Abstract
Activation of T-cell hybridomas, preactivated normal T cells, and peripheral blood lymphocytes (PBL) from human immunodeficiency virus (HIV)-infected individuals results in apoptosis. In the first two cases, apoptosis is caused by the upregulation of Fas ligand (FasL) and its subsequent interaction with Fas; the mechanism for the spontaneous and activation-induced death of lymph node cells and PBL from HIV+ blood is not known. A number of protease inhibitors have been shown to prevent T-cell apoptosis under all of these circumstances, but the mechanism of action has not been determined. Here we show that the cysteine protease inhibitor E64d prevents activation-induced T hybridoma cell death by inhibiting the upregulation of FasL. Quantitative polymerase chain reaction (PCR) demonstrated that mRNA for FasL is expressed at low levels in fresh PBL from HIV-infected blood, but increases in cultured PBL from both uninfected and HIV-infected donors. The ex vivo apoptosis of PBL from HIV+ donors was prevented by adding the soluble extracellular domain of Fas, demonstrating a requisite role for Fas/FasL interactions in this form of cell death. Furthermore, while having no effect on the death of PBL from HIV-infected blood stimulated directly via Fas, E64d inhibited FasL upregulation. Thus, aberrant apoptosis of cultured PBL from HIV-infected individuals is mediated by FasL and Fas, and E64d blocks this apoptosis by inhibiting the upregulation of FasL. These results are consistent with the hypothesis that the abnormal expression of Fas and the inducible expression of FasL contributes to the immunodeficiency of patients with acquired immune deficiency syndrome and suggest that modulation of FasL expression could be an effective target for therapeutic intervention.
ACTIVATION OF T CELLS via the T-cell receptor (TCR) results in apoptosis under certain circumstances, a response that plays an important role in the development and function of the immune system.1 For example, potentially autoreactive thymocytes are eliminated by activation-induced apoptosis (negative selection), a major mechanism for the establishment of self-tolerance.2 Activated peripheral T cells are also susceptible to activation-induced cell death, which serves to dampen ongoing immune responses.3 It has recently become clear that activation-induced death of cells such as murine T-cell hybridomas and preactivated human peripheral blood lymphocytes (PBL) is mediated by Fas, a transmembrane molecule belonging to the tumor necrosis factor receptor (TNFR) superfamily.4-8 In these cases, activation of T cells results in upregulation of Fas and FasL, and the interaction between this receptor/ligand pair causes cell death. Impairment of this activation-regulated death in mice, either by mutations in Fas (lpr mice) or FasL (gld mice), results in lymphoproliferation (the accumulation of CD4− CD8− TCR+ T cells) and autoimmune disease.9-11 Recently, mutations in Fas and impaired Fas-mediated apoptosis have been identified in patients with a lymphoproliferative syndrome.12 13 Thus, Fas-mediated apoptosis is a critical means of maintaining peripheral T-cell homeostasis and regulating the immune response.
Infection with human immunodeficiency virus (HIV) is associated with early CD4+ T-cell dysfunction and a later decrease in absolute CD4+ T-cell number.14 Many mechanisms have been proposed for the loss of CD4+ T cells, including a direct cytopathic effect of HIV on CD4+ T cells or their precursors, HIV gp120-mediated cross-linking of CD4 and subsequent cell death as a consequence of T-cell receptor (TCR) engagement, production of soluble cytotoxic molecules by infected cells, autoimmune phenomena, and superantigen-induced activation/death.15 An intriguing observation in this regard is that T cells from HIV+ individuals spontaneously undergo apoptosis when cultured in vitro and that, in some circumstances, this cell death can be increased by activation with mitogens or superantigens.16-18 This phenomenon was not observed in HIV-seronegative individuals suffering from acute or chronic infectious diseases.16 Furthermore, ex vivo cell death has also been observed in simian immunodeficiency virus (SIV)-infected rhesus macaques, which develop an acquired immune deficiency syndrome (AIDS)-like syndrome, but not HIV-infected chimpanzees that do not develop the disease.19,20 Inappropriate cell death is not simply an in vitro phenomenon: spontaneous apoptosis of HIV-negative bystander cells in lymph nodes from HIV-infected individuals has been found to be threefold to fourfold higher than in uninfected controls and correlates with the degree of activation of the cells.21,22 These findings have led to the speculation that activation-induced T-cell death, unrelated to direct viral cytolysis, accounts for at least some of the loss of CD4+ T cells in AIDS.23
Cysteine and serine protease inhibitors have been shown to prevent activation-induced T-cell apoptosis and the ex vivo death of PBL from HIV-infected individuals.24,25 Here we investigate the mechanism by which one of these cysteine protease inhibitors, E64d, mediates its effects. Our results demonstrate that E64d prevents activation-induced death of T hybridoma cells by inhibiting upregulation of FasL. Given that lymphocytes from HIV-infected individuals constitutively express abnormally high levels of Fas,26 27 the possible role of Fas and FasL in the death of PBL from HIV-infected individuals was also investigated. The data indicate that upregulated expression of FasL during culture and increased cellular sensitivity to Fas-mediated killing are responsible for ex vivo death of PBL from HIV-infected individuals, and that the inhibitory effect of E64d is due to inhibition of FasL upregulation.
MATERIALS AND METHODS
Reagents.Antihuman Fas antibody CH-11 was purchased from Kamiya Biomedical Co (Thousand Oaks, CA). Fas:Fc and TNFR:Fc are recombinant chimeric molecules made of the extracellular portions of human Fas or human 55-kD TNF receptor fused to the Fc portion of human IgG1, respectively.8 28 E64d, a cysteine protease inhibitor, was obtained from Sigma Chemical Co (St Louis, MO). Taq polymerase was purchased from Boehringer Mannheim (Indianapolis, IN).
Cells.2B4.11 is a murine T-cell hybridoma specific for the antigen pigeon cytochrome c.29 PC60-d11S (d11S), a T-cell hybridoma that expresses FasL following activation, mouse leukemia cell L1210, and L1210-Fas (L1210 cells transfected with fas cDNA) were kindly provided by Dr P. Golstein.30 Heparinized venous peripheral blood was obtained from HIV-infected individuals who were admitted to National Cancer Institute (NCI) clinic. All patients had absolute CD4+ T-cell counts of 200 to 500 cells/μL, and informed consent was obtained. Blood samples from HIV-negative individuals were obtained from NIH blood bank. PBL were isolated using Lymphocyte Separation Medium (Organon Teknika Corp, Durham, NC). Cells were cultured in complete medium (RPMI 1640 plus 10% fetal calf serum, 4 mmol/L glutamine, 100 μg/mL gentamicin, 100 U/mL penicillin, and 5 μmol/L 2-mercaptoethanol).
PCR primers and generation of mutated competitive cDNA. Amplification of a primer pair for FasL (primer 1: 5′ GCC TCT GGA ATG GGA AGA CAC C 3′; primer 2: 5′ CAT CTG CCC AGT AGT GCA GTA G 3′ ) yielded a 241-bp DNA fragment. To generate an internal deletion mutant of this polymerase chain reaction (PCR) product, an oligonucleotide (primer 3: 5′ TAC TTC CGG GGT CAA AAG GTC TAC ATG AGG 3′ ) was made, which includes the 15 nucleotides at the 5′ and 3′ ends of the center 57-bp portion of the cDNA (without the middle 27 bp). Two amplifications were then performed using the 241-bp cDNA as template and either primer 3 and 2, or primer 1 and an antisense oligonucleotide of primer 3, as primers. The two PCR products were annealed and reamplified using primers 1 and 2. The resulting 214-bp cDNA was used as competitor for quantitation of FasL mRNA. The specificity of each PCR-amplified product was confirmed by restriction enzymes digestion. The oligonucleotide primers and the generation of the competitive mutant for actin were generated as described.31
Quantitative competitive PCR. PBL were lysed with TE buffer containing 0.5% Triton-X 100, 4 U/mL of RNase inhibitor (Boehringer Mannheim), and 1 μmol/L dithiothreitol (DTT). After centrifugation at 14,000 rpm in Eppendorf tubes for 5 minutes, the supernatants were directly used for reverse transcription with an Invitrogen cDNA cycle kit (San Diego, CA). PCR amplification was then performed using primer 1 and 2 in the presence of increasing amounts of mutated cDNA (competitor). The reaction products were separated on a 4% agarose gel, stained with ethidium bromide, and visualized under ultraviolet (UV) light. Photographs were made with Polaroid Positive/Negative film (Polaroid, Cambridge, MA), and the negative scanned with a laser densitometer (PDI Imageware System, Huntington Station, NY). The ratios of the upper and lower bands were plotted as a function of the amount of mutated cDNA, and the amount of FasL cDNA was calculated from the linear regression line.
DNA fragmentation assay. DNA fragmentation was quantitated by adherence to fiberglass filters as described.32 Briefly, 2B4.11 cells or L1210 cells were labeled with [3H]thymidine (5 μCi/mL; 1 Ci = 37 GBq) for 3 hours in tissue culture. After thorough washing, 5 × 104 2B4 cells were distributed in triplicate into 96-well microtiter plates that had been coated with purified 2C11 at 1 μg/well as described33 or treated with complete medium. In some experiments, the labeled 2B4.11 cells were cocultured with different amounts of d11S cells, which had been activated with PMA (20 ng/mL) and ionomycin (3 μg/mL) for 3 hours and washed thoroughly. Following 16 hours of culture at 37°C in the presence or absence of E64d, the cells were hypotonically lysed and harvested onto fiberglass filters (PHD Cell Harvester; Cambridge Technology, Cambridge, MA). While intact chromatin DNA adheres to the filters, fragmented DNA passes through. The results are expressed as specific DNA fragmentation: [(M-E)/M] × 100%, where M is the retained label in cells cultured in medium and E is retained labeled in cells cultured under experimental conditions. To measure DNA fragmentation of L1210 and L1201-Fas cells, labeled cells were cocultured with activated or nonactivated 2B4.11 cells and harvested 6 hours later.
Northern blot analysis.Total RNA was made from 2B4.11 cells using acidic guanidine thiocyanate.34 The FasL probe was generated by PCR as described.8 The Pst fragment of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was kindly provided by Dr L. King (University of Pennsylvania School of Medicine, Philadelphia, PA). A total of 20 μg of RNA was loaded to each well of a 1% agarose gel and electrophoresed. After transfer to nylon membranes, hybridization with 32P-labeled FasL probe was performed in QuikHyb solution according to the procedures recommended by the manufacturer (Stratagene, San Diego, CA). The membrane was then stripped and reblotted for GAPDH.
Cell viability.Cell viability was assessed in duplicate by trypan blue exclusion and light microscopy. We have previously shown that this assay for cell viability correlates well with measurement of DNA fragmentation in PBL from HIV+ individuals.35
RESULTS
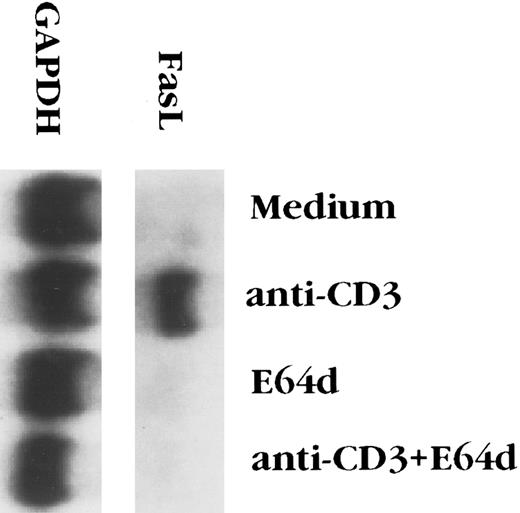
E64d prevents activation-induced apoptosis of T-cell hybridomas by inhibiting FasL upregulation.The cysteine protease inhibitor E64d prevents activation-induced apoptosis of T-cell hybridomas and PBL from patients with AIDS.24,25 Since activation-induced T hybridoma death is mediated by the interaction of Fas and FasL,6-8 cysteine protease inhibition might block signal transduction via Fas and/or alters the expression FasL. It has previously been reported that E64d has no effect on killing of T-cell blasts caused by ligation of Fas,36 a finding that we confirmed with the 2B4.11 T-cell hybridoma (data not shown), suggesting that E64d interfered with apoptosis by inhibiting FasL expression. The effect of the cysteine protease inhibitor on FasL expression was assessed in two ways. As previously reported,8 FasL transcripts were undetectable in unstimulated 2B4.11 cells, but were easily seen 4 hours after stimulation with anti-CD3 (Fig 1). Although E64d had no effect on mRNA levels of the housekeeping gene GAPDH, it completely inhibited the induction FasL mRNA. To determine the biological relevance of FasL mRNA inhibition, the level of functional FasL protein expressed on the surface activated 2B4.11 cells was assessed. L1210 is a mouse leukemia cell line that expresses a low level of Fas on its surface and is relatively insensitive to FasL-induced killing.8,30 In contrast, L1210 cells transfected with fas rapidly undergo apoptosis when cultured with FasL+ cells, such as d11S or activated 2B4.11 cells.8 Unactivated 2B4.11 cells did not kill L1210 or L1210-Fas cells (data not shown), whereas activated 2B4.11 caused extensive death of the Fas+ but not the Fas− target (Fig 2). To determine the effect of E64d on activation-induced FasL expression, 2B4.11 cells were activated with the indicated amounts of anti-CD3 antibody in the absence or presence of 100 μmol/L E64d for 2 hours, and [3H]thymidine labeled L1210 or L1210-Fas cells were then added into the culture and incubated for another 6 hours. As shown in Fig 2, L1210-Fas, but not L1210, cells were killed by activated 2B4.11. The killing was markedly inhibited when the T cells were activated in the presence of E64d. Furthermore, the inhibitory action of E64d was not due to inhibition of a Fas/FasL interaction or Fas-mediated killing because when the E64d was added together with L1210-Fas cells to activated 2B4.11 cells, the target cells still underwent apoptosis. Since E64d does not affect T-cell activation per se, as judged by interleukin-2 (IL-2) production24 (and our unpublished observation, June 1995), these data indicate that E64d specifically blocks activation-induced upregulation of FasL mRNA, which accounts for its ability to prevent activation-induced apoptosis.
E64d blocks the upregulation of Fas ligand mRNA induced by anti-CD3 antibody. 2B4.11 cells (106 cells/mL) were stimulated in 60-mm tissue culture dishes coated with 2C11 (20 μg/dish) for 4 hours in the presence or absence of 100 μmol/L E64d. Total RNA was extracted from the cells and Northern blot analysis performed.
E64d blocks the upregulation of Fas ligand mRNA induced by anti-CD3 antibody. 2B4.11 cells (106 cells/mL) were stimulated in 60-mm tissue culture dishes coated with 2C11 (20 μg/dish) for 4 hours in the presence or absence of 100 μmol/L E64d. Total RNA was extracted from the cells and Northern blot analysis performed.
E64d blocks anti-CD3–induced upregulation of Fas ligand protein. 2B4.11 cells were activated with the indicated concentrations of plastic-coated 2C11 in the presence or absence of 100 μmol/L E64d for 2 hours. [3H]thymidine-labeled L1210 cells or L1210-Fas cells were then added to the cultures. The parentheses in the legend indicate the time of addition of E64d: (2B4.11 + E64d), E64d added at time 0; (E64d + L1210-Fas), E64d added with the L1210-Fas cells, 2 hours after initiation of the culture. After another 6 hours of incubation, the cells were harvested and the percentage of DNA fragmentation determined. Similar results were obtained in three independent experiments.
E64d blocks anti-CD3–induced upregulation of Fas ligand protein. 2B4.11 cells were activated with the indicated concentrations of plastic-coated 2C11 in the presence or absence of 100 μmol/L E64d for 2 hours. [3H]thymidine-labeled L1210 cells or L1210-Fas cells were then added to the cultures. The parentheses in the legend indicate the time of addition of E64d: (2B4.11 + E64d), E64d added at time 0; (E64d + L1210-Fas), E64d added with the L1210-Fas cells, 2 hours after initiation of the culture. After another 6 hours of incubation, the cells were harvested and the percentage of DNA fragmentation determined. Similar results were obtained in three independent experiments.
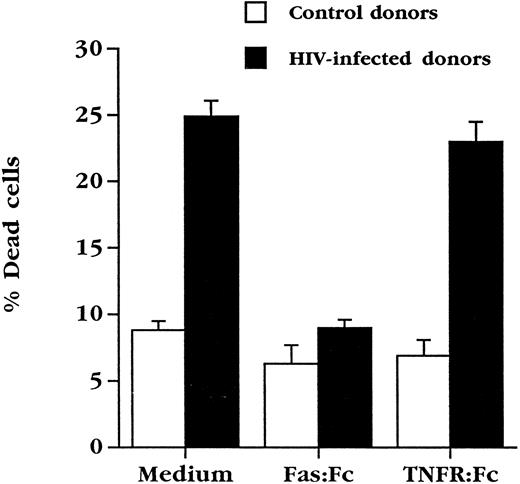
Soluble Fas inhibits apoptosis of cultured HIV+ PBL.PBL from HIV-infected individuals constitutively express high levels of Fas,26 and are sensitive to Fas engagement27 (and our unpublished observations, August 1995), which may reflect their chronically activated state.37 Since these cells undergo spontaneous death in culture, an event that was not significantly increased in our hands by further activation via the TCR,35 we attempted what contribution, if any, Fas and FasL might make to the ex vivo death of PBL from HIV-infected individuals. Use was made of chimeric molecules composed of the extracellular portion of Fas fused to the Fc portion of human IgG (Fas:Fc), which binds to FasL and prevents activation-induced death of activated human PBL4 and murine T-cell hybridomas.6-8 PBL from HIV+ individuals were cultured for 3 days in the presence or absence of Fas:Fc (Fig 3). As previously observed,35 5% to 10% of PBL from uninfected individuals die under these culture conditions, while approximately 25% of cells from HIV+ patients with absolute CD4+ cell counts of 200 to 500 cells/μL die over the same time course. Strikingly, culture in the presence of Fas:Fc prevented the death of PBL from HIV+ blood, bringing the level of spontaneous death to less than 10%. This effect was specific for FasL in that a TNFR:Fc fusion protein, a potent antagonist of the cytotoxic effects of TNF and LT-α,28 had no effect on cell death under this condition.
Soluble Fas chimeric molecules block death of PBL from HIV-infected individuals. PBL from uninfected (n = 5) or HIV+ (n = 9) individuals were cultured for 3 days in complete medium at a concentration of 106 cells/mL in duplicate cultures. Cultures were performed in medium alone or in the presence of Fas:Fc or TNFR:Fc (10 μg/mL final concentration) and cell viability assessed.
Soluble Fas chimeric molecules block death of PBL from HIV-infected individuals. PBL from uninfected (n = 5) or HIV+ (n = 9) individuals were cultured for 3 days in complete medium at a concentration of 106 cells/mL in duplicate cultures. Cultures were performed in medium alone or in the presence of Fas:Fc or TNFR:Fc (10 μg/mL final concentration) and cell viability assessed.
Expression of FasL by uninfected and HIV+ PBL.The requirement for Fas engagement in HIV-associated apoptosis raised the possibility that FasL is upregulated in cultured PBL from HIV+ blood. To examine this, a quantitative competitive reverse transcriptase (RT)-PCR assay was adapted to detect FasL mRNA in the limited number of cells that can be obtained from HIV+ individuals.31 38 For this assay, an internal deletion mutation of FasL cDNA was made, resulting in a sequence that competes with wild type FasL cDNA for the same pair of primers on an equimolar basis during PCR. When the mutant cDNA was amplified together with fresh or cultured PBL cDNA from HIV+ blood, agarose gel electrophoresis showed two bands (an example is shown in Fig 4) that correspond to the wild type (upper) and mutated (lower) cDNA, respectively. The ratio of the two bands changed in each sample as a constant amount of PBL cDNA was amplified in the presence of increasing amounts of competitor cDNA. Using laser densitometry, it was possible to determine the amount of FasL cDNA obtained from these cells. The analysis of PBL from multiple donors is summarized in Fig 5A. Interestingly, the levels of FasL mRNA present in fresh PBL from HIV− and HIV+ blood was similar, and culturing the cells in complete medium overnight resulted in a threefold to fivefold increase in FasL mRNA, both in PBL from uninfected and PBL from HIV-infected donors.
Competitive PCR for FasL mRNA in fresh and cultured PBL. mRNA in nonnuclear lysates of fresh or cultured (20 hours) PBL from an HIV-infected donor was reverse-transcribed using random primers and amplified by PCR in the presence of increasing amounts of mutated cDNA (lane 1 through lane 6, the amounts of mutated cDNA are 10−3, 2 × 10−3, 4 × 10−3, 10−2, 2 × 10−2, and 4 × 10−2 amol, respectively). The PCR products were analyzed by agarose gel electrophoresis. The wild type cDNA is seen as a 241-bp band, while the mutated cDNA migrates as a 214-bp band.
Competitive PCR for FasL mRNA in fresh and cultured PBL. mRNA in nonnuclear lysates of fresh or cultured (20 hours) PBL from an HIV-infected donor was reverse-transcribed using random primers and amplified by PCR in the presence of increasing amounts of mutated cDNA (lane 1 through lane 6, the amounts of mutated cDNA are 10−3, 2 × 10−3, 4 × 10−3, 10−2, 2 × 10−2, and 4 × 10−2 amol, respectively). The PCR products were analyzed by agarose gel electrophoresis. The wild type cDNA is seen as a 241-bp band, while the mutated cDNA migrates as a 214-bp band.
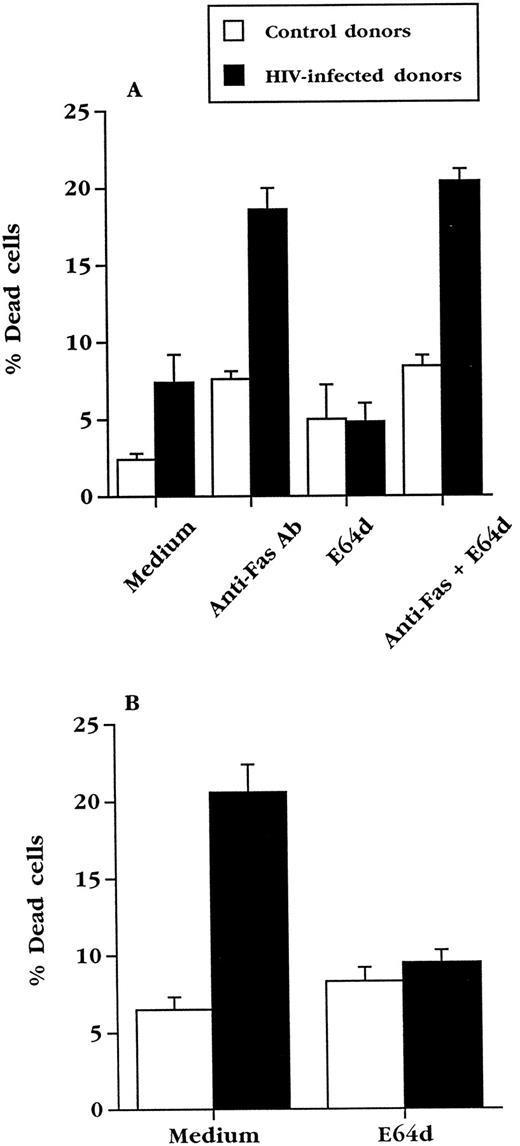
E64d does not inhibit apoptosis induced directly via Fas. Fresh PBL from uninfected (n = 3) or HIV+ (n = 4) individuals were treated with anti-Fas antibody CH11 (100 ng/mL) in the presence or absence of 50 μmol/L E64d. Cell viability was determined in duplicate wells by trypan blue exclusion after 20 hours of culture. (B) The PBL used in (A) were cultured in complete medium for 3 days in the presence or absence of 50 μmol/L E64d. Cell viability was determined in duplicate wells by trypan blue exclusion.
E64d does not inhibit apoptosis induced directly via Fas. Fresh PBL from uninfected (n = 3) or HIV+ (n = 4) individuals were treated with anti-Fas antibody CH11 (100 ng/mL) in the presence or absence of 50 μmol/L E64d. Cell viability was determined in duplicate wells by trypan blue exclusion after 20 hours of culture. (B) The PBL used in (A) were cultured in complete medium for 3 days in the presence or absence of 50 μmol/L E64d. Cell viability was determined in duplicate wells by trypan blue exclusion.
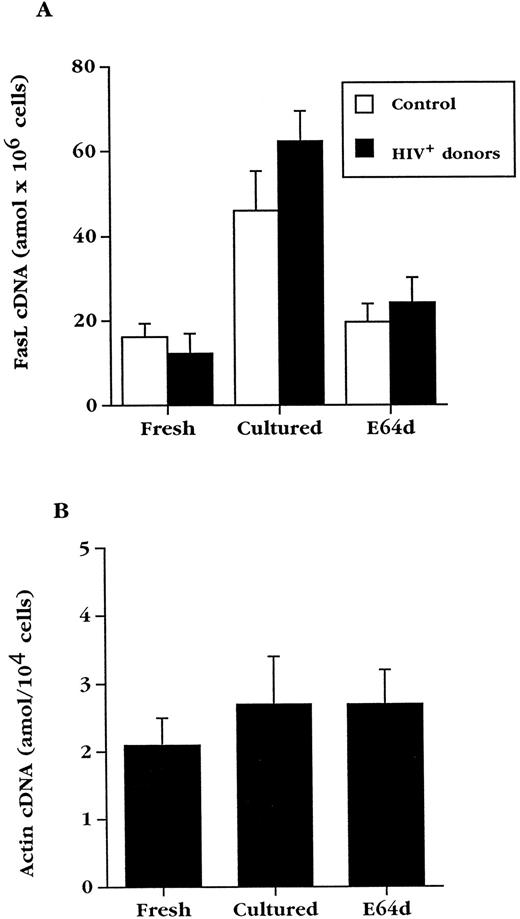
E64d prevents upregulation of FasL in normal and HIV+ PBL.E64d could exert its effects on Fas-mediated killing by inhibiting signaling via Fas or, as in T-cell hybridomas, by preventing the upregulation of FasL. In agreement with studies done with normal T-cell blasts,36 we found that E64d prevented the ex vivo death of cultured PBL from HIV-infected blood (Fig 5B), but did not inhibit apoptosis of the same cells caused by cross-linking Fas with an activating anti-Fas antibody (Fig 5A). To determine if the effect of E64d on apoptosis of PBL from HIV+ blood is due to an effect on FasL expression, E64d was added to cultured PBL from HIV+ and HIV− blood (Fig 6A). The induction of FasL mRNA in PBL from both uninfected and HIV-infected individuals during overnight culture was inhibited by E64d, which on average caused a greater than 75% decrease. In contrast, the levels of β-actin mRNA, measured by the same quantitative PCR technique, did not change significantly during these cultures (Fig 6B). Consistent with its effect on activation-induced apoptosis in T-cell hybridoma, these data demonstrate that E64d has no demonstrable effect on Fas-mediated signaling, but exerts its protective effect by prevention of FasL upregulation.
Culture of PBL results in upregulation of FasL mRNA, which is inhibited by E64d. (A) Mean FasL mRNA levels ± standard error of mean (SEM) in fresh PBL and PBL cultured for 20 hours in the absence or presence of E64d from uninfected (n = 6) and HIV-infected (n = 9) individuals. (B) Competitive PCR analysis of actin cDNA from HIV+ PBL (n = 4). Data are expressed as the arithmetic mean ± SEM.
Culture of PBL results in upregulation of FasL mRNA, which is inhibited by E64d. (A) Mean FasL mRNA levels ± standard error of mean (SEM) in fresh PBL and PBL cultured for 20 hours in the absence or presence of E64d from uninfected (n = 6) and HIV-infected (n = 9) individuals. (B) Competitive PCR analysis of actin cDNA from HIV+ PBL (n = 4). Data are expressed as the arithmetic mean ± SEM.
DISCUSSION
Several lines of evidence suggest that proteases play an important role in apoptosis. One of the early indications came from studies on proteases in the granules of cytotoxic T cells (granzymes). These cysteine proteases are required for killer cells to rapidly induce apoptotic death of target cells.39,40 Genetic studies in C elegans have found that the ced-3 gene is required for developmental programmed cell death.41 Interestingly, ced-3 shares some homology with IL-1β converting enzyme (ICE),42 a cysteine protease that processes pro–IL-1 into its active form. Although ICE itself appears not involved in cell death process,43 a number of ICE homologs have been identified, and their role in the induction of apoptosis is an area of intense investigation.44 Inhibitors of ICE-like proteases have been found to block Fas- and TNF-mediated apoptosis, as well as apoptosis induced by a number of drugs, indicating activation of this protease superfamily is required for multiple forms of cell death.45-47 In contrast to ICE family protease inhibitors, E64d and other cysteine, as well as some serine, protease inhibitors selectively prevent activation-induced T-cell apoptosis; they do not inhibit T-cell death caused by anti-Fas, γ-irradiation, etoposide, or extracellular adenosine triphosphate (ATP).24,36,48 One protease that is of particular interest is calpain, a Ca2+-dependent cysteine protease that is specifically inhibited by E64d.49 Calpain is an ubiquitously expressed neutral protease whose substrates include spectrin, the epidermal growth factor (EGF ) receptor, Src, calcineurin, c-Jun, and c-Fos.50 While expressed at a low level in resting T cells, calpain is highly expressed upon activation.51 Since increases in intracellular Ca2+ can activate calpain, it is likely that calpain activity is enhanced in activated T cells. Although E64d is a calpain inhibitor that prevents FasL upregulation, the question of whether this protease is the relevant target is unknown.
Activation of T-cell hybridomas and mature T cells causes apoptosis by upregulating FasL and, in some cases, Fas. The results in this report demonstrate that E64d prevents this form of apoptosis not by interfering with Fas signaling, but by inhibiting the upregulation of FasL mRNA and protein. Given the previously reported antiapoptotic effect of E64d on PBL from HIV+ blood, we investigated the role of Fas and FasL in this form of cell death. The increased sensitivity of lymphocytes to Fas engagement could result from direct HIV infection, the effects of soluble products of HIV infection, or their state of activation. However, the majority of cell death in patients in the later stages of the disease appears to be an indirect effect of HIV, since the number of dying cells far exceeds the number that is detectable infected, and in lymph nodes it is predominantly uninfected bystander cells that undergo apoptosis.21,22 One candidate for the cause of the enhanced sensitivity of bystander PBL to apoptosis is HIV-1 Tat, which is present in the serum of HIV-infected individuals. In vitro, Tat has been shown to cause the apoptotic death of PBL from uninfected individuals.52,53 Furthermore, Tat upregulates expression of FasL and sensitizes uninfected T cells to killing by TCR-induced apoptosis.54 Interestingly, cross-linking CD4-bound gp120 with anti-gp120 antibodies enhances susceptibility of CD4+ T cells to TCR-mediated apoptosis,55 an effect that is enhanced by Tat and blocked by noncytotoxic anti-Fas F(ab′ )2 or soluble Fas:Fc fusion molecules.54 However, we found no evidence that FasL levels are abnormally elevated in either fresh or cultured PBL from HIV+ individuals compared with PBL from uninfected controls. Therefore, if Tat is playing a role in ex vivo PBL apoptosis, it is likely to be at the level of the response to Fas ligation, not FasL upregulation. HIV infection of severe combined immunodeficiency (SCID)-hu mice has also been shown to cause the death of thymocytes, particularly CD4+ CD8− cells.56,57 In addition, while anti-Fas killed both CD4+ and CD8+ T cells from HIV-infected individuals, CD4+ memory T cells were found to be more sensitive.27 These observations may account, at least in part, for the relatively selective loss of CD4+ T cells observed in vivo.
Interestingly, the levels of FasL mRNA expressed by fresh PBL from HIV+ and HIV− individuals were comparable, as were the levels after upregulation in culture. Thus, in culture the effector molecule (FasL) was induced, but its receptor (Fas) was functionally present only on PBL from HIV+ blood. The ability of a soluble form of Fas to prevent cell death provides direct evidence that the interaction between these two molecules mediates HIV-associated T-cell apoptosis, demonstrating that PBL from individuals infected with HIV are abnormally sensitive to Fas-mediated killing by endogenously expressed FasL. Since FasL is normally upregulated by T-cell activation, this provides support for the hypothesis that periodic antigen-specific stimulation of peripheral CD4+ T cells may result in immune dysfunction and gradual cell loss.23,58 It is also noteworthy that while inhibitors of both cysteine and ICE family proteases can prevent Fas/FasL-dependent apoptosis, they appear to have different mechanisms of action; ICE family inhibitors prevent Fas-mediated killing,45,46 59 while the cysteine protease inhibitor E64d had no effect on Fas-mediated killing, but prevented upregulation of FasL. The finding that inhibition of FasL upregulation prevents HIV-associated PBL apoptosis may make it possible to determine to what extent inappropriate apoptosis contributes to the immunodeficiency of AIDS.
ACKNOWLEDGMENT
We are grateful to Dr H. Mitsuya and Dr L. J. Striker for their help and support; Dr R. Yarchoan, F. Merced-Galindez, and K. Wyvill for providing blood from HIV+ individuals; the NIH blood bank for providing uninfected blood; J. Jensen for making oligonucleotides; and Dr A. Weissman for critical review of the manuscript.
Supported in part by Grant No. IM663 from the American Cancer Society (Philadelphia, PA) and Grant No. AI33068 from the National Institutes of Health, Bethesda, MD, to C.F.W.
Address reprint requests to Jonathan Ashwell, MD, Rm 1B-40, Bldg 10, NIH, Bethesda, MD 20892-1152.

![Fig. 2. E64d blocks anti-CD3–induced upregulation of Fas ligand protein. 2B4.11 cells were activated with the indicated concentrations of plastic-coated 2C11 in the presence or absence of 100 μmol/L E64d for 2 hours. [3H]thymidine-labeled L1210 cells or L1210-Fas cells were then added to the cultures. The parentheses in the legend indicate the time of addition of E64d: (2B4.11 + E64d), E64d added at time 0; (E64d + L1210-Fas), E64d added with the L1210-Fas cells, 2 hours after initiation of the culture. After another 6 hours of incubation, the cells were harvested and the percentage of DNA fragmentation determined. Similar results were obtained in three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/2/10.1182_blood.v89.2.550/4/m_bl_0001f2.jpeg?Expires=1769756916&Signature=W2--YPZmgRlE0YRFbPqt-zi1WkJsWGVPO868g8zq1euGlQ~51VO6DRU54wKsOSdzmWsMrB~iHfpJ3DZaD6Y-suWHVR1Rxac-JpGvVfgNyURh-wLCXsFNmGlvGUVV2r9dHl6fLKz6uxLz9OPFtnn6-TWrmkIcLEmX0skcA74sXM-oeJNxmyEJ89ao-vMIVQZk-ECa9UYM7XXy9ZaTe6fcjW0c4q3cIuYphGZjetEiUgqVF5Dx8~okQ~1yVAG7ufPnvOrSZhG2fg43SmzNJqNrZxIWVvCx-3oFCv7oX-4oK1rMfNKhLPBDEE5XxgB07OMTofBgIqBRk-qCikoM-GPzYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)