Abstract
T cells that express the αβ T-cell receptor are thought to be the T-cell population primarily responsible for facilitating alloengraftment. The role of γδ+ T cells that comprise only a minority of mature T cells in promoting allogeneic engraftment, however, has not been extensively studied. The purpose of this study was to determine whether γδ T cells were capable of facilitating alloengraftment in murine recipients of major histocompatibility complex-mismatched marrow grafts. We developed a model where engraftment of C57BL/6 × 129/F2 (H-2b) marrow in sublethally irradiated (800 cGy) recipients (AKR/J, H-2k) is dependent on the presence of mature donor T cells in the marrow graft. In this model, donor T-cell engraftment was significantly augmented by as few as 1 × 105 αβ T cells. The role of γδ T cells was then investigated using transgenic donors (C57BL/6 × 129 background) in which a portion of the T-cell receptor–β chain gene was deleted by gene targeting so that these mice lack αβ T cells. Addition of 10 × 106 naive γδ T cells to T-cell depleted marrow grafts was required to significantly increase alloengraftment, although donor T cells averaged <50% of total splenic T cells. To determine whether higher doses of γδ T cells would improve donor engraftment and eradicate residual host T cells, γδ T cells were ex vivo expanded with a γδ T-cell–specific monoclonal antibody and interleukin-2 and then transplanted into irradiated recipients. Transplantation of ≥ 160 × 106 activated γδ T cells was necessary to consistently and significantly augment donor cell chimerism and enhance hematopoietic reconstitution when compared to control mice, but host T cells persisted in these chimeras. Addition of 2.5 × 104 mature αβ T cells, which alone were incapable of facilitating engraftment, to T-cell depleted marrow grafts containing 160 × 106 activated γδ T cells resulted in long-term (<100 day) complete donor engraftment, indicating that limiting numbers of αβ T cells were required in the marrow graft for the eradication of residual host T cells. Using serial weight curves and B-cell reconstitution as end points, clinically significant graft-versus-host disease was not observed in these chimeras under these experimental conditions. These data show that, whereas less potent than αβ T cells, γδ T cells are able to promote engraftment and enhance hematopoietic reconstitution in allogeneic marrow transplant recipients.
THE PROCESS of engraftment of allogeneic bone marrow (BM) can be conceptualized as the dynamic interplay between residual host immunity and transplanted donor immune effector cell populations, which must either eradicate or inactivate host cells for engraftment of donor hematopoietic stem cells to occur. A significant body of evidence indicates that donor-derived T cells play a critical role in this process. The most compelling evidence derives from the observation that T-cell depletion (TCD) of the donor marrow has, in most instances, led to higher rates of nonengraftment in human transplantation.1 This premise is also supported by murine models of transplantation where the addition of graded doses of donor T cells to the marrow inoculum has been able to overcome graft resistance in major histocompatibility complex (MHC)-incompatible donor/recipient strain combinations.2 Furthermore, in suboptimally conditioned murine recipients, increasing the number of donor T cells in the graft has been able to compensate for a less intense preparative regiment.3 Collectively, these data are evidence that donor T cells play a pivotal role in overcoming graft resistance across the MHC.
Donor T cells that express the αβ T-cell receptor (TCR) are thought to be primarily responsible for facilitating alloengraftment in both human and animal models because of the fact that these cells comprise the vast majority of mature T cells. In contrast, the role of T cells that express the γδ TCR and constitute a minor population of mature T cells in alloengraftment has not be extensively studied. αβ and γδ T cells share similarities in that both differentiate primarily in the thymus, possess common cell surface markers, and have a diversity of clonotypic receptors associated with the CD3 complex.4-6 Both αβ and γδ T cells are also able to secrete a variety of lymphokines7,8 and have cytotoxic capability.9,10 However, while having certain similarities, αβ and γδ T cells also have significant differences. Unlike αβ T cells, the majority of γδ T cells lack the functional expression of CD4 and CD8 molecules and the manner in which γδ T cells recognize alloantigen appears to be different from that of αβ T cells.11-16 These observations suggest that αβ and γδ T cells may each have unique roles. Moreover, from a clinical perspective, we have previously shown that patients transplanted with TCD marrow grafts in which γδ T cells are preferentially spared from the depletion procedure have high rates of engraftment, suggesting that γδ T cells may be important in facilitating engraftment.17 Therefore, the purpose of this study was to determine whether γδ T cells were capable of facilitating alloengraftment of rigorously TCD bone marrow in the absence of supplemental mature αβ T cells. We evaluated this question in a murine model where donor (C57BL/6 × 129/F2, H-2b) and recipient (AKR/J, H-2k) are mismatched at the major histocompatibility complex and where alloengraftment is dependent on the addition of mature donor T cells to the marrow graft.
MATERIALS AND METHODS
Mice.AKR/J (H-2k, Thy1.1+), C57BL/6 × 129/F2 (H-2b, Thy 1.2+), TCR β−/β− (C57BL/6 × 129/J background, αβ T-cell deficient), TCR δ−/δ− (C57BL/6 × 129/J background, γδ T-cell deficient), TCR β−/β− (pure C57BL/6 background) and normal C57BL/6 (H-2b, Thy1.2+) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in the American Association for Lab Animal Care (AALAC)-accredited Animal Resource Center of the Medical College of Wisconsin. The characteristics of the transgenic mice have been previously described by Mombaerts et al.18 Mice received regular mouse chow and acidified tap water ad libitum.
Flow cytometric analysis.Monoclonal antibodies (MoAbs) conjugated to either fluoroscein isothiocyanate (FITC) or phycoerythrin (PE) were used to assess chimerism in marrow transplant recipients. FITC-anti-Thy1.2 (CD90, rat IgG2b), and PE anti-L3T4 (CD4, rat IgG2b) were purchased from Collaborative Biomedical Products (Bedford, MA). FITC-anti-Ly5 (B220, rat IgG2a) and PE anti-Lyt2 (CD8, rat IgG2a) were obtained from Caltag (San Francisco, CA). PE anti-H57-597 (TCB αβ, hamster IgG), PE hamster IgG (isotype control), and FITC-anti-H-2Kb (Class I, mouse IgG2a) were all purchased from Pharmingen (San Diego, CA). γδ T cells were distinguished using a biotinylated antibody specific for the γδ TCR (3A10, hamster IgG, kindly provided by Dr S. Tonegawa, Massachusetts Institute of Technology, Cambridge)19 followed by secondary conjugation to PE-streptavidin. Spleen cells were obtained from chimeras at defined intervals posttransplant and stained for two-color analysis. Cells were analyzed on a FACS analyzer (Becton Dickinson, Mountain View, CA) with Consort 32 computer support and Lysis II software. Red cells and nonviable cells were excluded using forward and side scatter settings before analysis of spleen cell populations. Ten thousand cells were analyzed for each determination whenever possible.
Ex vivo expansion of γδ T cells.Spleen cells were obtained from TCR β−/β− donor animals and passed through nylon wool columns to remove B cells. The resulting population was typically comprised of approximately 50% cells expressing the γδ TCR. Cells were then resuspended in CDMEM plus 10% fetal bovine serum (FBS) and cultured in flasks precoated with an immobilized γδ T-cell–specific MoAb (GL4, hamster IgG; Pharmingen) at a concentration of 5 to 10 μg/mL. Twenty-four hours after the initiation of culture, human interleukin-2 (IL-2; Cetus Corp, Norwalk, CT) was added at a concentration of 20 U/mL (Cetus units). All cultures were split into fresh flasks as needed to maintain a cell concentration of 0.5 to 1.5 × 106 cells/mL. Cells were exposed to immobilized MoAb for the first 3 to 4 days of culture and thereafter grown only in medium plus IL-2 to allow for reexpression of the γδ TCR. After a total of 7 to 8 days in culture, cells were counted and the percentage of γδ T cells was analyzed by flow cytometry. Routinely, at total of 5 to 10 × 109 cells from C57BL/6 × 129/J donors was obtained with 95% to 99% of cells expressing the γδ TCR and <1% expressing the NK1.1 antigen. When γδ T cells were expanded from pure B6 background β−/β− mice, similar results were obtained for γδ T-cell percentages. However, a minority of activated γδ T cells (∼30%) from these animals coexpress NK1.1 and thus natural killer (NK) cells could not be independently quantified.
Assessment of hematopoietic reconstitution.Peripheral blood (PB) was obtained by retroorbital venipuncture and collected in EDTA-containing blood tubes. PB counts (ie, white blood cells, hematocrit, and platelet counts) were determined on a Coulter STK-S machine (Hialeah, FL). Because platelet counts greater ≥ 1 × 106/mm3 could not be accurately quantitated, mice whose platelets exceeded 1 million were arbitrarily defined as having a platelet count of exactly 1 × 106. All PB counts were subsequently corrected for the amount of diluent contained in the collection tube. In three instances, white blood cell (WBC) and/or platelet counts were not measurable on the Coulter machine because of technical problems. In these cases, the WBC count was estimated by counting contiguous fields on the blood smear under 40× magnification, averaging the number of cells/field, and multiplying by 2,500/mm3. Platelet counts were calculated similarly under oil (100× magnification) and multiplying the average by 15,000/mm3.
BM transplantation.BM was flushed from donor femurs and tibias with complete Dulbecco′s modified essential medium (CDMEM) and passed through sterile mesh filters to obtain single cell suspensions. BM was TCD in vitro with anti-Thy1.2 MoAb plus low toxicity rabbit complement (C6 Diagnostics, Mequon, WI). The hybridoma for 30-H12 (anti-Thy1.2, rat IgG2b) antibody was obtained from the American Tissue Culture Center (Rockville, MD) and grown in CDMEM plus 5% FBS. The culture supernatants were then harvested, precipitated in ammonium sulfate, and dialyzed against phosphate-buffered saline before use in in vitro depletion experiments. The efficacy of TCD was confirmed with a cytolytic limiting dilution assay (LDA), which indicated precursor T-cell frequencies of 1/13, 777, and >1/400,000 before and after TCD, respectively. Each succeeding lot of antibody that was used in these studies was similarly screened in an LDA before in vitro depletion with equivalent results. BM cells were then washed and resuspended in DMEM before injection.
Spleen cell suspensions were obtained by pressing spleens through wire mesh screens. Erythrocytes were removed from spleen cell suspensions by hypotonic lysis with sterile distilled water. T cells (either αβ or γδ T cells) for admixture with TCD BM before transplantation were then obtained by passing spleen cells once or twice through nylon wool columns (Robbins Scientific, Sunnyvale, CA) to remove B cells. The percentage of αβ+ T cells from B6129 or B6 donors and γδ+ T cells from β−/β− donors was quantified by flow cytometry. αβ+ T cells were defined as Thy1.2+ αβ+ and γδ T cells as Thy1.2+ γδ+. The average number of naive αβ or γδ T cells in the spleen cell suspensions after nylon wool depletion is indicated in the respective Table legends. The remaining cells as assessed by flow cytometry consisted of NK cells (NK1.1+) and residual B cells not removed by the nylon wool columns.
AKR recipient mice were given varying doses of total body irradiation (TBI) as a single exposure at a dose rate of 79 cGy using a Shepherd Mark I Cesium Irradiator (J.L. Shepherd and Associates, San Fernando, CA). Irradiated AKR/J recipients then received a single intravenous injection of TCD BM (10 × 106) with or without added naive or ex vivo activated spleen cells.
Experimental design.Analysis of the role of γδ T cells on alloengraftment is dependent on the ability to obtain sufficient numbers of γδ T cells and to insure that these cells are not contaminated by αβ T cells. To this end, we employed transgenic mice (129/J × C57BL/6, H-2b), in which a portion of the β chain had been deleted by gene targeting, as donors for these experiments. Henceforth, these mice are referred to as β0, reflecting the fact that they do not make αβ T cells. Normal 129 × C57BL/6/F2 mice in which there are no qualitative or quantitative abnormalities in αβ T cells were used as comparable donor control animals, and are referred to as B6129. In latter experiments, availability of pure background C57BL/6 transgenic mice from Jackson Labs allowed us to validate our findings with a genetically homogenous donor strain. In these studies, normal C57BL/6 (B6) mice were employed as comparable donor control animals. AKR/J (H-2k) mice served as recipients in all these studies. Thus, donor and recipient differed at both class I and class II, as well as at multiple minor histocompatibility loci.
Statistics.Group comparisons of mean donor T-cell chimerism, donor cell chimerism, overall spleen cell content, splenic B-cell content, and PB counts were performed using the unpaired Student′s t-test. The correlation between the percentage of donor T-cell engraftment and the number of splenic B cells was assessed using Pearson′s correlation coefficient. A two-tailed P value ≤ .05 was deemed to be significant.
RESULTS
AKR mice reject TCD B6129 marrow grafts when conditioned with < 900 cGy.Initial experiments were conducted to determine the dose of TBI, which would allow engraftment of TCD B6129 BM in AKR recipients. Recipient animals were conditioned with doses of TBI ranging from 700 to 1,000 cGy in 100 cGy increments and then transplanted with 10 × 106 TCD B6129 marrow cells. The rationale for using B6129 as opposed to β0 BM was to allow for the normal reconstitution of BM-derived αβ T cells, analogous to what occurs in clinical marrow transplantation. At 45 to 62 days posttransplant, mice were sacrificed and the extent of donor T-cell chimerism in the spleen was determined by two-color immunofluorescence (Table 1). All recipients were reconstituted with donor T cells when conditioned with 1,000 cGy. At a dose of 900 cGy, only one-half of the recipients had evidence of donor T-cell engraftment, whereas all mice conditioned with 700 to 800 cGy rejected their grafts. Based on these data, a dose of 800 cGy was determined to be the maximal dose that would result in uniform rejection of TCD marrow grafts from B6129 donors.
Effect of TBI Dose on Engraftment of TCD B6129 BM
| Group . | No. of Mice . | Dose of TBI (cGy) . | (%) Donor T Cells in Spleen . |
|---|---|---|---|
| I | 4 | 700 | 1, 2, 1, 1 |
| II | 4 | 800 | 1, 1, 1, 1 |
| III | 4 | 900 | 4, 70, 1, 59 |
| IV | 4 | 1,000 | 74, 68, 66, 72 |
| Group . | No. of Mice . | Dose of TBI (cGy) . | (%) Donor T Cells in Spleen . |
|---|---|---|---|
| I | 4 | 700 | 1, 2, 1, 1 |
| II | 4 | 800 | 1, 1, 1, 1 |
| III | 4 | 900 | 4, 70, 1, 59 |
| IV | 4 | 1,000 | 74, 68, 66, 72 |
AKR recipients were irradiated with increasing exposures of TBI and then transplanted with 10 × 106 TCD B6129 BM. Animals were sacrificed 45 to 62 days posttransplant and T-cell chimerism in the spleen was assessed. Donor T cells were defined as Thy 1.2+/L3T4+ and Thy 1.2+/Lyt2+.
Alloengraftment Is Dependent on Mature Donor T Cells in The Marrow Graft
| Group . | No. of Mice . | Donor αβ T Cells Added . | (%) Donor T Cells* . | (Mean) . | No. of Splenic B Cells (×10−6)* . | (Mean) . |
|---|---|---|---|---|---|---|
| I | 11 | None | 1, 1, 1, 2, 3, 4, 1, 2, 2, 11, 1 | (3) | 2, 12, 0, 1, 3, 1, 1, 0, 0, 6, 1 | (2) |
| II | 4 | 1 × 104 | 3, 2, 5, 1 | (3) | 1, 1, 1, 1 | (1) |
| III | 7 | 5 × 104 | 79, 8, 0, 3, 57, 1, 1 | (21) | 17, 0, 0, 1, 37, 1, 0 | (8) |
| IV | 5 | 1 × 105 | 85, 90, 89, 69, 1 | (67†) | 32, 12, 24, 33, 2 | (21) |
| V | 4 | 5 × 105 | 90, 94, 62, 90 | (84†) | 14, 15, 1, 39 | (17) |
| VI | 4 | 1 × 106 | 93, 82, 88, 91 | (88†) | 40, 24, 24, 32 | (30) |
| VII | 4 | 2.3 × 106 | 96, 93, 95, 95 | (95†) | 0, 10, 2, 3 | (4) |
| Group . | No. of Mice . | Donor αβ T Cells Added . | (%) Donor T Cells* . | (Mean) . | No. of Splenic B Cells (×10−6)* . | (Mean) . |
|---|---|---|---|---|---|---|
| I | 11 | None | 1, 1, 1, 2, 3, 4, 1, 2, 2, 11, 1 | (3) | 2, 12, 0, 1, 3, 1, 1, 0, 0, 6, 1 | (2) |
| II | 4 | 1 × 104 | 3, 2, 5, 1 | (3) | 1, 1, 1, 1 | (1) |
| III | 7 | 5 × 104 | 79, 8, 0, 3, 57, 1, 1 | (21) | 17, 0, 0, 1, 37, 1, 0 | (8) |
| IV | 5 | 1 × 105 | 85, 90, 89, 69, 1 | (67†) | 32, 12, 24, 33, 2 | (21) |
| V | 4 | 5 × 105 | 90, 94, 62, 90 | (84†) | 14, 15, 1, 39 | (17) |
| VI | 4 | 1 × 106 | 93, 82, 88, 91 | (88†) | 40, 24, 24, 32 | (30) |
| VII | 4 | 2.3 × 106 | 96, 93, 95, 95 | (95†) | 0, 10, 2, 3 | (4) |
Irradiated (800 cGy) AKR recipients were transplanted with 10 × 106 TCD B6129 marrow cells with or without the indicated number of αβ T cells. Enriched populations of αβ T cells were obtained by nylon wool depletion of spleen cells from B6129 donors. The average number of αβ+ T cells in the splenic inocula after nylon wool depletion was 86%. The number of αβ T cells administered to recipients was quantitated by multiplying the percent of total cells that stained positively with Thy 1.2 and H57-597 antibodies by the total spleen cell dose. Mice were sacrificed at 26 to 35 days posttransplant and chimerism in the spleen was assessed. Donor T cells were defined as Thy 1.2+/L3T4+ and Thy 1.2+/Lyt2+.
Numbers represent data for individual mice.
P < .02 relative to group I (no added T cells).
Engraftment is dependent on the presence of donor T cells in the marrow graft.Having defined the maximal dose of TBI, which resulted in uniform rejection of TCD B6129 BM in AKR recipients, we then evaluated whether addition of donor T cells to the marrow graft was able to facilitate alloengraftment. Irradiated recipients were transplanted with TCD B6129 BM with or without graded doses of T cells from B6129 donors (Table 2). Because the majority of T cells in the spleens of these mice are αβ+ (>98%), this was essentially a functional assessment of the capability of αβ T cells to promote engraftment. As expected, animals in group I that received TCD B6129 BM only all rejected their grafts, as evidenced by the absence of donor T cells. Recipients transplanted with 1 × 104 αβ T cells (P = .44) or 5 × 104 αβ T cells (P = .17) had levels of T-cell engraftment that were not significantly different from control animals (Table 2). However, when mice were transplanted with ≥ 1 × 105 αβ T cells, donor T-cell engraftment was significantly enhanced (P < .02). Although there was a trend toward increased donor T-cell chimerism with higher doses of αβ T cells, this was not statistically significant (P = .17, group IV v VII). We then evaluated whether there was a correlation between donor T-cell engraftment and engraftment of other non T-cell populations in the spleens of transplanted recipients to determine whether donor T cells facilitated hematopoietic reconstitution. We employed B-cell reconstitution as an end point because this cell constitutes the major cell population in the spleen. A statistically significant correlation between the percentage of splenic donor T cells and the magnitude of B-cell repopulation in the spleen of these animals was observed (Table 2) (r = .66, P < .00001), indicating that donor T-cell engraftment promotes B-cell reconstitution in this model.
Facilitation of alloengraftment by γδ T-cell–enriched spleen cells.Studies were then conducted to determine if γδ T cells were capable of preventing graft rejection in this model. Irradiated (800 cGy) AKR recipients were transplanted with TCD B6129 BM with or without graded doses of γδ T-cell enriched spleen cells. Animals transplanted with TCD BM only all rejected their grafts (6% mean donor T cells, n = 18) (Table 3). The addition of spleen cells containing ≥ 10 × 106 γδ T cells to the marrow graft was required to significantly enhance donor T-cell engraftment (P < .02), whereas doses below this threshold were ineffective in promoting alloengraftment. However, there was some variability in the degree of donor T-cell engraftment observed between animals transplanted with ≥ 10 × 106 γδ T cells as half (7/14) of these mice had ≥ 50% donor T-cell chimerism in the spleen whereas 3 of 14 had < 10% donor T cells. As was observed in mice transplanted with αβ+ T cells, there was a significant correlation between the percentage of donor T cells and the number of splenic B cells (Pearson′s correlation coefficient r = .72, P < .00001). These data suggested that γδ T cells were capable of facilitating engraftment of donor-derived B cells.
Facilitation of Alloengraftment by Naive γδ T Cells
| Group . | No. of Mice . | Donor γδ T Cells Added . | (%) Donor T Cells3-150 . | (Mean) . | No. of Splenic B Cells (×10−6)3-150 . | (Mean) . |
|---|---|---|---|---|---|---|
| I | 18 | None | Mean 6 ± 5 | Mean 2 ± 3 | ||
| II | 5 | 1.3 × 106 | 3, 2, 1, 1 | (3) | 1, 1, 1, 0, 0 | (1) |
| III | 4 | 2.8 × 106 | 3, 3, 11, 2 | (5) | 1, 1, 1, 0 | (1) |
| IV | 4 | 5 × 106 | 12, 30, 45, 4 | (23) | 1, 20, 14, 1 | (9) |
| V | 7 | 10 × 106 | 62, 67, 66, 63, 6, 4, 28 | (42†) | 38, 40, 38, 12, 4, 1, 49 | (26) |
| VI | 7 | 20 × 106 | 43, 74, 79, 66, 1, 31, 45 | (48‡) | 3, 44, 18, 44, 1, 9, 32 | (22) |
| Group . | No. of Mice . | Donor γδ T Cells Added . | (%) Donor T Cells3-150 . | (Mean) . | No. of Splenic B Cells (×10−6)3-150 . | (Mean) . |
|---|---|---|---|---|---|---|
| I | 18 | None | Mean 6 ± 5 | Mean 2 ± 3 | ||
| II | 5 | 1.3 × 106 | 3, 2, 1, 1 | (3) | 1, 1, 1, 0, 0 | (1) |
| III | 4 | 2.8 × 106 | 3, 3, 11, 2 | (5) | 1, 1, 1, 0 | (1) |
| IV | 4 | 5 × 106 | 12, 30, 45, 4 | (23) | 1, 20, 14, 1 | (9) |
| V | 7 | 10 × 106 | 62, 67, 66, 63, 6, 4, 28 | (42†) | 38, 40, 38, 12, 4, 1, 49 | (26) |
| VI | 7 | 20 × 106 | 43, 74, 79, 66, 1, 31, 45 | (48‡) | 3, 44, 18, 44, 1, 9, 32 | (22) |
Irradiated (800 cGy) AKR recipients were transplanted with 10 × 106 TCD B6129 marrow cells with or without the indicated number of γδ T cell enriched spleen cells (obtained from β0 donors on a B6129 background). The average number of γδ+ T cells in the splenic inocula after nylon wool depletion was 53%. The number of γδ T cells administered to recipients was quantitated by multiplying the percent of total cells which stained positively for Thy 1.2 and 3A10 antibodies by the total spleen cell dose. Donor T cells in the spleen were defined as Thy 1.2+/Lyt2+, Thy 1.2+/L3T4+, and Thy 1.2+/γδ TCR+. All mice in these experiments were evaluated 26 to 35 days posttransplant, except for the first four mice in group VI that were tested 59 to 61 days after transplant.
Numbers represent data for individual mice.
P < .02 (Relative to group I).
P < .007 (Relative to group I).
Graft Enhancing Effect of Activated γδ T Cells
| Group . | No. of Mice . | Donor γδ T . | (%) Donor T Cells . | (Mean) . | No. of Splenic B Cells (×10−6) . | (Mean) . | (%) Donor (H-2b) Cells . | (Mean) . |
|---|---|---|---|---|---|---|---|---|
| . | . | Cells Added . | . | . | . | . | . | . |
| I | 12 | None | 2, 3, 2, 3, 3, 4, 6, 3, 4, 3, 2, 2 | (3) | 1, 8, 5, 0, 1, 1, 1, 0, 0, 1, 2, 0 | (1) | 4, 5, 2, 27, 5, 2, 8, 5, 5‡ | (7) |
| II | 5 | 5 × 106 | 7, 25, 4, 2, 16 | (11) | 0, 23, 2, 2, 1 | (6) | ND | |
| III | 5 | 10 × 106 | 5, 9, 8, 3, 3 | (6) | 4, 1, 10, 0, 1 | (3) | ND | |
| IV | 5 | 20 × 106 | 2, 3, 1, 5, 42 | (11) | 17, 0, 2, 1, 22 | (8) | ND | |
| V | 5 | 40 × 106 | 45, 25, 40, 5, 48 | (33*) | 36, 4, 28, 1, 8 | (15) | ND | |
| VI | 5 | 80 × 106 | 37, 37, 45, 4, 2 | (25) | 20, 44, 6, 1, 1 | (14) | 76, 85, 74, 11, 5 | (50) |
| VII | 4 | 160 × 106 | 57, 53, 20, 53 | (46*) | 49, 39, 1, 36 | (31) | 90, 88, 43, 90 | (78†) |
| VIII | 7 | 240 × 106 | 72, 69, 67, 64, 29, 0, 62 | (52*) | 52, 68, 61, 50, 4, 0, 28 | (38) | 94, 93, 92, 91, 82, 3, 91 | (78†) |
| Group . | No. of Mice . | Donor γδ T . | (%) Donor T Cells . | (Mean) . | No. of Splenic B Cells (×10−6) . | (Mean) . | (%) Donor (H-2b) Cells . | (Mean) . |
|---|---|---|---|---|---|---|---|---|
| . | . | Cells Added . | . | . | . | . | . | . |
| I | 12 | None | 2, 3, 2, 3, 3, 4, 6, 3, 4, 3, 2, 2 | (3) | 1, 8, 5, 0, 1, 1, 1, 0, 0, 1, 2, 0 | (1) | 4, 5, 2, 27, 5, 2, 8, 5, 5‡ | (7) |
| II | 5 | 5 × 106 | 7, 25, 4, 2, 16 | (11) | 0, 23, 2, 2, 1 | (6) | ND | |
| III | 5 | 10 × 106 | 5, 9, 8, 3, 3 | (6) | 4, 1, 10, 0, 1 | (3) | ND | |
| IV | 5 | 20 × 106 | 2, 3, 1, 5, 42 | (11) | 17, 0, 2, 1, 22 | (8) | ND | |
| V | 5 | 40 × 106 | 45, 25, 40, 5, 48 | (33*) | 36, 4, 28, 1, 8 | (15) | ND | |
| VI | 5 | 80 × 106 | 37, 37, 45, 4, 2 | (25) | 20, 44, 6, 1, 1 | (14) | 76, 85, 74, 11, 5 | (50) |
| VII | 4 | 160 × 106 | 57, 53, 20, 53 | (46*) | 49, 39, 1, 36 | (31) | 90, 88, 43, 90 | (78†) |
| VIII | 7 | 240 × 106 | 72, 69, 67, 64, 29, 0, 62 | (52*) | 52, 68, 61, 50, 4, 0, 28 | (38) | 94, 93, 92, 91, 82, 3, 91 | (78†) |
Irradiated (800 cGy) AKR recipients were transplanted with 10 × 106 TCD B6129 marrow cells with or without the indicated number of activated γδ T cells. Activated γδ T cells were obtained from β0 donor animals (B6129 background) as described in Materials and Methods. Animals transplanted with 160 × 106 γδ T cells received two intravenous injections of 80 × 106 cells 1 hour apart. Mice transplanted with 240 × 106 γδ T cells received an additional injection of 80 × 106 cells 1 day posttransplant. All mice were analyzed 29 to 34 days after transplant.
P < .02 (Relative to group I).
P ≤ .002 (Relative to group I).
Only 9 of 12 mice in this group were tested.
Activated γδ T cells facilitate alloengraftment.The administration of higher doses of naive γδ T cells (ie, > 20 × 106) to recipients was limited by constraints pertaining to the number of cells, which could be feasibly obtained from transgenic donors. Therefore, we examined an alternative strategy to obtain larger numbers of γδ T cells. Specifically, we evaluated the ability of γδ T cells that were activated ex vivo with a γδ T-cell specific MoAb and then selectively expanded in recombinant IL-2 to facilitate alloengraftment. This also allowed us to assess the role of a purer population of γδ T cells in facilitating engraftment as well as determine the effect of activation of γδ T cells on alloengraftment. Transplantation of ≤ 20 × 106 activated γδ T cells failed to enhance donor T-cell engraftment in sublethally irradiated recipients (Table 4), in contrast to what was observed with naive γδ T cells. The addition of ≥ 160 × 106 γδ T cells to TCD BM was required before a significant increase in donor T cell (P ≤ .02) and overall donor cell (P ≤ .002) engraftment was observed. Seven of eleven animals in these groups (VII and VIII) had ≥90% H-2b cells and 8 of 11 ≥50% donor T cells. The extent of donor T-cell chimerism was also significantly correlated with the magnitude of splenic B-cell reconstitution in these chimeras as individual mice with higher percentages of splenic donor T cells also had greater numbers of B cells (r = .88, P < .00001). These data indicated that activated γδ T cells can prevent graft rejection and that activation of γδ T cells per se does not preclude these cells from facilitating engraftment.
Limiting numbers of mature αβ T cells are required in the marrow graft in addition to activated γδ T cells for complete allogeneic engraftment.Despite the administration of large numbers of activated γδ T cells, residual host T cells persisted in these chimeras. Since prior studies by other investigators20 have indicated that γδ T cells may require the presence of αβ T cells for optimal function, experiments were performed to determine whether the addition of limiting numbers of αβ T cells to the marrow graft was required to eradicate remaining host cells. Because αβ T cells themselves can facilitate alloengraftment when present in sufficient numbers (Table 2), the dose of αβ T cells had to be low enough so as not to be able to facilitate measurable engraftment. We had previously shown that AKR mice consistently rejected TCD B6129 BM if less than 50,000 αβ T cells were present in the marrow graft. Therefore, we added 25,000 αβ T cells to these grafts to determine if donor T-cell chimerism could be further enhanced. As expected, mice transplanted with TCD B6129 only all rejected their grafts (Table 5). When AKR recipients were transplanted with TCD B6129 BM and 160 × 106 γδ T cells, both donor T cell and overall donor cell engraftment were significantly enhanced (P < .0001). We then assessed whether the addition of limiting numbers of mature αβ T cells to the marrow inoculum could further enhance donor cell engraftment. αβ T cells were obtained from δ0 donors (B6129 background) and contained no γδ T cells. Animals transplanted with TCD BM plus 2.5 × 104 αβ T cells rejected their grafts indicating that this number of αβ T cells could not facilitate measurable engraftment. The addition of 2.5 × 104 αβ T cells to 160 × 106 γδ T cells, however, enhanced donor T-cell chimerism and overall donor cell engraftment when compared to mice transplanted with activated γδ T cells alone (P ≤ .03, Group II v IV). These data indicated that to achieve complete (>98% H-2b+ cells) allogeneic engraftment after transplantation with activated γδ T cells, small numbers of mature αβ T cells were also required to be present in the graft.
Limiting Numbers of Mature αβ T Cells Are Required for Complete Donor Chimerism
| Group . | No. of Mice . | Splenic T Cells Added . | (%) Donor T Cells5-150 . | (Mean) . | (%) Donor (H-2b) Cells5-151 . | (Mean) . | |
|---|---|---|---|---|---|---|---|
| . | . | αβ . | γδ . | . | . | . | . |
| I | 2 | None | None | 1, 2 | (2) | 5, 3 | (4) |
| II | 5 | None | 160 × 106 | 74, 69, 83, 82, 82 | (78) | 92, 92, 98, 96, 94 | (94) |
| III | 6 | 2.5 × 104 | None | 4, 3, 1, 1, 1, 3 | (2) | 8, 6, 9, 6, 3, 68 | (17) |
| IV | 6 | 2.5 × 104 | 160 × 104 | 91, 89, 93, 88, 90, 89 | (90) | 99, 98, 99, 98, 98, 98 | (98) |
| Group . | No. of Mice . | Splenic T Cells Added . | (%) Donor T Cells5-150 . | (Mean) . | (%) Donor (H-2b) Cells5-151 . | (Mean) . | |
|---|---|---|---|---|---|---|---|
| . | . | αβ . | γδ . | . | . | . | . |
| I | 2 | None | None | 1, 2 | (2) | 5, 3 | (4) |
| II | 5 | None | 160 × 106 | 74, 69, 83, 82, 82 | (78) | 92, 92, 98, 96, 94 | (94) |
| III | 6 | 2.5 × 104 | None | 4, 3, 1, 1, 1, 3 | (2) | 8, 6, 9, 6, 3, 68 | (17) |
| IV | 6 | 2.5 × 104 | 160 × 104 | 91, 89, 93, 88, 90, 89 | (90) | 99, 98, 99, 98, 98, 98 | (98) |
Irradiated (800 cGy) AKR recipients were transplanted with 10 × 106 TCD B6129 BM marrow cells with or without the indicated number of activated γδ T cells from β 0 donors or naive αβ T cells from δ0 donors. All donor mice were on a B6129 genetic background. Animals transplanted with a total of 160 × 106 γδ T cells received intravenous injections of 80 × 106 cells on the day of transplant and 1 day posttransplant. αβ T cells were obtained by nylon wool depletion of spleen cells from γδ T-cell–deficient (δ0) mice. All mice were analyzed 28 days posttransplant.
Key statistical comparisons: groups II and IV v I, P < .0001; group I v III, P = .54, group II v IV, P = .014.
Groups II and IV v I, P < .001, group I v III, P = .53, group II v IV, P = .03.
γδ T cells facilitate long-term alloengraftment.Because activated γδ T cells were able to facilitate complete allogeneic engraftment when animals were sacrificed at ∼30 days posttransplant, we assessed whether engraftment was durable when tested more than three months post BMT. To permit a comparative analysis of the relative potency of αβ and γδ T cells in facilitating long-term engraftment, groups of mice were also transplanted with either TCD BM alone or TCD BM plus graded doses of αβ T cells that had been shown to promote short-term alloengraftment (Table 2). The majority of animals transplanted with TCD BM or TCD BM plus 1 × 105 αβ T cells rejected their grafts after 100 days (Table 6). A minimum dose of 5 × 105 αβ T cells was required in this model for durable donor engraftment (P < .002 relative to TCD BM only). The addition of 20 × 106 naive γδ T cells was ineffective in promoting long-term engraftment. Only one of four mice evaluable after 86 days had donor cell engraftment; however, this animal also had evidence of graft-versus-host disease (GVHD) (∼30% loss of body weight). One additional animal died 43 days posttransplant because of GVHD. In contrast, mice transplanted with 160 × 106 activated γδ T cells had augmented donor T-cell chimerism and donor cell repopulation in the spleen (P < .02 v group I). The addition of limiting numbers of αβ T cells (2.5 × 104) to grafts containing 160 × 106 activated γδ T cells (group VIII) further enhanced donor T cell (P < .0001) and overall donor cell chimerism (P < .05), and resulted in complete allogeneic engraftment (ie, 100% H-2b+ cells), when compared to mice transplanted with 160 × 106 activated γδ T cells only (group VI).
Facilitation of Long-Term Alloengraftment (<100 Day) by γδ T Cells
| Group . | No. of Mice . | Donor αβ T Cells Added . | Donor γδ T Cells Added . | (%) Donor T Cells6-150 . | (%) Donor (H-2b) Cells6-151 . | Splenic B Cells (×10−6) . | Spleen Size . | Day 100 . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | (×10−6) . | No. Mice Surviving . | No. Mice Engrafting . |
| I | 6 | None | None | 49, 3, 1, 1, 3 | 94, 6, 3, 3, 3 | 77, 26, 24, 28, 23 | 111, 45, 56, 65, 54 | 5/6 | 1/6 |
| II | 4 | 1 × 105 | None | 83, 2 | 96, 4 | 29, 23 | 52, 34 | 2/4 | 1/4 |
| III | 4 | 5 × 105 | None | 87, 82, 90, 88 | 99, 98, 99, 98 | 21, 41, 8, 16 | 56, 78, 19, 39 | 4/4 | 4/4 |
| IV | 3 | 1 × 106 | None | 97, 80, 90 | 94, 99, 95 | 0, 5, 1 | 1, 16, 2 | 3/3 | 3/3 |
| V | 5 | None | 20 × 106 | 1, 0, 86‡, 0 | 4, 3, 98, 10 | 18, 7, 15, 3 | NA | 3/5 | 1/5 |
| VI | 3 | None | 160 × 106 | 83, 86, 84 | 98, 97, 96 | 120, 63, 36 | 204, 147, 73 | 3/3 | 3/3 |
| VII | 3 | 2.5 × 104 | None | 3, 3, 89 | 3, 2, 98 | 18, 14, 58 | 44, 31, 144 | 3/3 | 1/3 |
| VIII | 4 | 2.5 × 104 | 160 × 106 | 95, 95, 96, 94 | 100, 100, 100, 100 | 58, 87, 10, 54 | 154, 175, 39, 117 | 4/4 | 4/4 |
| Group . | No. of Mice . | Donor αβ T Cells Added . | Donor γδ T Cells Added . | (%) Donor T Cells6-150 . | (%) Donor (H-2b) Cells6-151 . | Splenic B Cells (×10−6) . | Spleen Size . | Day 100 . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | (×10−6) . | No. Mice Surviving . | No. Mice Engrafting . |
| I | 6 | None | None | 49, 3, 1, 1, 3 | 94, 6, 3, 3, 3 | 77, 26, 24, 28, 23 | 111, 45, 56, 65, 54 | 5/6 | 1/6 |
| II | 4 | 1 × 105 | None | 83, 2 | 96, 4 | 29, 23 | 52, 34 | 2/4 | 1/4 |
| III | 4 | 5 × 105 | None | 87, 82, 90, 88 | 99, 98, 99, 98 | 21, 41, 8, 16 | 56, 78, 19, 39 | 4/4 | 4/4 |
| IV | 3 | 1 × 106 | None | 97, 80, 90 | 94, 99, 95 | 0, 5, 1 | 1, 16, 2 | 3/3 | 3/3 |
| V | 5 | None | 20 × 106 | 1, 0, 86‡, 0 | 4, 3, 98, 10 | 18, 7, 15, 3 | NA | 3/5 | 1/5 |
| VI | 3 | None | 160 × 106 | 83, 86, 84 | 98, 97, 96 | 120, 63, 36 | 204, 147, 73 | 3/3 | 3/3 |
| VII | 3 | 2.5 × 104 | None | 3, 3, 89 | 3, 2, 98 | 18, 14, 58 | 44, 31, 144 | 3/3 | 1/3 |
| VIII | 4 | 2.5 × 104 | 160 × 106 | 95, 95, 96, 94 | 100, 100, 100, 100 | 58, 87, 10, 54 | 154, 175, 39, 117 | 4/4 | 4/4 |
Irradiated (800 cGy) AKR recipients were transplanted with 10 × 106 TCD B6129 BM with or without the indicated number of naive αβ, naive γδ, or activated γδ T cells. Naive αβ T cells were procured from normal B6129 mice. Activated γδ T cells (groups VI and VIII) were obtained from spleens of β 0 donors on a B6129 background, whereas naive γδ T cells (group V) were obtained from β 0 donors on a pure B6 background. Animals were sacrificed 99 to 103 days posttransplant and assessed for overall spleen size, B-cell reconstitution, and the extent of overall donor and T-cell chimerism.
Key statistical comparisons: groups III, IV, VI, and VIII v I, P < .002; VI v VIII, P < .0001.
Groups III, IV, VI, and VIII v I, P < .02; VI v VIII, P < .05.
One animal in this group was evaluated at day 86 immediately antemortem.
To further assess the efficacy of γδ T cells in promoting long-term engraftment, we evaluated B-cell reconstitution in mice transplanted with activated γδ T cells (Table 6, groups VI and VIII). No significant differences were observed in B-cell reconstitution between animals transplanted with activated γδ T cells (mean 61 × 106, n = 7) versus those receiving TCD BM only (mean 36 × 106, n = 6, P = .19). Transplantation with activated γδ T cells, however, did result in an increased overall spleen size (mean 130 × 106, n = 7) when compared to animals transplanted with TCD marrow only (mean 66 × 106, n = 6, P < .05). These data suggested that γδ T cells enhanced overall splenic reconstitution.
γδ T cells facilitate alloengraftment in a pure background B6→AKR model.In the previous studies, we evaluated the role of γδ T cells using mixed background (B6129) donors. Because there is some donor-to-donor genetic variability in these F2 animals that could theoretically be a confounding factor, we subsequently performed experiments employing γδ T cells derived from pure background (C57BL/6) β0 donors. In the initial experiment, similar to the experimental design in Table 4, irradiated (800 cGy) AKR recipients were transplanted with or without graded doses of activated γδ T cells. PB counts were also measured in these chimeras 5-weeks posttransplant to assess the effect of γδ T cells on hematopoietic reconstitution. Sublethally irradiated recipients transplanted with TCD B6 BM all rejected their grafts, similar to what was observed with TCD B6129 BM. Although supplementation of 40 × 106 γδ T cells to the graft failed to augment these parameters of engraftment, the addition of 80 × 106 activated γδ T cells to TCD marrow grafts significantly enhanced donor T cell and overall donor chimerism (P < .03) (Table 7). Hematopoietic reconstitution was also augmented as both WBC and platelet counts were greater in these animals (group III) than in mice that rejected their grafts (group I) (P < .03).
Activated γδ T Cells From Pure Background β0 Donors Facilitate Alloengraftment and Enhance Hematopoietic Reconstitution
| Group . | No. of Mice . | Donor γδ T Cells Added . | (%) Donor T Cells7-150 . | (Mean) . | (%) (H-2b) Cells . | (Mean) . | WBC . | HCT . | Platelets . |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | (per mm3) . | . | (×1,000) . |
| I | 3 | None | 1, 1, 3 | (2) | 5, 7, 9 | (7) | 4.7 ± 1.2 | 40 ± 9.7 | 474 ± 159 |
| II | 5 | 40 × 106 | 2, 62, 24, 47, 1 | (27) | 7, 91, 54, 85, 5 | (48) | 6.9 ± 3.7 | 46 ± 5.2 | 765 ± 484 |
| III | 5 | 80 × 106 | 69, 58, 55, 3, 54 | (48†) | 91, 87, 89, 5, 85 | (71†) | 8.6 ± 1.67-151 | 46 ± 1.6 | 1,089 ± 1377-151 |
| Group . | No. of Mice . | Donor γδ T Cells Added . | (%) Donor T Cells7-150 . | (Mean) . | (%) (H-2b) Cells . | (Mean) . | WBC . | HCT . | Platelets . |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | (per mm3) . | . | (×1,000) . |
| I | 3 | None | 1, 1, 3 | (2) | 5, 7, 9 | (7) | 4.7 ± 1.2 | 40 ± 9.7 | 474 ± 159 |
| II | 5 | 40 × 106 | 2, 62, 24, 47, 1 | (27) | 7, 91, 54, 85, 5 | (48) | 6.9 ± 3.7 | 46 ± 5.2 | 765 ± 484 |
| III | 5 | 80 × 106 | 69, 58, 55, 3, 54 | (48†) | 91, 87, 89, 5, 85 | (71†) | 8.6 ± 1.67-151 | 46 ± 1.6 | 1,089 ± 1377-151 |
Irradiated (800 cGy) AKR recipients were transplanted with 10 × 106 TCD B6 marrow cells with or without the indicated number of activated γδ T cells from β 0 donors (pure B6 background). Animals were evaluated 35 to 37 days posttransplant. Donor T cells were defined as Thy 1.2+/CD3+ and donor cells as H-2b+. PB counts are reported as the mean ± 1 SD. Normal ranges for blood counts for comparison were determined by obtaining blood samples from normal nontransplanted C57BL/6 mice (n = 4). These measurements were as follows: WBC 10.3 ± 1.5, HCT 49 ± 1.1, and platelets 1,070 ± 62.
Numbers represent data for individual mice.
P ≤ .03 relative to group I.
In a second experiment, we evaluated whether the presence of a limiting number of mature αβ T cells was required for complete allogeneic engraftment, using an experimental design identical to that in Table 5. Mice transplanted with TCD BM plus 160 × 106 activated γδ T cells had significantly enhanced donor T cell (P < .001) and donor cell engraftment (P < .0001), although host T cells persisted in these chimeras (Table 8). The addition of 2.5 × 104 αβ T cells to the same marrow graft further augmented engraftment (P < .05, group IV v group III, Table 8) with 99% overall donor cell chimerism. Engraftment parameters in mice transplanted with TCD BM plus 2.5 × 104 αβ T cells did not differ from those of animals receiving TCD BM only (P ≥ .50, group I versus II for donor T cell and donor cell chimerism). Collectively, these data validate the results of prior experiments performed using mixed background donors, substantiate that activated γδ T cells can facilitate alloengraftment, and confirm that limiting numbers of mature αβ T cells are required for complete allogeneic engraftment.
Limiting Numbers of Mature αβ T Cells Are Required for Complete Allogeneic Engraftment in B6 → AKR Chimeras
| Group . | No. of Mice . | Donor αβ T Cells Added . | Donor γδ T Cells Added . | (%) Donor T Cells8-150 . | (Mean) . | (%) Donor (H-2b) Cells8-151 . | (Mean) . |
|---|---|---|---|---|---|---|---|
| I | 3 | None | None | 6, 0, 8 | (5) | 8, 3, 15 | (9) |
| II | 3 | 2.5 × 104 | None | 1, 1, 61 | (21) | 2, 3, 91 | (32) |
| III | 3 | None | 160 × 106 | 59, 59, 64 | (61) | 90, 84, 91 | (88) |
| IV | 3 | 2.5 × 104 | 160 × 106 | 97, 96, 97 | (97) | 98, 99, 99 | (99) |
| Group . | No. of Mice . | Donor αβ T Cells Added . | Donor γδ T Cells Added . | (%) Donor T Cells8-150 . | (Mean) . | (%) Donor (H-2b) Cells8-151 . | (Mean) . |
|---|---|---|---|---|---|---|---|
| I | 3 | None | None | 6, 0, 8 | (5) | 8, 3, 15 | (9) |
| II | 3 | 2.5 × 104 | None | 1, 1, 61 | (21) | 2, 3, 91 | (32) |
| III | 3 | None | 160 × 106 | 59, 59, 64 | (61) | 90, 84, 91 | (88) |
| IV | 3 | 2.5 × 104 | 160 × 106 | 97, 96, 97 | (97) | 98, 99, 99 | (99) |
Irradiated (800 cGy) AKR recipients were transplanted with 10 × 106 TCD B6 BM with or without the indicated number of activated γδ T cells from β0 donors (pure B6 background) or naive αβ T cells from B6 donors. After ex vivo activation, 99.4% of cultured cells expressed the γδ TCR. Numbers represent data for individual mice. Donor T cells were defined as Thy 1.2+ CD3+ and donor cells as H-2b+. Animals were evaluated 35 to 37 days posttransplant.
Key statistical comparisons: group I v II, P = .50, groups III and IV v I, P < .001; group III v IV; P < .0001.
Group I v II, P = .51, groups III and IV v I, P < .002; group III v IV, P = .043.
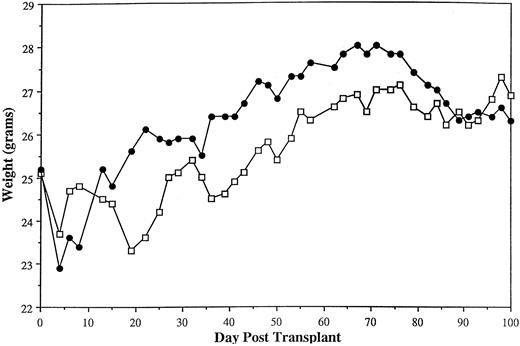
Transplantation with activated γδ T cells does not result in clinically significant GVHD.Given the large doses of activated γδ T cells required to facilitate alloengraftment, we evaluated whether these cells were able to induce GVHD under the conditions used to evaluate engraftment. Weight curves of engrafting mice transplanted with ≥160 × 106 activated γδ T cells with or without 2.5 × 104 αβ T cells were virtually indistinguishable from mice transplanted with TCD BM only, consistent with a lack of clinically significant GVHD (Fig 1). No mortality was noted in these animals. As another parameter of GVHD, we observed in this model (B6129→AKR) that B-cell reconstitution was a sensitive indicator of GVH reactivity. Specifically, AKR recipients transplanted with either 2.2 × 106 (n = 14) or 5 × 106 αβ T cells (n = 5) had a mean of 7.2 × 106 splenic B cells/mouse when evaluated 26 to 35 days posttransplant (data not shown). This was significantly less than engrafting animals transplanted with ≤1 × 106 αβ T cells that averaged 19 × 106 splenic B cells (n = 17, Table 3) (P = .0001) when assessed at similar time points posttransplant. We inferred from these data that the reduced number of splenic B cells in these mice was likely due to GVHD, as has been shown in other murine models.21 22 The subsequent observation that mice transplanted with 160 × 106 activated γδ T cells with or without 2.5 × 104 αβ T cells averaged 61 × 106 splenic B cells/mouse (n = 7) (Table 6) provided further evidence that these chimeras lacked clinically significant GVHD.
Transplantation of activated γδ T cells does not result in clinically significant GVHD in long-term chimeras. Irradiated (800 cGy) AKR mice were transplanted with 10 × 106 TCD B6129 BM alone (n = 3, □) or TCD B6129 BM plus 160 × 106 activated γδ T cells (from β0 donors, B6129 background) with or without 2.5 × 104 naive αβ T cells (n = 7, •). The mean weights of animals for the first 100 days posttransplant are shown. Weight curves are from mice shown in Table 6.
Transplantation of activated γδ T cells does not result in clinically significant GVHD in long-term chimeras. Irradiated (800 cGy) AKR mice were transplanted with 10 × 106 TCD B6129 BM alone (n = 3, □) or TCD B6129 BM plus 160 × 106 activated γδ T cells (from β0 donors, B6129 background) with or without 2.5 × 104 naive αβ T cells (n = 7, •). The mean weights of animals for the first 100 days posttransplant are shown. Weight curves are from mice shown in Table 6.
DISCUSSION
This study was designed to examine the role of γδ T cells in facilitating engraftment across the MHC under experimental conditions where donor T cells in the marrow graft are required for alloengraftment. To this end, we employed transgenic donors which lacked the ability to make αβ T cells as a source of γδ T cells. This allowed us to examine the role of γδ T cells without the confounding presence of mature αβ T cells and also to dissect the relative contributory role of both αβ and γδ T cells in this model.
Addition of spleen cells containing ≥10 × 106 naive γδ T cells to TCD marrow grafts significantly enhanced donor T-cell engraftment in sublethally irradiated recipients. These results indicated that engraftment of a thoroughly TCD marrow graft could occur in the absence of mature splenic αβ T cells. We concluded that γδ T cells were most likely responsible for facilitating donor engraftment although our data do not conclusively support this interpretation. This is based on the fact that approximately 50% of the spleen cells administered to animals consisted of γδ T cells, whereas the remaining cells consisted of NK and residual B cells still present after nylon wool depletion. Thus, mice did not receive a pure population of γδ T cells. Based on prior studies by Blazar et al23 who failed to show a facilitory role for NK cells in allogeneic engraftment, we believe it unlikely that NK cells prevented graft rejection in this model and consider the most plausible interpretation to be that alloengraftment was facilitated by γδ T cells. However, these data do not exclude the possibility that NK cells may have played a graft facilitory role.
Transplantation with doses of naive γδ T cells as high as 20 × 106, while enhancing donor engraftment, still failed to eradicate all host T cells in this model. Although it is possible that higher numbers of naive γδ T cells may have further enhanced engraftment, dose escalation was constrained by limitations in the number of cells that could be feasibly obtained from β0 donors, since γδ T cells represent only 3% to 4% of all spleen cells in transgenic donors. Therefore, we examined the efficacy of activated γδ T cells in facilitating engraftment, because this was a clinically feasible approach and allowed us to examine the effect of transplanting augmented numbers of γδ T cells. This also served as a means of obtaining a relatively pure population of γδ T cells since after ex vivo activation greater than 95% to 99% of all B6129 cells expressed the γδ TCR and less than 1% expressed the NK 1.1 antigen. The use of activated γδ T cells therefore allowed the graft facilitory role of γδ T cells to be more definitively addressed.
Results from several different experiments using both mixed background and pure background transgenic donors confirmed that addition of large numbers of activated γδ T cells (in excess of 40 to 80 × 106) significantly enhanced engraftment. These data strongly suggested that γδ T cells could facilitate alloengraftment. The relative purity of the γδ T-cell population used in these experiments is important in light of studies by Murphy et al24 who showed that IL-2 activated NK cells can promote allogeneic engraftment in mice. In their work, transplantation of 20 to 30 × 106 activated NK cells plus the in vivo administration of exogenous IL-2 were required for donor cell engraftment to be observed. In the present studies, because NK1.1 expressing cells represented less than 1% of the B6129 activated cell population, these cells would have constituted no more than 2.4 × 106 NK cells at the highest dose administered (ie, 240 × 106) and no more than 0.4 × 106 cells at the lowest dose (ie, 40 × 106), which was shown to enhance donor engraftment. Therefore, we believe the likelihood that IL-2 activated NK cells promoted engraftment in this model was small and the preponderance of data support a direct role for activated γδ T cells in facilitating alloengraftment.
Our conclusion that γδ T cells can facilitate engraftment of H-2 disparate TCD BM is supported by prior studies of Blazar et al25 who also showed that γδ T cells could enhance alloengraftment of TCD scid BM. In their experiments, γδ T cells expressing a single TCR were able to promote engraftment by recognition of H-2Tb antigens presumably on host hematopoietic cells. Significant differences between the two studies, however, were that we examined the graft facilitory effect of a heterogeneous population of activated γδ T cells and observed that a much higher dose of γδ T cells was required for engraftment. The relative significance of each of these studies for human marrow transplantation is presently unknown.
Paradoxically a greater number of activated than naive γδ T cells were necessary in this model for an equivalent degree of donor cell engraftment. Since activation of γδ T cells in vitro has been shown to augment cytolytic activity,26 we would have surmised that fewer cells would have been required to facilitate engraftment. That this was not the case suggested that either the life span of these cells was reduced in vivo, their homing properties altered, or their functional capabilities affected by activation with TCR-specific antibody and IL-2. Prior studies with lymphokine activated killer cells have shown early cell death and altered trafficking,27,28 raising the possibility that activated γδ T cells may have shared a similar fate. Alternatively, other studies have showed that activated γδ T cells are more susceptible than naive cells to apoptosis after religation of the T-cell receptor in vitro.29 30 Experiments are currently underway to explain the requirement for larger cell doses of activated γδ T cells in this model.
One of the most striking aspects of this study was that very high doses of both naive and activated γδ T cells were required to augment engraftment. Specifically, on a cell to cell comparison, γδ T cells were 100- to 500-fold less potent than αβ T cells at preventing graft rejection in this model. Because we had shown that engraftment in this model was dependent on the presence of donor T cells in the marrow graft (Table 2), this was evidence that donor T cells were required to interact with the host microenvironment to eradicate or inactivate residual host T-cell populations capable of rejecting the graft. These data indicated that αβ T cells are functionally more competent to perform this role than γδ T cells. There are several possible explanations for this observation. First of all, if one assumes that donor T-cell recognition and destruction of host T cells is one mechanism by which engraftment is facilitated,2 then this would require that donor T cells have the capability to be cytolytic. The relative ability of αβ and γδ T cells to facilitate engraftment would therefore be contingent, in part, on their cytotoxic capabilities. This question has been indirectly examined by Kabelitz et al31 who showed that the frequency of allocytotoxic γδ T-cell clones in the PB in man is significantly lower than for αβ T-cell clones. In their study, the majority of γδ T-cell clones did not discriminate between autologous and allogeneic target cells. The authors concluded that most γδ T cells lacked specific allocytotoxicity. Given the broader reactivity and lower allocytotoxic frequency of γδ T cells at the clonal level, this would predict that higher doses of these cells would be required to facilitate equivalent degrees of donor cell engraftment.
A second possibility is that γδ T cells are inherently competent to promote engraftment either by destruction or inactivation of host T cells but require help from αβ T cells for optimal function. We observed that transplantation of γδ T cells in the absence of mature αβ T cells were limited in their ability to eradicate residual host T cells and facilitate complete allogeneic engraftment. Subsequent studies indicated that limiting numbers of αβ T cells were required for complete engraftment and eradication of host T cells. The manner by which limiting numbers of αβ T cells enhanced engraftment in this model is not known, but the data suggest that αβ T cells play an important facilitory role. Several possible mechanisms can be advanced. First of all, prior studies have shown that the CD4+ γδ+ T-cell subset that secretes IL-2 represents only 0% to 5% of all γδ T cells.20 This is a much lower percentage than found for αβ T cells, and indicates that the γδ T-cell subpopulation that is capable of providing “help” is markedly diminished. Similarly, αβ T cells may secrete other cytokines necessary for γδ T-cell proliferation.32 This would presume that the inability of lower numbers of γδ T cells to inactivate or eradicate host T-cell populations is caused by a deficit of appropriate αβ T-cell help that is cytokine-mediated. Alternatively, small numbers of αβ T cells may be necessary to compensate for γδ T cells, which are relatively deficient in generating a cytolytic response against host target cells.33 In this instance, αβ T cells that are cytotoxic may be required to eradicate residual host T cells, which are not completely removed by γδ T cells.
Although αβ+ T cells are thought to play a primary role in the pathophysiology of GVHD,34,35 the role of γδ T cells has not been defined. Blazar et al36 showed that transgenic γδ T cells could cause GVHD by specific recognition of nonclassical class Ib antigens in mice. Meanwhile, Ellison et al37 have postulated a role for a subset of γδ T cells, which coexpress the NK1.1 antigen in mediating GVH reactivity. In the present study, which employed a heterogeneous population of γδ T cells, high doses of naive γδ T cells (20 × 106) were able to cause GVHD in chimeras, but since most of these animals failed to engraft, GVHD was not further assessable. In contrast, clinically significant GVHD was not observed when animals were transplanted with large numbers of activated γδ T cells. Weight curves over 3 months in these engrafting mice were similar to those obtained from TCD BM controls. Moreover, B-cell reconstitution that appeared to be a more sensitive indicator of GVH reactivity in this model was increased relative to control animals. Since in this engraftment model recipients were sublethally irradiated, it remains to be seen whether more intensively conditioned recipients would develop GVHD under similar circumstances. Although additional studies are needed, these data do provide evidence that, under certain conditions, γδ T cells may be able to facilitate alloengraftment without escalating toxicity from GVHD. Whether activation of γδ T cells per se functionally alters these cells in such a way as to minimize their propensity to cause GVH reactivity will require further study.
The results of this study may have implications for human marrow transplantation. Although obtaining large numbers of naive γδ T cells in humans is problematic, transplantation of ex vivo activated γδ T cells is a clinically feasible approach. If one assumes that γδ T cells are 500-fold less potent on a cell to cell basis than αβ T cells, the required number of γδ T cells for engraftment would still be well within the range of what has been shown to be technically feasible in adoptive immunotherapy studies using in vitro polyclonally expanded anti-CD3 stimulated T cells.38-40 Furthermore, because limiting numbers of αβ T cells are graft facilitory, it is conceivable that the requisite number of γδ T cells for complete allogeneic engraftment may be lower than the dose of 160 × 106 activated γδ cells which we tested in this model. This would commensurately reduce the γδ T-cell requirement in humans. It is also worth noting that even if activated γδ T cells are only able to facilitate mixed T-cell chimerism in humans, this may still be of potential clinical benefit, if there is a corresponding reduction in GVHD in transplanted recipients.41,42 Specifically, in nonmalignant disorders amenable to BM transplantation (eg, immunodeficiency states, BM failure syndromes, metabolic, and red blood cell disorders), the establishment of partial yet durable donor T-cell chimerism might allow for correction of the underlying disorder.43 Thus, the supplementation of the marrow graft with activated γδ T cells may represent a unique approach for the facilitation of BM engraftment in humans.
ACKNOWLEDGMENT
The authors thank Dr Paul Martin for helpful discussions. This manuscript was prepared with the assistance of Deanna Cherubini.
Supported by Grant No. CA01534 from the National Institutes of Health, The Ralph and Marian Falk Medical Research Trust, and The Florence Carter Fellowship from The American Medical Association.
Address reprint requests to William R. Drobyski, MD, Bone Marrow Transplant Program, Froedtert East Hospital, 9200 W Wisconsin Ave, Milwaukee, WI 53226-3596.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal