity in EPO, Arg 14, residues between 100 and 110, and some residues near the C-terminus. Interpretation of the results of these experiments is difficult, because the crystal or nuclear magnetic resonance structures of EPO have not been reported. In addition, interpreting mutagenesis results is complicated by the difficulty of distinguishing between changes in bioactivity caused by changes in the active site and changes in protein folding that indirectly affect bioactivity. The effect of mutations on folding of EPO variants was not established in previous studies, except for an Arg103 to Ala mutation.31
We wished to accurately map the active site of rHuEPO and determine whether one or two binding sites could be identified. We constructed 141 different rHuEPO variants and tested them for biologic activity. We have previously reported the development of an immunologic approach that allowed testing of the effect of mutations on rHuEPO conformation.20,21 Analysis of the effect of mutations in rHuEPO on its immunoreactivity with this method has been used previously to identify buried residues in rHuEPO and those important for the rHuEPO structure. With this strategy, we have identified mutations that are either predicted to be on the exposed surface of rHuEPO or only modestly affect the rHuEPO structure. Thus, we have identified residues that appear to be part of the rHuEPO active site. According to models that suggest that rHuEPO is a four-helical bundle,20,27,33 34 the residues important for bioactivity can be localized to two different regions. This suggests that rHuEPO has two different binding sites.
MATERIALS AND METHODS
Construction and expression of rHuEPO variants.The rHuEPO cDNA was obtained from COS-1 cells transformed with a human rHuEPO genomic clone.2,35 The coding region was recovered as an 810-nucleotide BstEII-Bgl II DNA fragment and was cloned into an SV40 vector.4 The vector contained a dihydrofolate reductase (DHFR) gene for selection in Chinese hamster ovary (CHO) DHFR-cells. All the variants were expressed in COS-1 cells. Transfection was by either the calcium phosphate method20,36 or electroporation.20 Conditioned medium was collected after 3 to 5 days. Aliquots were made and stored at −70°C. In cases for which larger amounts of material were needed, stable CHO cell transformants were made. CHO cells were transfected by the calcium phosphate method, and transformants were selected for DHFR expression.
To construct rHuEPO variants, in vitro oligo-directed mutagenesis was performed.20 In brief, a DNA segment containing human EPO coding sequences was transferred to M13mp18, and single-stranded DNA was produced in a dut ung Escherichia coli strain (RZ1032). The single-stranded DNA was annealed with synthetic oligonucleotides containing the desired mutations, and was then extended with Klenow polymerase in the presence of T4 DNA ligase. The DNA was then transfected into E. coli strain JM109. Variants with the desired mutations were identified by differential hybridization using the mutant oligonucleotide. The presence of the mutations was confirmed by DNA sequencing, and then the DNA segment was transferred to the expression vector.
Analysis of rHuEPO expression.The rHuEPO concentration (units per milliliter) in conditioned medium for each rHuEPO variant was determined by four to six different immunoassays. The EPO standard used in the assays was rHuEPO produced in CHO cells. 125I-EPO was obtained from Amersham (Arlington Heights, IL). The isolation and characterization of antibodies and immunoassays has been described elsewhere.20,21 These immunoassays included a radioimmunoassay (RIA) using an antibody raised to purified rHuEPO (RIA-P); a RIA using an antibody raised to a synthetic peptide corresponding to the first 20 amino acids of rHuEPO (RIA-N); and enzyme-linked immunosorbent assays (ELISAs) using two different monoclonal antibodies that recognize different rHuEPO epitopes (EIA-D11 and EIA-F12). The ELISA format involved capture of rHuEPO by the monoclonal antibody, and then an incubation with one of two different signal polyclonal antibodies raised to rHuEPO. Two different polyclonal signal antibodies raised in goats or rabbits were available. The RIA-N immunoassay is largely unaffected by rHuEPO conformation. In addition, the epitopes recognized by these immunoassays have been determined.20 Therefore, it was possible to determine the rHuEPO concentration in samples by selecting assays that were unaffected by the introduced mutation. Each rHuEPO variant was also assayed with the monoclonal antibody 9G8A (RIA-9G8A). The specific 9G8A immunoreactivity was determined as units of 9G8A immunoreactive EPO divided by total units of immunoreactive EPO as determined earlier.
Increased immunoreactivity measured by RIA-9G8A is indicative of altered folding. Completely denatured rHuEPO yields an immunoreactivity measured by this antibody that was 20- to 40-fold higher than expected.21 In addition, immunoreactivity appears to increase in proportion to the degree of unfolding.20 Immunoreactivity measured by this assay that was 2.5 times higher than expected was considered indicative of altered folding.
Purification of rHuEPO variants.Conditioned medium from CHO cells expressing the various rHuEPO variants was purified as previously described.37
In vitro bioassays.In vitro bioactivity was determined by measuring thymidine uptake in a factor-dependent cell line, 32D,38,39 that had been made dependent on EPO for growth by introduction of a murine EPO receptor gene (32D + EPOR40 ). Assays were performed as previously described40 with the following modifications. 32D cells were factor-starved for 3 to 5 hours in assay medium (RPMI 1640 medium; GIBCO BRL, Grand Island, NY) containing 10% fetal bovine serum and 1% Glutamine Pen-Strep (Irvine Scientific, Santa Ana, CA) lacking EPO. Test samples or EPO standard (rHuEPO) were added to wells in a 96-well microtiter plate; 50 μL starved cells were then added (15,000 cells/well), and the plates were incubated in a humidified incubator at 37°C and 6% CO2 . After 18 to 24 hours, 50 μL methyl-3H-thymidine (1 mCi/mL, 20 Ci/mmol) diluted 1:100 in assay medium was added. Plates were incubated for an additional 4 hours at 37°C and 6% CO2 . Labeled cells were harvested onto glass fiber filtermats using a PHD cell harvester (Cambridge Technology Inc, Watertown, MA) and deionized water as a washing solution. Filters were rinsed a final time with 2-propanol, and then were dried and counted in a Beckman (Fullerton, CA) model LS6000IC scintillation counter. Biologic activity was expressed in units using rHuEPO as the standard. The specific bioactivity was units of bioactive EPO divided by units of immunoreactive EPO.
Receptor binding.Receptor binding activity was determined by cold-displacement assays with high–specific activity 125I-EPO (Amersham) on a human cell line (OCIM141 ). Cells were grown to 2 to 5 × 105/mL, collected by centrifugation, washed two times in binding buffer (RPMI 1640 medium/1% bovine serum albumin/25 mmol/L HEPES, pH 7.3), and then resuspended at 1 to 2 × 107/mL in binding buffer containing 0.1% azide and 10 μg/mL cytochalisin B. Cells were incubated with varying amounts of sample and 125I-EPO at 37°C. After 3 hours, cells were centrifuged through phthalate oil (60:40 vol/vol dibutyl:dinonyl phthalate). The tubes were then quick-frozen in dry ice ethanol, the pellets were clipped, and the amount of bound 125I-EPO was determined by counting in a gamma counter. Receptor binding activity was determined by comparison of the displacement by the rHuEPO variants to a cold-displacement standard curve generated with a rHuEPO standard. Values were thus relative to the standard and are reported in units per milliliter. Specific receptor binding activity was determined by dividing by the amount of immunoreactive EPO determined by immunoassay.
RESULTS
Construction and analysis of rHuEPO variants.We have previously constructed over 200 different rHuEPO mutations. These have been analyzed to determine which ones affect rHuEPO structure.20 We have also used immunologic data to predict residues thought to be on the solvent-exposed surface of rHuEPO. Thus, we selected from this group 141 individual mutations with substitutions at 118 of 165 amino acid positions in rHuEPO and tested them for in vitro biological activity. At several positions, multiple substitutions were made and the rHuEPO variants were assayed. Thus, the additional mutations would confirm the importance of the position for bioactivity and provide information on the amino acid requirements at that site.
Each variant was transiently expressed in COS-1 cells, and conditioned medium was collected. In several cases, stable expression was obtained in CHO cells to produce larger quantities of rHuEPO variants. To perform the analysis on a large scale, most of the variants were not purified. Instead, each supernatant was assayed by up to six different immunoassays. Therefore, it was possible to determine the concentration of variants in the cell-conditioned medium using the assays that were unaffected by the introduced mutation. The effect of each mutation on rHuEPO conformation was also estimated with RIA-9G8A. The immunoreactivity measured by this assay increases when rHuEPO is denatured.21
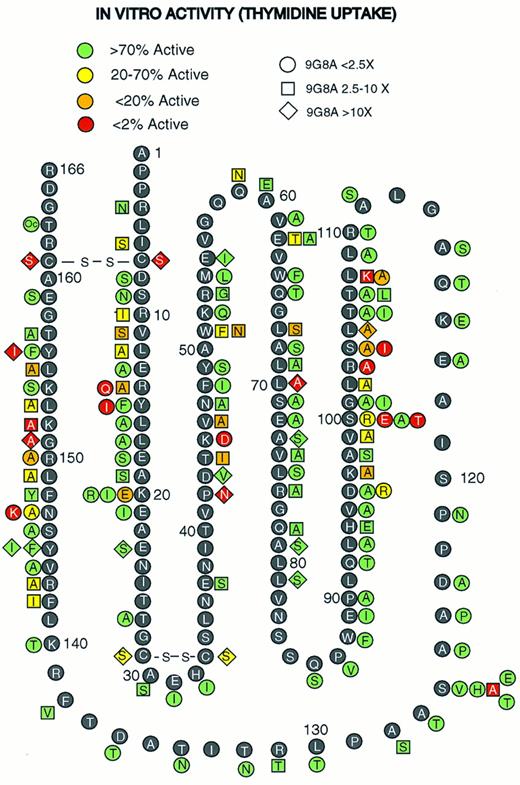
Figure 1 shows results of the in vitro bioassays that measured thymidine uptake in the EPO-dependent cell line 32D + EPOR. Figure 1 also shows the effect of each mutation on folding according to the results of RIA-9G8A immunoassays. Several amino acid segments in rHuEPO could be mutated without affecting bioactivity. These included residues 21 to 44, 52 to 95, and 109 to 140. Thus, these regions do not appear to play a major role in bioactivity. In addition, a termination codon introduced at Thr163 also had no apparent effect on bioactivity, which suggests that the C-terminal residues are not important for bioactivity. Introduction of a proline at −2 relative to the amino-terminal Ala affects cleavage of the signal peptide, resulting in a three–amino acid extension (Val Pro Gly20,42,43 ). In vitro activity and folding measured by RIA-9G8A with a Pro-2 variant was normal (data not shown), which suggests that the amino terminus is not important for bioactivity. This variant has an altered amino terminus as evidenced by a lack of immunoreactivity with antibodies that bind to the amino terminus.20 Forty-three mutations at 36 positions did have an affect on bioactivity. These mutations were concentrated in four regions: residues 10 to 20, 44 to 51, 96 to 108, and 142 to 156. Mutations that reduced the bioactivity by over 50-fold were found in all four regions. Mutations at several other scattered positions also reduced bioactivity. These included the mutations Gln59, Glu62, Leu67, and Leu70 and disulfide bonds. These latter mutations also affect folding and/or are predicted to be in buried positions.20 Thus, the mutations in these residues may affect bioactivity indirectly.
Mutagenesis of rHuEPO. Amino acid residues in the rHuEPO sequence (black circles with white letters) and rHuEPO variants (adjacent to rHuEPO sequences with shaded symbols) are indicated. Oc, substitution of a termination codon, TGA. The shape of the symbols indicates the effect of the mutation on RIA-9G8A immunoreactivity. The shading code represents the effect on in vitro bioactivity.
Mutagenesis of rHuEPO. Amino acid residues in the rHuEPO sequence (black circles with white letters) and rHuEPO variants (adjacent to rHuEPO sequences with shaded symbols) are indicated. Oc, substitution of a termination codon, TGA. The shape of the symbols indicates the effect of the mutation on RIA-9G8A immunoreactivity. The shading code represents the effect on in vitro bioactivity.
All rHuEPO variants in Fig 1 were also tested for the ability to stimulate formation of erythroid bursts using erythroid precursors from human bone marrow. The results were qualitatively similar to those in Fig 1 (data not shown). Mutations in all the residues that reduced thymidine uptake in 32D + EPOR also reduced erythroid burst formation. In addition, variants identified as having the greatest reduction in thymidine uptake activity also had the greatest reduction in erythroid burst formation. The fact that human cells were used in this assay suggests that the residues identified as being important for activation of 32D + EPOR (murine cells) are also important for activation of human cells. Thus, the mutations in the putative active site affect both proliferation and differentiation and are important in both species.
Effect of mutations on structure and function for residues 5 to 23. A helical wheel presentation shows amino acids found in rHuEPO (closest to the circle). Arrows point to mutations (in boxes). RIA-9G8A immunoreactivity for each variant is shown in parentheses adjacent to the mutation. Shading indicates the effect of the mutation on in vitro bioactivity.
Effect of mutations on structure and function for residues 5 to 23. A helical wheel presentation shows amino acids found in rHuEPO (closest to the circle). Arrows point to mutations (in boxes). RIA-9G8A immunoreactivity for each variant is shown in parentheses adjacent to the mutation. Shading indicates the effect of the mutation on in vitro bioactivity.
Role of Tyr and acidic and basic residues in bioactivity.It has been reported that chemical modification of tyrosines can inactivate rHuEPO.44 45 To see if we could identify which tyrosines were responsible for this effect, each of the four Tyr residues were individually mutated. Mutations at Tyr49 or Tyr145 had no effect on bioactivity (Fig 1). The Tyr15 and Tyr156 mutations included Phe and Ile substitutions. The Phe15 and Phe156 mutations had no effect on either bioactivity or rHuEPO folding, as evidenced by a normal immunoreactivity measured by RIA-9G8A. However, an Ile156 substitution affected both RIA-9G8A immunoreactivity (20-fold increase) and bioactivity (11-fold decrease), and an Ile15 substitution reduced bioactivity over 200-fold while at the same time having no apparent effect on RIA-9G8A. These results suggest that an aromatic amino acid is required at position 15 for bioactivity, and that an aromatic amino acid at position 156 may be required for proper folding.
Chemical modification of amino groups (eg, Lys) has also been reported to affect bioactivity.44,45 In addition, electrostatic interactions between charged residues in four–helix-bundle proteins and their receptors have been reported to play a role in hormone receptor recognition.46 We therefore examined the effect of substitutions in the charged residues in rHuEPO. rHuEPO has six Asp, 12 Glu, eight Lys, 13 Arg, and two His residues.1 2 All the charged residues except Arg162 were individually mutated to uncharged and/or oppositely charged residues. The Asp at 165 was removed by substituting a termination codon at Thr163 (Fig 1). Some substitutions of the acidic residues affected folding. However, only a Glu62 to Thr mutation resulted in a decrease in bioactivity twofold. This variant also had a 7.5-fold increase in RIA-9G8A immunoreactivity, and an Ala62 mutation had no effect on bioactivity. This suggests that Glu62 may be important for structure but not for bioactivity, and that acidic residues as a group do not have a major role to play in bioactivity. The two His residues at 32 and 94 were also mutated. Mutations in these residues also had no effect on bioactivity. The results were different when the lysine and arginine residues were mutated. Mutations at four of eight lysine residues (positions 20, 45, 97, and 152) and five of 13 arginine residues (positions 10, 14, 103, 143, and 150) affected bioactivity. Some of the mutations that affected bioactivity also affected folding (eg, at Lys97, Lys152, Arg10, and Arg143). Therefore, we cannot eliminate the possibility that for these latter four residues the reduced bioactivity was due to indirect effects. Mutations at Arg14, Arg103, Arg150, and Lys45 had dramatic effects on bioactivity with no apparent effect on folding. This suggests that these are part of the active site. Substitutions at Lys20 revealed additional information about charge requirements. An isoleucine substitution at Lys20 had no effect on bioactivity; however, a glutamic acid substitution reduced bioactivity approximately 11-fold. Receptor binding activity was also reduced eightfold for the Glu20 mutation (data not shown). None of these mutations significantly affected RIA-9G8A immunoreactivity. This suggests that there is not a requirement for a positively charged residue at this position, but that a negatively charged residue disrupts receptor binding and thus bioactivity. This observation in combination with the fact that only mutations in the positively charged lysine and arginine residues affected bioactivity suggests that the electrostatic interactions between rHuEPO and its receptor may be modulated primarily by positive charges on rHuEPO and presumably by negative charges on the EPO receptor.
Effect of mutations on structure and function for residues 62 to 79. A helical wheel presentation shows amino acids found in rHuEPO (closest to the circle). Arrows point to mutations (in boxes). RIA-9G8A immunoreactivity for each variant is shown in parentheses adjacent to the mutation. Shading indicates the effect of the mutation on in vitro bioactivity. Predicted buried and exposed surfaces are indicated.
Effect of mutations on structure and function for residues 62 to 79. A helical wheel presentation shows amino acids found in rHuEPO (closest to the circle). Arrows point to mutations (in boxes). RIA-9G8A immunoreactivity for each variant is shown in parentheses adjacent to the mutation. Shading indicates the effect of the mutation on in vitro bioactivity. Predicted buried and exposed surfaces are indicated.
Identification of active site mutants.With an analysis of this type, one would expect many of the mutations to result in a reduction in biologic activity because the rHuEPO structure was affected. For example, disruption of disulfide bonds has been shown to alter rHuEPO structure and activity, and thus they presumably are not part of the active site.21,27 32 Some of these latter types of mutations were identified according to high immunoreactivity as measured by RIA-9G8A. For example, at position 126, five different substitutions were evaluated. Only an Ala126 mutation affected RIA-9G8A immunoreactivity, and this was the only mutation that affected biologic activity even though the other substitutions were nonconservative. Thus, it is unlikely that Ser126 is part of the active site.
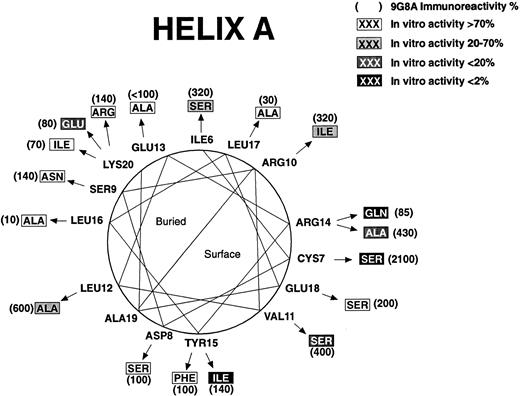
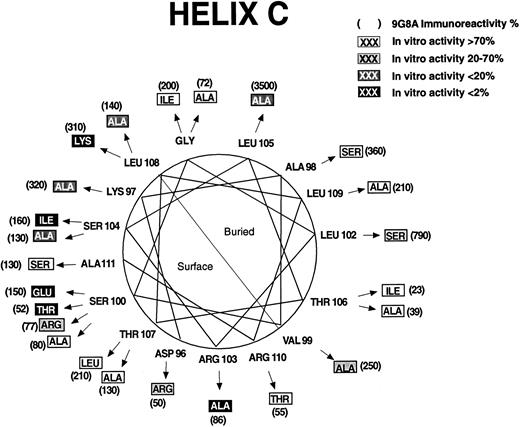
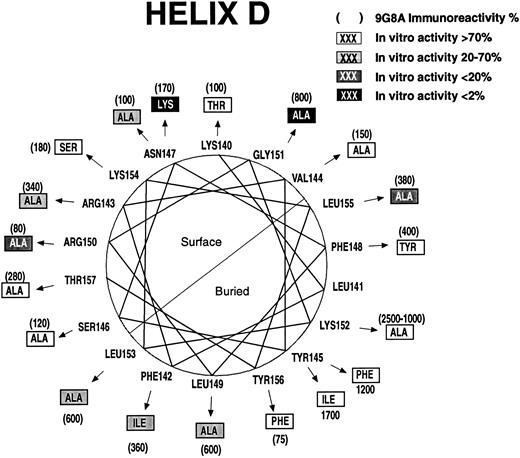
Some of the regions in which mutations affect biologic activity are in potential α-helices.20 Mutations that disrupt protein structure as evidenced by increased RIA-9G8A immunoreactivity are concentrated on the buried sides of the predicted helices. The predicted helices have been designated helix A (Fig 2), helix B (Fig 3), helix C (Fig 4), and helix D (Fig 5). Thus, it is possible to discriminate between direct and indirect effects of the mutations on activity by examining the locations of the affected residues on helical wheels.
Effect of mutations on structure and function for residues 94 to 111. A helical wheel presentation shows amino acids found in rHuEPO (closest to the circle). Arrows point to mutations (in boxes). RIA-9G8A immunoreactivity for each variant is shown in parentheses adjacent to the mutation. Shading indicates the effect of the mutation on in vitro bioactivity. Predicted buried and exposed surfaces are indicated.
Effect of mutations on structure and function for residues 94 to 111. A helical wheel presentation shows amino acids found in rHuEPO (closest to the circle). Arrows point to mutations (in boxes). RIA-9G8A immunoreactivity for each variant is shown in parentheses adjacent to the mutation. Shading indicates the effect of the mutation on in vitro bioactivity. Predicted buried and exposed surfaces are indicated.
Effect of mutations on structure and function for residues 140 to 157. A helical wheel presentation shows amino acids found in rHuEPO (closest to the circle). Arrows point to mutations (in boxes). RIA-9G8A immunoreactivity for each variant is shown in parentheses adjacent to the mutation. Shading indicates the effect of the mutation on in vitro bioactivity. Predicted buried and exposed surfaces are indicated.
Effect of mutations on structure and function for residues 140 to 157. A helical wheel presentation shows amino acids found in rHuEPO (closest to the circle). Arrows point to mutations (in boxes). RIA-9G8A immunoreactivity for each variant is shown in parentheses adjacent to the mutation. Shading indicates the effect of the mutation on in vitro bioactivity. Predicted buried and exposed surfaces are indicated.
Mutations in three positions in helix A (Fig 2) had large effects on bioactivity and were concentrated on the same side of the helix: Val11, Arg14, and Tyr15. Gln14 and Ile15 mutations did not affect immunoreactivity measured by RIA-9G8A but reduced the bioactivity over 50-fold, which suggests that they may be folded normally. Therefore, these residues are probably part of the active site. The Ser11 mutation also affected bioactivity, and it is on the same face of the predicted helix as Arg14 and Tyr15. However, the mutation had a fourfold increased immunoreactivity measured by RIA-9G8A, suggesting an effect on folding. Mutations in residues Arg6, Arg10, and Leu12 had modest effects on bioactivity. Mutations in these residues had an effect on rHuEPO folding as evidenced by their increased RIA-9G8A immunoreactivity, and they are on the same face of the helix as the bulk of the hydrophobic amino acids (eg, Leu12, Leu16, and Leu17). Therefore, these mutations may be in residues that are buried. The Cys7 to Ser mutation had an effect on biologic activity, but it also had a dramatic effect on RIA-9G8A immunoreactivity, presumably because it is involved in formation of a Cys7-Cys161 disulfide bond that is important for rHuEPO structure.20 27
Mutations at three positions in helix B affected bioactivity: Glu62, Leu67, and Leu70. However, mutations in all three positions affected RIA-9G8A immunoreactivity and are predicted to be in buried positions. This suggests that mutations in helix B that affect bioactivity do so indirectly (Fig 3).
Ser100, Arg103, Ser104, and Leu108 (helix C, Fig 4) are predicted to be on the protein surface, and mutations in them appear to be folded normally but dramatically affect bioactivity. Four substitutions were examined at Ser100, including Glu, Thr, Arg, and Ala. Only the Ala100 mutation had normal bioactivity. Mutations in residues on the opposite face of the predicted helix, at Leu102 and Leu105, severely affect folding as evidenced by high RIA-9G8A. Thus, it is unlikely that Leu102 or Leu105 are part of the active site. On the predicted exposed surface of helix D (Fig 5), mutations at Asn147, Arg150, and Gly151 had the greatest effect on bioactivity. An Arg143 to Ala mutation had a modest effect on bioactivity, but this mutation also affected folding. A Leu155 to Ala mutation also affected bioactivity. This residue may be partially buried or may be important for structure, since mutations in it had an effect on folding. An Ala151 substitution resulted in greater than a 150-fold decrease in bioactivity. However, the Ala151 mutation affected folding significantly. Several mutations in predicted buried positions affected RIA-9G8A immunoreactivity and bioactivity: Phe142, Leu149, Lys152, Leu153, and Tyr156.
Mutations between residues 42 and 51 also affected bioactivity. This region is thought to be in a connecting loop between helices A and B. An Asp45 mutation resulted in reduced bioactivity but appeared to be folded normally, suggesting that it may play a role in bioactivity (Table 1). The substitutions Asn42, Ile44, Ala46, Asn51, and Phe51 affected bioactivity also. However, the mutations also affected folding, so we cannot definitively conclude which ones are part of the active site.
Receptor binding activity and biologic activity of rHuEPO variants reveal two classes.The residues thought to play a role in in vitro bioactivity were examined to see what effect they had on binding to the EPO receptor. Several rHuEPO variants were expressed in both COS-1 and CHO cells (Table 1). All the mutants that affected bioactivity in the A/B connecting loop are shown, even though we do not know which of them may be directly involved in biologic activity. The helix A, C, and D variants shown include only those with mutations in residues thought to be on the solvent-exposed surface. We purified three different rHuEPO variants and natural-sequence rHuEPO to confirm the methods used to calculate activities. This was necessary because we determined the rHuEPO concentration in conditioned medium by immunoassay. The results are shown in Table 2. In all cases, results on purified material compared favorably with results obtained with unpurified material. This was true for effects on both biologic activity and receptor binding activity.
Some of the mutations had modest effects on receptor binding activity but no detectable biologic activity (Tables 1 and 2). Similar results were observed for both COS and CHO cell-expressed rHuEPO variants. This result suggests that rHuEPO binding by itself is not sufficient for activation of the EPO receptor. The four regions important for bioactivity could be divided into two groups according to the differential effects on biologic activity and receptor binding activity. The variants with changes in helices A and C retained substantial receptor binding activity (≥5% of normal) even though bioactivity in many cases was low or not detectable (Arg14/Gln, Tyr15/Ile, Ser100/Glu, Ser100/Thr, Arg103/Ala, Ser104/Ile, and Lys108/Ala; Table 1). Thus, a relatively small change in receptor binding activity due to mutations in these regions resulted in much greater effects on bioactivity. In contrast, substitutions in residues between 42 and 51 and in residues in helix D had receptor binding activity that more closely correlated with biologic activity (Val46/Ala, Trp51/Phe, Lys147/Ala, and Arg150/Ala). Mutations in these two regions also differed from mutations in helices A and C in that some mutations resulted in dramatic decreases in receptor binding activity (≥100-fold decrease; Lys45/Asp, Asn147/Lys, and Gly151/Ala). One explanation for these observations is that the two groups may represent residues in different receptor binding sites, and each receptor binding site has a different role to play in activity.
DISCUSSION
We have devised a strategy to evaluate the effect on folding and bioactivity of 141 different rHuEPO mutations at 118 positions, and found that four regions were important for bioactivity. These have been localized to helix A (Val11, Arg14, and Tyr15), helix C (Ser100, Arg103, Ser104, and Leu108), helix D (Asn147, Arg150, Gly151, and Leu155), and the A/B connecting loop (residues 42 to 51).
Previous studies implicated three of four regions we identified as having an important role to play in biological activity. These regions included residues between 10 and 15, 100 and 108, and 150 and 159.30-32 In particular, alanine-scanning mutagenesis data suggested that amino acids 14, 103, 104, 105, 108, and 151 were important for bioactivity and that residues 100, 101, 102, 106, 107, and 109 were not.31,32 We have also introduced alanines in all these positions and obtained qualitatively similar results. At three of these positions, other substitutions were made. These substitutions, Glu100/Ser, Ser104/Ile, and Leu108/Lys mutations, reduced bioactivity over 1,000-, 500-, and 5,000-fold, respectively, whereas Ala substitutions resulted in modest or no effects. Thus, at these positions Ala can substitute for the existing amino acid, resulting in effective activation of the EPO receptor, but other amino acids cannot. Our results and those of Wen et al32 and Grodberg31 are in disagreement with another study that reported that an Ala103 mutation resulted in a modest effect on bioactivity.30 An Ala154 mutation was reported to reduce bioactivity 10-fold.30 We examined Arg154 and Ser154 substitutions and found no effect on bioactivity. In addition, Wen et al32 tested an Ala154 mutation and reported no effect on bioactivity. The same group30 reported that a Glu159 to Ala mutation reduced bioactivity fourfold. We tested a Ser159 mutation and found no effect on bioactivity. The reasons for the discrepancies are not known, but may be due to the different amino acid substitutions, the different methods of determining EPO concentration, or the methods of expression and/or storage.
A recent analysis of hematopoietic cytokines and their receptors suggests that electrostatic interactions are important for receptor binding. Complementary electrostatic interactions between the binding surfaces of growth hormone and IL-4 and their corresponding receptors have been suggested.46 In addition, mutations in both positively charged and negatively charged residues in growth hormone affect activity.18 We examined the effect of mutations in acidic and basic residues, and found that only mutations in the basic residues affected bioactivity of rHuEPO. This differs from what is suggested for growth hormone, which uses both positive and negative charges for interaction with the growth hormone receptor. However, the difference in the importance of negatively charged residues for rHuEPO and human growth hormone is consistent with the difference in the charge of growth hormone and rHuEPO; growth hormone has a net charge of −4e, and the protein portion of rHuEPO has a net charge of +5e. EPO is more like IL-4, which has a net charge of +7e. In this case, the charge in the IL-4 receptor at the predicted IL-4 receptor-hormone interface is primarily negative.46 This suggests that the positively charged (basic) residues on rHuEPO may interact with the negatively charged (acidic) residues on the EPO receptor.
We and others have reported models of rHuEPO structure based on the four–helix-bundle motif.20,27,33 The four regions important for bioactivity identified by ourselves and others30-32 map to two sites on the rHuEPO models. These include site 1, containing the residues between amino acids 41 and 52 and amino acids 147, 150, and 151. The second site includes residues 11, 14, 15, 100, 103, 104, and 108. Mutations in site 1 have substantial effects on both receptor binding and in vitro biological activity. Mutations in site 2 have modest effects on receptor binding activity and much greater effects on in vitro biological activity.
This pattern of active site residues is analogous to that of growth hormone, which has two binding sites, exists as a monomer in solution, and also has a four–helix-bundle structural motif.19
Mutations in the two different sites in rHuEPO and human growth hormone behave similarly with respect to receptor binding activity and biologic activity. The observations can be explained by a sequential binding model, whereby the residues in site 1 have high affinity and are responsible for the initial interaction with the human growth hormone receptor. The residues in site 2 have less effect on receptor binding, but are required for dimerization. One consequence of the human growth hormone site 2 mutations is that they can act as antagonists.47 We have found that mutations in site 2 in rHuEPO can also act as antagonists (data not shown). Also consistent with this proposal is the recent report that the extracellular domain of the EPO receptor and rHuEPO can form a 2:1 complex in solution.48 In addition, both high- and low-affinity binding can be detected. Thus, it appears that EPO receptor dimerization may proceed in a manner similar to that of growth hormone.
ACKNOWLEDGMENT
We wish to thank T. Papayannopoulou (University of Washington, Seattle, WA) for OCIM1 cells; R. Pacifici for 32D + EPOR cells; B. Aguero, J. Grant, D. Greene, and J. Egrie for expert technical assistance and helpful discussions; K. Chen, S. Suggs, A. Harcourte, J. Katzowitz, A. Janssen, and L. Antonio for DNA sequence analysis; K. Aoki and S. Asher for purification of rHuEPO variants; and T. Jones and B. Goodman for synthetic oligonucleotides.
Address reprint requests to Steve Elliott, PhD, Amgen, Amgen Center, Thousand Oaks, CA 91320.