To the Editor:
Aman et al1 recently reported that interferon-α (IFN-α) upregulates the production of interleukin-10 (IL-10) by activated monocytes or CD4+ T cells. These data are in line with our previously published findings2 which showed that IFN-α downregulates IL-5 production by human CD4+ T cells while it significantly increases IL-10 secretion. These effects of IFN-α were observed both on resting CD4+ T cells from normal individuals and on differentiated Th2-like cells isolated from patients with the hypereosinophilic syndrome (HES).
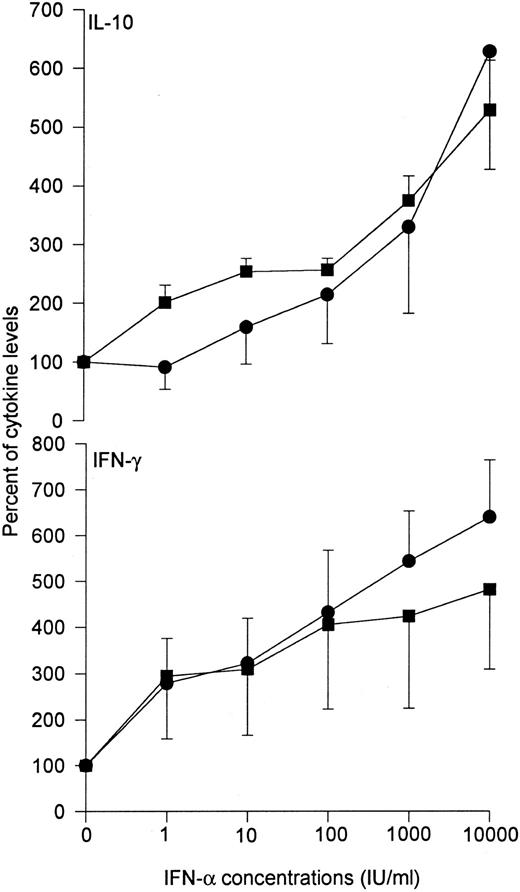
Although upregulation of IL-10 secretion might indeed contribute to some antiinflammatory properties of IFN-α, we would like to underline that IFN-α also upregulates the synthesis of IFN-γ, a well-known proinflammatory cytokine. Firstly, it has been well established that, when present in the induction phase of the immune response, IFN-α favors the differentiation of CD4+ T cells into Th1 cells.3 Secondly, we found that IFN-α directly upregulates the production of IFN-γ by CD4+ T cells. As shown in Fig 1, IFN-α stimulates in a dose-dependent manner the production of both IL-10 and IFN-γ by resting CD4+ T cells activated either by phorbol myristate acetate (PMA) and anti-CD28 monoclonal antibody (MoAb) or anti-CD3 MoAb cross-linked on fibroblasts transfected with the B7-1. gene.
Effects of recombinant IFN-α on the production of IL-10 and IFN-γ by CD4+ T cells. Graded doses of recombinant IFN-α (Schering-Plough, Innishannon, Ireland) were added to CD4+ T cells purified from peripheral blood of five healthy volunteers and stimulated with either PMA (1 ng/mL) and anti-CD28 MoAb (1 μg/mL) (•), or the CLB-T3/4.1 anti-CD3 MoAb (100 ng/mL) cross-linked on fibroblasts transfected with the B7-1 gene (▪), as previously described.2 After 48 hours, culture supernatants were harvested for determination of immunoreactive IL-10 and IFN-γ by ELISA, as previously described.2 Data are shown as mean ± SEM.
Effects of recombinant IFN-α on the production of IL-10 and IFN-γ by CD4+ T cells. Graded doses of recombinant IFN-α (Schering-Plough, Innishannon, Ireland) were added to CD4+ T cells purified from peripheral blood of five healthy volunteers and stimulated with either PMA (1 ng/mL) and anti-CD28 MoAb (1 μg/mL) (•), or the CLB-T3/4.1 anti-CD3 MoAb (100 ng/mL) cross-linked on fibroblasts transfected with the B7-1 gene (▪), as previously described.2 After 48 hours, culture supernatants were harvested for determination of immunoreactive IL-10 and IFN-γ by ELISA, as previously described.2 Data are shown as mean ± SEM.
Interestingly, IL-12 like IFN-α also promotes both IFN-γ and IL-10 synthesis.4 This parallel induction of IFN-γ and IL-10 might be important for the natural regulation of immune responses. Moreover, it could be relevant to the therapeutic applications of IFN-α, especially in HES. Indeed, we found that IFN-α increased IFN-γ production by in vivo differentiated CD4+CD3− Th2 clones isolated from two patients with HES. As IFN-γ inhibits differentiation of eosinophils and their recruitment into tissue,5,6 we suggest that the beneficial effects of IFN-α in this condition7 might be related not only to inhibition of IL-5 and upregulation of IL-10 synthesis but also to IFN-γ upregulation.