THE LAST 3 decades have witnessed profound changes in the management of patients with thalassemia major. Regular red blood cell (RBC) transfusions eliminate the complications of anemia and compensatory bone marrow (BM) expansion, permit normal development throughout childhood, and extend survival.1 In parallel, transfusions result in a “second disease” while treating the first,2 that of the inexorable accumulation of tissue iron that, without treatment, is fatal in the second decade of life. Further altering the prognosis of thalassemia major over the last 20 years has been progress in the development of iron-chelating therapy for iron overload. Deferoxamine mesylate, first introduced in short-term studies in iron-loaded patients in the early 1960s, gained acceptance as standard therapy over a decade later in countries able to support the high costs of this therapy. Twenty years later, extended survival free of iron-induced complications, and dramatically improved quality of life, are observed in well-chelated patients. Indeed, over this period, iron-chelating therapy for thalassemia major has resulted in one of the most dramatic alterations in morbidity and mortality associated with a genetic disease. In this review the experience gained in the use of deferoxamine, the benefits of and problems associated with this agent in the treatment and prevention of iron overload, and recent progress in the development of orally effective iron-chelating drugs will be reviewed.
ADJUNCTS TO THE USE OF CHELATING THERAPY
Adjuncts to the use of chelating therapy to reduce iron accumulation in patients with thalassemia major include the judicious use of transfusion to minimize iron loading while adequately suppressing endogenous erythropoiesis, the appropriate timing of splenectomy to minimize administration of transfusions, and the specific therapy of complications that may result from iron-induced organ damage. These will be briefly reviewed here.
Transfusion Regimens
Untransfused children with homozygous β-thalassemia usually exhibit some or all of the complications of anemia and ineffective erthropoiesis1; the prevention of these complications is the goal of regular transfusions.2-5 The transfusion regimen itself appears critical in the control of body iron loading. Maintenance of a regimen in which pretransfusion hemoglobin concentrations do not exceed 9.5 g/dL has been shown to result in a reduced transfusion requirement and improved control of body iron burden,6,7 compared with a transfusion schedule (termed “supertransfusion”) in which baseline hemoglobins are permitted to exceed 11 g/dL.8
Type of RBC Concentrates
Early studies of the use of neocytes, or young RBCs, predicted that prolonged survival in vivo of these concentrates should reduce the RBC mass required to maintain appropriate baseline hemoglobin concentrations.8-11 Clinical investigations confirmed that an extension of transfusion interval of 13% to 25% in thalassemia could be achieved with the use of neocytes.12-16 A recent study found that a 15% extension of transfusion interval during administration of neocyte concentrates, expected to minimally reduce the requirement for iron chelation therapy, could be achieved but at the cost of an increased exposure to donated units and a fivefold increment in preparation expenses over those of standard concentrates.16 Hence, the use of neocytes should have a limited impact on the long-term management of most chronically transfused patients. In contrast, the use of automated RBC exchange transfusions in regularly transfused patients with sickle cell disease has been reported to substantially reduce transfusional iron accumulation, permitting reduction in the intensity of chelation therapy.17 Pilot studies in thalassemia major18 suggest that a similar approach may warrant careful evaluation in this disorder.
Splenectomy
In patients with thalassemia in whom yearly transfusion requirements exceed 200 mL packed cells per kilogram body weight, splenectomy should significantly diminish RBC requirements and iron accumulation.19,20 Hypersplenism may be avoided by early and regular transfusion; many patients reaching adolescence in this decade have not required splenectomy.21 Because of the risk of postsplenectomy infection, splenectomy should generally be delayed until the age of 5 years or later.22
Treatment of Hepatitis
Liver disease is reported as a common cause of death after age 15 years in patients with thalassemia.23 Iron-induced hepatic damage is exacerbated by a second complication of transfusions, infection with hepatitis C virus,24,25 the most frequent cause of hepatitis in thalassemic children.26 The high incidence of liver failure and hepatocellular carcinoma in patients who have acquired the virus through transfusions27 supports the use of antiviral therapy for patients with thalassemia. The results of recent trials of interferon-α in hepatitis-C–infected patients with thalassemia28,29 suggest that the clinical and pathologic responses to this agent may be inversely related to body iron burden.29 The effectiveness of antiviral therapy in thalassemia may therefore depend on that of iron-chelating therapy; such therapy should be intensified in hepatitis-C–infected patients.
Iron Overload
The most important consequence of life-saving transfusions in thalassemia is the inexorable accumulation of iron within tissues, causing progressive organ dysfunction that is fatal without chelating therapy.2 The toxicity of iron has been thoroughly reviewed previously.30 Here, the sites and toxicity of chelatable iron important in patients with thalassemia will be briefly considered.
SITES AND TOXICITY OF IRON IN VIVO
Nontransferrin-Bound Iron
The toxicity of iron is mediated, in part, by its catalysis of reactions which generate free hydroxyl radicals, propagators of oxygen-related damage.30-32 Hydroxyl radicals induce lipid peroxidation of cellular organelles including mitochondria, lysosomes, and sarcoplasmic membranes. Evidence of peroxidant damage has been demonstrated in vivo in the tissues of iron-loaded animals33 and of thalassemic patients.34 Iron unbound to storage or transport proteins is particularly toxic in this regard; in normal individuals, tight binding of plasma iron to the transport protein transferrin prevents the catalytic activity of iron in free radical production.35,36 In very heavily iron-loaded patients, transferrin becomes fully saturated and a nontransferrin-bound fraction of iron becomes detectable in plasma.37-43 Nontransferrin-bound iron may accelerate the formation of free hydroxyl radical41 and facilitate uptake of iron by tissues.35 44 The effectiveness of an iron-chelating agent depends in part on its ability to bind nontransferrin-bound iron over sustained periods of time, thereby decreasing tissue uptake and iron-catalyzed toxic reactions.
Chelatable Tissue Iron
On delivery to cells by transferrin, iron is immediately available for chelation from a low-molecular-weight iron pool through which the intracellular traffic of iron may pass.45 When this pool is large, it may be toxic to cells with a limited capacity to generate iron storage proteins.46,47 Excess iron is deposited in reticuloendothelial cells, where it appears to be relatively harmless, or in parenchymal tissues, where it may cause significant damage.30
Deferoxamine
Iron overload may be prevented or treated with a chelating agent capable of complexing with iron and promoting its excretion. The only iron-chelating agent presently available for clinical use is deferoxamine B, a trihydroxamic acid produced by Streptomyces pilosus, with relative specificity for ferric iron.48 Deferoxamine is poorly absorbed orally49 and rapidly metabolized in plasma,50 conferring on the drug its principal drawback: the requirement for prolonged parenteral infusions during which plasma concentrations reach a plateau at 12 hours.30 The sources of iron chelatable by deferoxamine have been thoroughly reviewed.22,30,51,52 Iron bound by deferoxamine is rendered virtually inactive metabolically and deferoxamine can prevent or reverse effects of free radical formation and lipid peroxidation in many experimental systems.33,44 53-57
Clinical Use of Deferoxamine
Substantial iron excretion was first reported after administration of intramuscular, intravenous (IV),58,59 and subcutaneous bolus injections60 of deferoxamine in the early 1960s. A decade later, chronic intramuscular administration was shown to slow iron accumulation and arrest hepatic fibrosis in transfused patients.61 Over the next 10 years, the effectiveness of 24-hour infusions of IV62,63 and subcutaneous deferoxamine,64,65 the efficacy and feasibility of 12-hour subcutaneous infusions,66 and the substantial fecal iron excretion induced by deferoxamine67 were reported. Together, these studies permitted the design of regimens of nightly subcutaneous deferoxamine using portable ambulatory pumps.64-66 Clinical studies important in our understanding of the use and benefits of deferoxamine are outlined in Table 1.
CLINICAL COMPLICATIONS OF IRON OVERLOAD AND THE IMPACT OF IRON-CHELATING THERAPY
The Heart
In the absence of chelating therapy, myocardial disease remains the life-limiting complication of transfusional iron overload. As detailed over 30 years ago, irregularly transfused, unchelated children frequently developed left ventricular hypertrophy and conduction disturbances by late childhood, and ventricular arrhythmias and refractory congestive failure by the mid-teens.68 Within the heart, even small amounts of unbound iron may generate reactive harmful oxygen metabolites and toxicity, while both chronic pulmonary hypertension69 and myocarditis70 may accelerate iron-induced cardiac failure in thalassemia. These observations may explain the variable correlation observed between the severity of myocardial iron deposition and that of cardiac fibrosis.71 72
The Impact of Iron-Chelating Therapy on Cardiac Disease and Survival
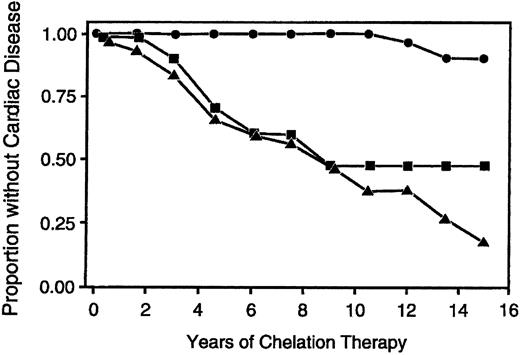
The beneficial effects of deferoxamine therapy on survival and cardiac disease in patients with thalassemia were first reported in the early 1980s.23,73-80 Over the subsequent decade, several studies observing reduction in morbidity and mortality examined periods of follow-up too short to provide definitive conclusions regarding the long-term benefits of deferoxamine on cardiac disease.81-90 Only in the present decade did patients who started deferoxamine in early childhood reach an age at which long-term survival could be assessed with greater certainty. Two recent trials, both of over 10 years duration, have demonstrated unequivocally that effective long-term use of deferoxamine in thalassemia major is associated with long-term survival free of the complications of iron overload.91,92 Both studies identified the magnitude of the body iron burden as the principal determinant of clinical outcome. One trial used the serum ferritin to evaluate iron loading.91 Over the period of follow-up, patients with most serum ferritin concentrations less than 2,500 μg/L had an estimated cardiac disease free survival of 91% after 15 years, in contrast to patients in whom most serum ferritin concentrations had exceeded 2,500 μg/L, who had an estimated cardiac disease free survival after 15 years of less than 20% (Fig 1). The other trial quantitatively examined the relationship between the total amount of iron administered by transfusion, the cumulative use of deferoxamine and the magnitude of the body iron burden, as assessed by measurements of hepatic iron stores.92 Using a threshold for transfused iron and deferoxamine use that is equivalent to a hepatic storage iron of about 80 μmol iron per gram liver, wet weight (about 15 mg iron per gram liver, dry weight),93 patients were classified as having received ineffective or effective chelation therapy. Ineffective chelating-therapy was associated with the greatest risk of clinical complications and early death in patients with thalassemia major; the probability of survival to at least the age of 25 years was only 32% among patients above the threshold. By contrast, effective chelation helped protect against impaired glucose tolerance, diabetes mellitus, cardiac disease, and early death; no deaths had occurred among patients below the threshold.
Survival without cardiac disease according to the proportion of serum ferritin measurements greater than 2,500 μg/dL. The circles show cardiac disease free survival among patients in whom less than 33% of serum ferritin measurements exceeded 2,500 μg/L; squares show survival among patients in whom 33% to 67% of ferritin measurements exceeded 2,500 μg/L; and triangles show survival among patients in whom more than 67% of ferritin measurements exceeded 2,500 μg/L. (Reprinted by permission of The New England Journal of Medicine, Olivieri NF, Nathan DG, MacMillan JH, et al. Volume 331, pp 574-578, 1994. Copyright 1994. Massachusetts Medical Society. All rights reserved.)91
Survival without cardiac disease according to the proportion of serum ferritin measurements greater than 2,500 μg/dL. The circles show cardiac disease free survival among patients in whom less than 33% of serum ferritin measurements exceeded 2,500 μg/L; squares show survival among patients in whom 33% to 67% of ferritin measurements exceeded 2,500 μg/L; and triangles show survival among patients in whom more than 67% of ferritin measurements exceeded 2,500 μg/L. (Reprinted by permission of The New England Journal of Medicine, Olivieri NF, Nathan DG, MacMillan JH, et al. Volume 331, pp 574-578, 1994. Copyright 1994. Massachusetts Medical Society. All rights reserved.)91
The Liver
The liver is a major repository of transfused iron. Hepatic parenchymal iron accumulation, demonstrated after only 2 years of transfusion therapy,2 may rapidly result in portal fibrosis in a significant percentage of patients: one center has observed portal fibrosis in a high percentage of biopsies in children under the age of 3 years.94 In young adults with thalassemia major, in whom liver disease remains a common cause of death,23 viral infection24,25 and alcohol ingestion95 may act synergistically with iron in accelerating the development of liver damage.
The Impact of Iron-Chelating Therapy on Liver Disease
Reports of reduction in liver iron concentration, improvement in laboratory abnormalities of liver function, and arrest of hepatic fibrosis provide evidence for the beneficial effects of subcutaneous deferoxamine on iron loading within the liver.61,96-101 High-dose IV deferoxamine has been reported to achieve the same benefits in patients with massively elevated hepatic iron concentrations.102
Endocrine Function and Growth
The most common endocrine abnormalities in patients with thalassemia in the modern era include hypogonadotropic hypogonadism, growth hormone deficiency, and diabetes mellitus.103,104 Variable incidences of hypothyroidism,105 hypoparathyrodism,106 and low levels of adrenal androgen secretion with normal glucocorticoid reserve,107 have been less commonly reported. Although normal rates of prepubertal linear growth may be observed in patients maintained on regular transfusion programs,108-110 poor pubertal growth and impaired sexual maturation have been observed in well-transfused patients.107,110-114 Poor pubertal growth has been attributed to iron-induced selective central hypogonadism,105,110,115,116 interference of iron with the production of insulin-like growth factor (IGF-1),117-119 or both. The role of iron is supported by histologic findings of selective iron deposition in pituitary gonadotropes120 and by the reversibility of hypogonadism in primary hemochromatosis with intensive phlebotomy.121,122 Poor pubertal growth has also been attributed to several other causes, including impaired growth hormone responses to growth hormone-releasing hormone,123 abnormalities in growth hormone secretion124 or in the growth hormone-receptor itself125 in the presence of normal growth hormone reserves in most patients126-128; growth may improve with administration of exogenous growth hormone.129,130 Hyposecretion of adrenal androgen,106,107 delay in pubertal development itself,131,132 zinc deficiency,133-135 and free-hemoglobin–induced inhibition of cartilage growth136 have also been implicated in impairment of growth in patients with thalassemia major.
Impact of Iron-Chelating Therapy on Endocrine Function and Growth
The effectiveness of deferoxamine in the prevention of growth failure and gonadal dysfunction was first reported in a cohort of patients regularly treated since mid-childhood, 90% of whom reached normal puberty. In contrast, in a group of patients who had administered a relatively lower total dose of deferoxamine beginning in the early teens, only 38% achieved normal pubertal status. In both cohorts, final height did not differ significantly from mid-parental height.137 In parallel, a striking increase in fertility in men and women with thalassemia has been reported over the last decade.138 These findings contrast with older,107,110,113 and some recent,139 studies in which a high incidence of gonadal dysfunction in chelated patients has been reported. While insufficient length or intensity of therapy in some of these studies almost certainly explains the lack of reported benefit of deferoxamine in the preservation of pubertal function, it is disappointing to note that secondary ammenorrhea may eventually develop in many thalassemic women with previous evidence of normal pituitary function.139 Intensive deferoxamine administration itself may be associated with impaired linear growth.140-144
Diabetes mellitus in thalassemia has been attributed to impaired secretion of insulin secondary to chronic pancreatic iron overload,106,145-150 and to insulin resistance151-154 as a consequence of iron deposition within liver152 or skeletal muscle.155 Diabetes has also been linked temporally to episodes of acute viral hepatitis in some patients.149,150 In most studies there exists a direct relationship between the development of diabetes and the severity and duration of iron overload.150,156 Iron-induced free hydroxyl radical-induced islet cell damage, shown to induce diabetes in animals,157 may also play a role in the development of this complication in iron-loaded patients.
Impact of Iron-Chelating Therapy on Diabetes
Reduction in the risk of diabetes mellitus and glucose intolerance has been reported in patients who used more deferoxamine in relationship to their transfusional iron load, compared to a group who had begun deferoxamine at a more advanced age and had administered therapy less intensively.92
Reversal of Iron-Induced Organ Dysfunction
Evidence that established iron-induced dysfunction of the heart78,79,85,87 and liver96-101 may improve during intensive deferoxamine therapy has been presented in several reports. Even if administration of deferoxamine does not reverse iron-induced cardiac dysfunction altogether, the outlook for patients who develop cardiac disease in the modern era but who thereafter comply with chelating therapy is strikingly improved, compared with the prognosis reported 30 years ago in similar patients.68 A recent study reported that iron-induced cardiac disease was fatal in most patients in whom body iron burdens remained high, but that extended survivals were observed in patients who had reduced iron stores, as estimated by serum ferritin concentration, 2 years after the onset of this complication.158 In patients with true “end-stage” iron-related disease, both cardiac transplantation159 and combined cardiac and liver transplantation160 has been successful in extending survival in patients with thalassemia major.
Although pituitary growth hormone reserve has been reported to improve after deferoxamine therapy in adults with acquired transfusional iron overload,84 reversal of established pituitary failure has not been reported in thalassemia major. In contrast, improvement in both thyroid function75 and glucose intolerance22 has been reported following deferoxamine treatment in this disorder.
Management of Chelation Therapy
Several practical problems are associated with long-term chelation therapy. One of the most important of these is the accurate assessment of body iron burden, essential to the evaluation of the effectiveness of deferoxamine, as well as to that of new chelators entering clinical trials. As well, issues regarding the appropriate age for the initiation of deferoxamine treatment, the maintenance of balance between its effectiveness and toxicity, and the problems of compliance with deferoxamine arise frequently in the management of patients with thalassemia.
ASSESSMENT OF BODY IRON
Both direct and indirect means for the assessment of body iron are available but no single indicator or combination of indicators is ideal for the evaluation of iron status in all clinical circumstances (Table 2). Measurement of hepatic iron stores provides the most quantitative means of assessing the body iron burden in patients with thalassemia major161 and may be considered the reference method for comparison with other techniques. Data that have accumulated over the past 10 years permit a quantitative approach to the management of iron overload, and provide guidelines for the control of body iron burden in individual patients treated with chelating therapy.
Indirect Assessment
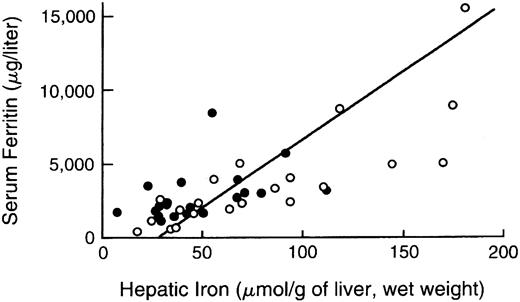
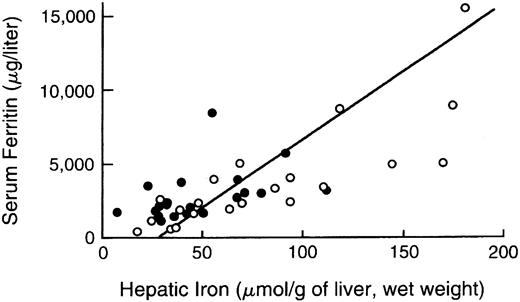
Serum or plasma estimates of body iron burden.The measurement of plasma or serum ferritin is the most commonly used indirect estimate of body iron stores.52,162-166 Normally, ferritin concentrations decrease with depletion of storage iron and increase with storage iron accumulation. A maximum glycosylated plasma ferritin concentration of about 4,000 μg/L may represent the upper physiologic limit of the rate of synthesis167; higher concentrations are thought to be caused by the release of intracellular ferritin from damaged cells. Interpretation of ferritin values may be complicated by a variety of conditions that alter concentrations independently of changes in body iron burden, including ascorbate deficiency, fever, acute infection, chronic inflammation, acute and chronic hepatic damage, hemolysis, and ineffective erythropoiesis,168 169 all of which are common in thalassemia major. In one study of patients with thalassemia major or sickle cell disease, the 95% prediction intervals for hepatic iron concentration, given the plasma ferritin, were so broad as to make determination of plasma ferritin a poor predictor of body stores. As a consequence, reliance on ferritin alone can lead to inaccurate assessment of body iron burden in individual patients (Fig 2). The serum iron, transferrin, transferrin saturation, and transferrin receptor concentration do not quantitatively reflect body iron stores.
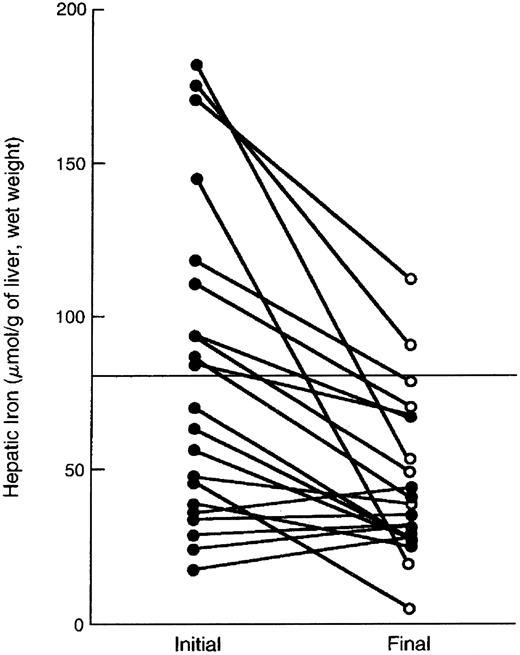
Comparison of hepatic iron and serum ferritin concentrations in patients with thalassemia major. Indirect estimation of body iron load, based on serum ferritin concentration, is compared with the reference method, direct measurement of hepatic iron concentration (by chemical analysis or magnetic-susceptibility studies) in patients with thalassemia major treated with deferiprone. Open circles denote the values determined prior to deferiprone therapy and solid circles those at the time of final analysis after 1 to 5 years of treatment. The diagonal line denotes the simple linear least-squares regression between the two variables. (Reprinted by permission of The New England Journal of Medicine, Olivieri NF, Brittenham GM, Matsui D, et al. Volume 332, pp 918-922, 1995. Copyright 1995. Massachusetts Medical Society. All rights reserved.)93
Comparison of hepatic iron and serum ferritin concentrations in patients with thalassemia major. Indirect estimation of body iron load, based on serum ferritin concentration, is compared with the reference method, direct measurement of hepatic iron concentration (by chemical analysis or magnetic-susceptibility studies) in patients with thalassemia major treated with deferiprone. Open circles denote the values determined prior to deferiprone therapy and solid circles those at the time of final analysis after 1 to 5 years of treatment. The diagonal line denotes the simple linear least-squares regression between the two variables. (Reprinted by permission of The New England Journal of Medicine, Olivieri NF, Brittenham GM, Matsui D, et al. Volume 332, pp 918-922, 1995. Copyright 1995. Massachusetts Medical Society. All rights reserved.)93
Twenty-four–hour deferoxamine-induced urinary iron excretion.The usefulness of measurement of the amount of chelated iron in the urine induced by a single intramuscular dose or prolonged subcutaneous infusion of deferoxamine112 has several limitations in the accurate assessment of body iron burden. Most important is the poor correlation between urinary iron excretion and hepatic iron concentration, in part because the relative amounts of iron excreted into stool and urine vary with the dose of deferoxamine administered, body iron burden, and erythroid activity.51 Chelator-induced urinary iron excretion is also vulnerable to extraneous influences by infection, inflammation, the activity and effectiveness of erythropoiesis, extramedullary hematopoiesis, liver disease, and ascorbic acid deficiency.
Imaging of tissue iron.Computed tomography,170-174 nuclear resonance scattering (NRS) from manganese-56,175 and the most widely used modality, magnetic resonance imaging,176-193 have all been used to evaluate tissue iron stores in vitro and in vivo, but none is clinically available for the measurement of hepatic iron concentrations. Biopsy-demonstrated reductions in hepatic iron have been reflected by magnetic resonance imaging (MRI) in individual patients192 (Fig 3), but correlations between hepatic iron concentrations determined by biopsy and those estimated by magnetic resonance have varied with differences in both equipment and method. Magnetic resonance represents the only imaging method in clinical use with the potential to detect iron within the heart189,192,193 (Fig 4). Although imprecision in the quantitation of cardiac iron obtained at biopsy194,195 prevents direct correlation with values of cardiac iron estimated by MRI in humans, good correlation between MRI-derived, and biopsy-determined, cardiac iron has been observed in a thalassemic mouse model.193 Furthermore, MRI changes consistent with the reduction of cardiac iron (Fig 5) that are paralleled by improvement in cardiac function have been reported in individual patients.192 Similarly, MRI studies of the iron-loaded anterior pituitary gland196,197 have reported variations in pituitary iron that are correlated with pituitary reserve in individual patients with thalassemia.198 In summary, although many studies show that MRI can reflect the presence of, and changes in, tissue iron in vivo, this method has not been validated as one that provides measurements of tissue iron that are quantitatively equivalent to those determined at tissue biopsy.
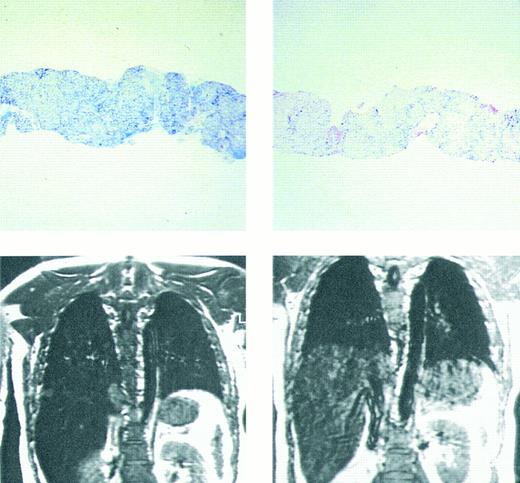
Prussian blue stain showing (top figures) hepatic iron in hepatocytes and portal macrophages, before (left) and after (right) 9 months of chelating therapy with the orally active chelating agent deferiprone in a patient with homozygous β thalassemia. Hepatic iron concentration in the sample on left was approximately 16 mg/g dry weight liver tissue; in that on the right hepatic iron concentration was less than 2 mg/g dry weight tissue. Coronal MRI (lower figures) of hepatic iron before (left) and after (right) therapy with the orally active iron chelating agent deferiprone in the same patient. Complete absence of liver signal in the MRI on the left is compatible with significant iron deposition, while improvement in signal intensity after 9 months of therapy (right) indicates that the liver iron content is reduced compared with that of the previous study. (Reprinted with permission.192 )
Prussian blue stain showing (top figures) hepatic iron in hepatocytes and portal macrophages, before (left) and after (right) 9 months of chelating therapy with the orally active chelating agent deferiprone in a patient with homozygous β thalassemia. Hepatic iron concentration in the sample on left was approximately 16 mg/g dry weight liver tissue; in that on the right hepatic iron concentration was less than 2 mg/g dry weight tissue. Coronal MRI (lower figures) of hepatic iron before (left) and after (right) therapy with the orally active iron chelating agent deferiprone in the same patient. Complete absence of liver signal in the MRI on the left is compatible with significant iron deposition, while improvement in signal intensity after 9 months of therapy (right) indicates that the liver iron content is reduced compared with that of the previous study. (Reprinted with permission.192 )
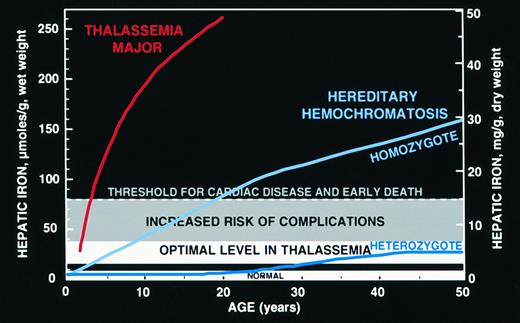
Hepatic iron concentrations shown are those in normal individuals (approximately 0.6 to 1.2 mg iron per gram liver, dry weight)209; concentrations observed in heterozygotes for hereditary hemochromatosis associated with normal survival free of the complications of iron overload (approximately 3.2 to 7 mg iron per gram liver, dry weight),214 designated “optimal” (see text) and considered a goal for transfusion-dependent patients in whom phlebotomy cannot safely decrease body iron burden; concentrations associated with an increased risk of iron-induced complications including hepatic fibrosis and diabetes mellitus (exceeding 7 mg iron per gram liver, dry weight)215,217,218; and concentrations associated with a greatly increased risk for iron-induced cardiac disease and early death (at or exceeding 15 mg iron per gram liver, dry weight).91 Mean hepatic iron concentrations for patients with thalassemia major studied before the availability of iron-chelating therapy,61 and those observed in homozygotes and heterozygotes for hereditary hemochromatosis.214
Hepatic iron concentrations shown are those in normal individuals (approximately 0.6 to 1.2 mg iron per gram liver, dry weight)209; concentrations observed in heterozygotes for hereditary hemochromatosis associated with normal survival free of the complications of iron overload (approximately 3.2 to 7 mg iron per gram liver, dry weight),214 designated “optimal” (see text) and considered a goal for transfusion-dependent patients in whom phlebotomy cannot safely decrease body iron burden; concentrations associated with an increased risk of iron-induced complications including hepatic fibrosis and diabetes mellitus (exceeding 7 mg iron per gram liver, dry weight)215,217,218; and concentrations associated with a greatly increased risk for iron-induced cardiac disease and early death (at or exceeding 15 mg iron per gram liver, dry weight).91 Mean hepatic iron concentrations for patients with thalassemia major studied before the availability of iron-chelating therapy,61 and those observed in homozygotes and heterozygotes for hereditary hemochromatosis.214
Sagittal MRI of the heart in three patients with homozygous β thalassemia and transfusional iron overload. (A, left) Normal signal from the septum (long arrow) and posterior wall of the heart, consistent with the presence of very mild cardiac iron loading, in a transfused patient regularly complianct with iron chelating therapy. The homogenous signal of the liver, consistent with very mild iron loading in this organ (short arrow), is also seen below the image of the heart. (B, middle): Imhomogenity of signal from the septum (long arrow) and posterior wall, consistent with moderate iron deposition in a transfused patient erratically compliant with iron chelating therapy. Loss of liver signal (short arrow) is consistent with heavier iron loading in this organ. (C, right): Absence of signal from the septum (arrow), posterior wall and liver (short arrow), compatible with heavy iron deposition in a transfused patient who has been noncompliant with iron chelating therapy over several years.
Sagittal MRI of the heart in three patients with homozygous β thalassemia and transfusional iron overload. (A, left) Normal signal from the septum (long arrow) and posterior wall of the heart, consistent with the presence of very mild cardiac iron loading, in a transfused patient regularly complianct with iron chelating therapy. The homogenous signal of the liver, consistent with very mild iron loading in this organ (short arrow), is also seen below the image of the heart. (B, middle): Imhomogenity of signal from the septum (long arrow) and posterior wall, consistent with moderate iron deposition in a transfused patient erratically compliant with iron chelating therapy. Loss of liver signal (short arrow) is consistent with heavier iron loading in this organ. (C, right): Absence of signal from the septum (arrow), posterior wall and liver (short arrow), compatible with heavy iron deposition in a transfused patient who has been noncompliant with iron chelating therapy over several years.
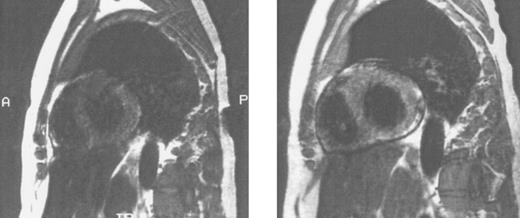
Sagittal MRI of cardiac iron before (left) and after (right) therapy with the orally active iron chelating agent deferiprone in the same patient with homozygous β thalassemia whose liver histology and hepatic MRI are shown in Fig 3. Imhomogenity of cardiac signal in the MRI on the left is compatible with significant iron deposition, while improvement in signal intensity after nine months of chelating therapy indicates that the cardiac iron content is reduced compared with that of the previous study. (Reprinted with permission.192 )
Sagittal MRI of cardiac iron before (left) and after (right) therapy with the orally active iron chelating agent deferiprone in the same patient with homozygous β thalassemia whose liver histology and hepatic MRI are shown in Fig 3. Imhomogenity of cardiac signal in the MRI on the left is compatible with significant iron deposition, while improvement in signal intensity after nine months of chelating therapy indicates that the cardiac iron content is reduced compared with that of the previous study. (Reprinted with permission.192 )
Assessment of Organ Function
Cardiac function.Electro- or resting echo-cardiograms may be normal late in the course of iron-induced cardiac disease, and therefore are not sufficiently sensitive for the early detection of iron-induced cardiac dysfunction.199-203 Decreased left ventricular contractile reserve in clinically asymptomatic patients can be demonstrated with multi-gated exercise cardiac radionuclide angiography203 or with low-dose dobutamine stimulation204; these modalities may be useful in the diagnosis of early iron-induced cardiac disease. Diastolic dysfunction in asymptomatic individuals204-207 has been shown to have prognostic significance for the development of symptomatic iron-induced cardiac disease in some,205,206 but not all,70 studies.
Anterior pituitary reserve.Measurement of peak serum lutenizing hormone following a bolus of gonadotropin releasing hormone may be useful in the evaluation of pituitary reserve.137 In one study, 72% of patients with absent or very mild pituitary iron loading had a normal increase of lutenizing hormone, while only 5% of those with moderate or severe pituitary iron loading had a normal response.198
Direct Assessment
Measurement of hepatic iron concentration is the most quantitative, specific, and sensitive method for determining the body iron burden in patients with thalassemia major.208 Liver biopsy is the best direct means of assessing iron deposition, permitting chemical measurement of the nonheme (storage) iron concentration and histochemical examination of the pattern of iron accumulation in hepatocytes and Kupffer cells as well as evaluation of the extent of inflammation, fibrosis, and cirrhosis. Magnetic susceptometry using a superconducting quantum interference device (SQUID) magnetometer provides a direct measure of hepatic storage iron that is based on a fundamental physical property of ferritin and hemosiderin.209-212 Use of the magnetic susceptibility of a tissue to determine the storage iron is much simpler than the use of the resonance behavior produced by the application of the oscillating magnetic fields used in magnetic resonance studies. When body iron stores are increased, the results of noninvasive determinations of magnetic susceptibility and of the chemical analysis of hepatic tissue obtained by biopsy are quantitatively equivalent.209-211 Magnetic susceptometry has been useful in clinical investigation of iron overload but is not generally available, in part because only two sites, one in the United States209 and one in Germany,212 have the specialized equipment needed for measurements of hepatic magnetic susceptibility.
OPTIMAL BODY IRON IN PATIENTS WITH THALASSEMIA MAJOR
Because the magnitude of the body iron burden seems to be the principal determinant of clinical outcome,91-93 the prime goal of iron-chelating therapy in patients with thalassemia major is the control of body iron. The optimal body iron should minimize both the risk of adverse effects from the iron-chelating agent and the risk of complications from iron overload. With stable transfusion requirements and in the absence of other confounding factors, the lower the level of body iron desired, the higher the dose of iron chelator needed. As detailed below, with many of the adverse reactions encountered with deferoxamine, the higher the dose, the greater the risk of adverse reactions. As a consequence, therapy to maintain a normal body iron, corresponding to a hepatic iron of about 1 to 9 μmol iron per gram liver, wet weight (about 0.2 to 1.6 mg iron per gram liver, dry weight)209 might abate the likelihood of complications of iron overload but greatly increase the probability of dose-related drug toxicity. At the opposite extreme, with high body iron burdens corresponding to hepatic iron concentrations greater than 80 μmol iron per gram liver, wet weight (about 15 mg iron per gram liver, dry weight),92 93 deferoxamine toxicity is rare but the risk of cardiac disease and early death is greatly increased.
In the absence of prospective clinical trials in patients with thalassemia major adequate for the evaluation of life-long therapy, guidance about the risk of complications associated with lower levels of body iron may be derived from the clinical experience with hereditary hemochromatosis. In this autosomal recessive disorder, the iron overload is the result of an abnormality affecting the regulation of iron absorption that produces an inappropriate increase in iron uptake, with homozygotes developing a chronic progressive increase in body iron stores.52 A candidate gene for this disorder has been recently identified.213 Minor iron loading develops in about one quarter of those heterozygous for hereditary hemochromatosis, but body iron stores in these individuals do not seem to increase beyond about two to four times the upper limit of normal.214 Body iron loads of the magnitude found in heterozygotes for hereditary hemochromatosis have no apparent ill effects and are associated with a normal life expectancy.214 By contrast, homozygotes who develop greater iron burdens have an increased risk of cardiac disease, hepatic fibrosis, diabetes mellitus, endocrine abnormalities, and other complications of iron overload. Just as for transfusional iron overload, in the iron overload of hereditary hemochromatosis, the greater the body iron excess, the higher the risk of adverse consequences.215-218 The toxic manifestations of iron overload depend not only on the amount of excess iron but also on (1) the rate of iron accumulation, (2) the duration of exposure to increased iron, (3) the partition of the iron burden between relatively benign sites in the macrophage and more hazardous deposits in parenchymal cells, (4) ascorbate status, which helps determine the allocation of iron between macrophage and parenchymal cells, (5) the extent of internal redistribution of iron between macrophage and parenchymal sites, and (6) noniron-related factors, such as alcohol and viral hepatitis.52 Nonetheless, the considerations above would suggest that a conservative goal for iron chelation therapy in patients with thalassemia major is to maintain an “optimal” body iron corresponding to hepatic storage iron concentrations of about 18 to 38 μmol iron per gram liver, wet weight (about 3.2 to 7 mg iron per gram liver, dry weight), in the range found in heterozygotes for hereditary hemochromatosis. The risks of deferoxamine toxicity associated with regimens to maintain body iron within this range are likely minor (see below) but are almost certainly increased at lower body iron burdens. Patients with higher body iron burdens, up to about 80 μmol iron per gram liver, wet weight (about 15 mg iron per gram liver, dry weight) are considered to be at an increased risk of hepatic fibrosis, diabetes mellitus, and other complications and need more intensive iron chelation therapy. Patients with still higher body iron burdens are recognized as having a greatly increased risk of cardiac disease and early death and are candidates for continuous IV ambulatory deferoxamine219 or other special programs of management.102 These ranges are shown graphically in Fig 6 and suggestions for management are summarized in Table 3.
If measurement of the hepatic iron concentration is not feasible, serum ferritin concentrations provide an alternative but less reliable means of determining if the body iron is in a optimal range (Fig 2). As noted above, a serum ferritin concentration of about 2,500 μg/L may be used as a threshold value to identify patients at an increased risk of cardiac disease and early death.91 Patients with most serum ferritin concentrations in excess of 2,500 μg/L had an estimated cardiac disease free survival after 15 years of less than 20% (Fig 1). The serum ferritin concentrations corresponding to the optimal range for hepatic iron shown in Fig 6 are less clearly defined. Preliminary analysis of studies of a large number of adults with thalassemia major over more than 15 years of deferoxamine therapy found that very rigorous control of body iron burden — as estimated by maintenance of serum ferritin concentrations under 1,000 μg/L — was associated with a very low incidence of iron-induced complications. Iron-related morbidity increased strikingly with even slightly less effective iron-chelating therapy.220
INITIATION OF CHELATING THERAPY
Uncertainties as to the optimal age for the start of chelation therapy continue to exist. Reports of abnormal linear growth and metaphyseal dysplasia observed in children treated with deferoxamine before the age of 3 years141-144 have prompted recommendations for later therapy.141 In parallel, ultrastructural observations of liver biopsy specimens in transfused patients with thalassemia, including a unique study of three infants whose biopsies at this early age, have revealed moderate to severe iron overload.94 Furthermore, elevated hepatic iron concentrations associated with hepatic fibrosis, not uniformly evident by determinations of serum ferritin or laboratory abnormalities of liver function, have been observed in transfused thalassemic children less than 3 years of age.221 222 These data suggest that that some modified program of chelating therapy is likely indicated before this age (below and Table 3).
How can one identify the patient for whom iron chelation therapy should be initiated? Because of the imprecision of indirect measurements, we recommend that initiation of therapy be based on hepatic iron concentration obtained after 1 year of regular transfusions. Liver biopsy under ultrasound guidance is a safe procedure in children, with a complication rate of 0 in patients aged less than 5 years in a series of 1,184 biopsies performed before marrow transplantation for thalassemia.221 Although this must be viewed as an estimate with certain confidence limits, a similar experience has been observed from two other centers, including our own, with large numbers of patients regularly undergoing liver biopsies under ultrasound guidance223 (and Olivieri N.F., unpublished data, November 1996).
Few guidelines exist with respect to the initiation of iron-chelating therapy. In practice, the approach of most clinicians is to determine the serum ferritin concentration after a period of regular transfusions and, based on the value of this parameter, to begin a regimen of nightly subcutaneous deferoxamine therapy. As emphasized above, reliance on serum ferritin measurements alone can lead to inaccurate assessment of body iron burden in individual patients.165 Therefore, we recommend that all children with thalassemia major undergo determination of liver iron concentration after 1 year of regular transfusions. The value of hepatic iron that should prompt therapy is in the range of the same concentrations which should be ideally maintained during chronic iron-chelating therapy, as discussed above.
If liver biopsy is not available at the start of therapy, therapy with subcutaneous deferoxamine, not exceeding 25 to 35 mg deferoxamine per kilogram body weight/24 hours in young children, should be initiated after approximately 1 year of regular transfusions. The basis for this recommendation, and a titration scheme that has provided ideal chelating efficacy while attempting to circumvent drug toxicity, is detailed below and in Table 4.
BALANCE BETWEEN EFFECTIVENESS AND TOXICITY OF DEFEROXAMINE
It has been recognized that most toxic effects of deferoxamine have been observed in patients during administration of doses exceeding 50 mg per kilogram body weight, or smaller doses in the presence of very modestly elevated body iron burdens.224 The observation that the toxicity of deferoxamine is enhanced as the serum ferritin concentration declines, and deferoxamine dose increases, is supported by most analyses of this complication.141,142 224-226 As emphasized above, attempts to maintain normal hepatic iron concentrations with deferoxamine in patients with thalassemia major may be associated with increased deferoxamine toxicity.
Adverse effects associated with deferoxamine include ocular and auditory abnormalities,225,227-235 sensorimotor neurotoxicity,236 changes in renal function,237,238 and pulmonary toxicity.239,240 A toxic manifestation of deferoxamine therapy of great concern in young children is failure of linear growth (Fig 7), associated with evidence of cartilagenous dysplasia of the long bones (Fig 8) and spine (Fig 9).141,142,241-247 Over the past 3 years, it has been recognized that short stature, primarily related to disproportionate truncal growth and loss of sitting height in thalassemic children,141,142 may be due to the effect of deferoxamine on spinal cartilage.244-246 At the same time, the findings of iron overload and hepatic damage in young transfused children outlined above have prompted our recommendations of the use of deferoxamine early in life, using reduced doses as a balance between risk and benefit. This practice is supported by studies of children who have received low-dose deferoxamine (15 to 35 mg/kg/night) since the age of 3 years, all of whom had normal sitting heights, standing heights, and normal spinal x-rays. By contrast, in a second cohort of children in which deferoxamine, administered at standard doses (50 mg/kg) from an equally early age had induced a comparable reduction in body iron burden, mean sitting height was markedly abnormal and significant x-ray abnormalities were observed.246 These data suggest that abnormal linear growth may be a direct toxic effect of prolonged administration of higher doses of deferoxamine, unrelated to changes in body iron. Because improvement in linear growth of patients with spinal abnormalities has not been observed even with reduction of deferoxamine dose, it would appear important to prevent this toxicity.
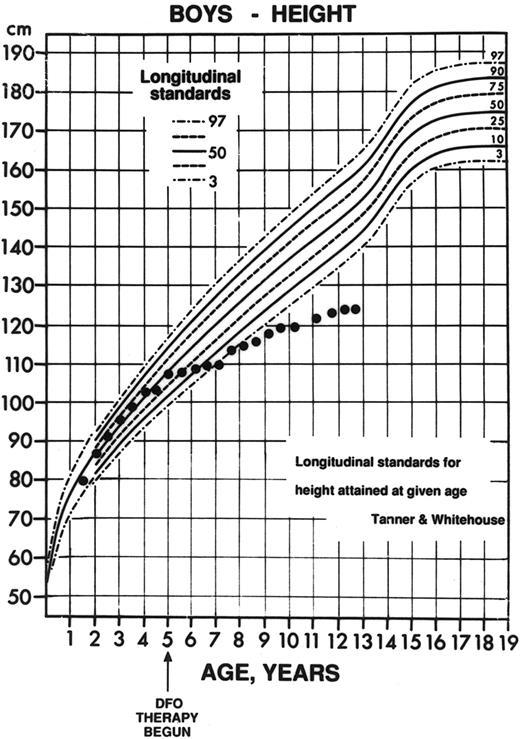
Decline in height percentile observed in a child with thalassemia major. The patient began therapy at age 4 years, 11 months (arrow) with nightly subcutaneous deferoxamine (initial dose, 11 mg deferoxamine per kilogram per day; mean dose over the first 3 years of therapy, 55 ± 17 mg/kg/d). This patient had normal radiographs before the start of deferoxamine (see Fig 8A) but subsequently developed marked growth failure with a dramatic decline in height percentile, from the 37th percentile for age 6 months before initiation of deferoxamine, to less than the 3rd percentile 36 months later. (Reprinted with permission.144 )
Decline in height percentile observed in a child with thalassemia major. The patient began therapy at age 4 years, 11 months (arrow) with nightly subcutaneous deferoxamine (initial dose, 11 mg deferoxamine per kilogram per day; mean dose over the first 3 years of therapy, 55 ± 17 mg/kg/d). This patient had normal radiographs before the start of deferoxamine (see Fig 8A) but subsequently developed marked growth failure with a dramatic decline in height percentile, from the 37th percentile for age 6 months before initiation of deferoxamine, to less than the 3rd percentile 36 months later. (Reprinted with permission.144 )
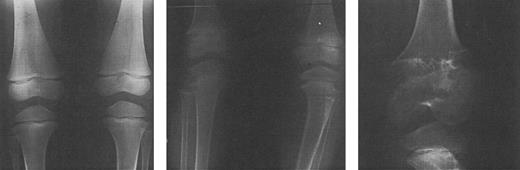
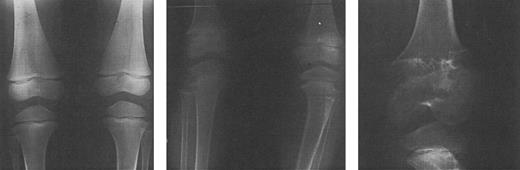
Radiographs of the femoral and tibial metaphyses of a child treated with deferoxamine therapy. Shown are the metaphyses prior to initiation of nightly subcutaneous deferoxamine (Fig 7A); 3 years after initiation of deferoxamine therapy (Fig 7B); and 6 years after initiation of deferoxamine therapy (Fig 7C). Radiographs show evidence of progressive widening and irregularity of the unossified metaphyseal matrix, which has irregular sclerotic margins. Similar processes in the proximal tibial metaphyses produced both varus and valgus deformities requiring bracing and osteotomy. (Reprinted with permission.144 )
Radiographs of the femoral and tibial metaphyses of a child treated with deferoxamine therapy. Shown are the metaphyses prior to initiation of nightly subcutaneous deferoxamine (Fig 7A); 3 years after initiation of deferoxamine therapy (Fig 7B); and 6 years after initiation of deferoxamine therapy (Fig 7C). Radiographs show evidence of progressive widening and irregularity of the unossified metaphyseal matrix, which has irregular sclerotic margins. Similar processes in the proximal tibial metaphyses produced both varus and valgus deformities requiring bracing and osteotomy. (Reprinted with permission.144 )
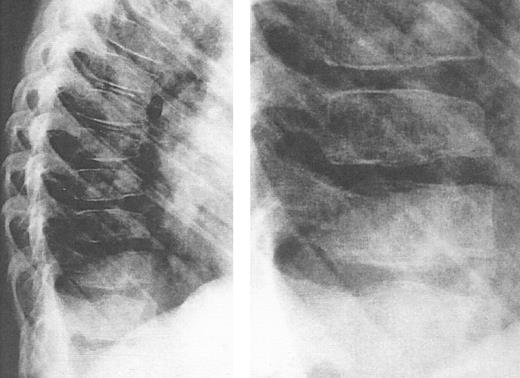
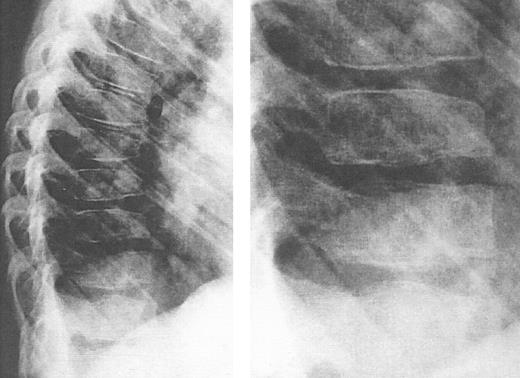
Lateral view of the thoracic spine in an 11-year, 9-month-old girl with thalassemia major treated with intensive deferoxamine throughout childhood. The spine shows decreased vertebral height with intervertebral disc calcification, flattening and lengthening and anterior tapering or the vertebrae, wedging and moderate kyphosis in this region. Detailed inset shows a bone-within-bone appearance, demarcating a zone of pronounced calicification. (Reprinted by permission from Pediatric Radiology, Spinal deformities in deferoxamine-treated beta-thalassemia major patients, Hartkamp MJ, Babyn PS, Olivieri NF, Volume 23, pp 525-528, Figure 2, 1993, Copyright Springer-Verlag GmbH & Co, KG. 1993.)244
Lateral view of the thoracic spine in an 11-year, 9-month-old girl with thalassemia major treated with intensive deferoxamine throughout childhood. The spine shows decreased vertebral height with intervertebral disc calcification, flattening and lengthening and anterior tapering or the vertebrae, wedging and moderate kyphosis in this region. Detailed inset shows a bone-within-bone appearance, demarcating a zone of pronounced calicification. (Reprinted by permission from Pediatric Radiology, Spinal deformities in deferoxamine-treated beta-thalassemia major patients, Hartkamp MJ, Babyn PS, Olivieri NF, Volume 23, pp 525-528, Figure 2, 1993, Copyright Springer-Verlag GmbH & Co, KG. 1993.)244
In summary, deferoxamine-induced toxicity can be avoided by regular, direct assessment of body iron burden with regular evaluation of the hepatic iron concentration. If hepatic iron concentration is not regularly assessed, a “toxicity” index, defined as the mean daily dose of deferoxamine (mg/kg) divided by the serum ferritin concentration (μg/L) should be calculated for each patient every 6 months, and should not exceed 0.025.226 We recommend that doses of deferoxamine not exceed 50 mg/kg/d. Although higher doses have been used in an attempt to “rescue” patients with severe iron-related organ failure, such attempts have not infrequently been associated with deferoxamine toxicity, including permanent hearing loss and fatal pulmonary toxicity.225 240 Hence, it is difficult to justify the use of higher doses, especially because very few centers now administer deferoxamine to patients in whom the body iron burden has been determined precisely using the hepatic iron concentration, rather than estimated using the serum ferritin concentration. Regular evaluation of deferoxamine toxicity (Table 4) is strongly recommended in all patients maintained on any dose of deferoxamine.
Ascorbate supplementation.The dilemma of ascorbate supplementation has been thoroughly reviewed.30 Low ascorbic acid levels have been found in iron-loaded thalassemic patients248-250 in whom ascorbate supplementation results in a marked improvement in deferoxamine-induced iron excretion251 by expansion of the chelatable iron pool to which deferoxamine has access.248-253 In parallel, ascorbate-induced expansion of this pool may enhance free radical formation, and aggravate the toxicity of iron in vivo.254-257 Although routine ascorbate supplementation has been therefore discouraged in patients with thalassemia,22 observation of loss of sustained efficacy of deferoxamine in an unsupplemented patient should prompt determination of tissue ascorbate concentrations. If these are reduced, 100 mg ascorbic acid per day should be administered. If possible, patients should administer ascorbic acid approximately 30 minutes to 1 hour after the start of an infusion of deferoxamine, only on days during which deferoxamine is administered. The toxicity of ascorbate supplementation during therapy with other chelating agents is presently unknown.
COMPLIANCE WITH DEFEROXAMINE AND ALTERNATIVES TO SUBCUTANEOUS INFUSIONS
The most common difficulty associated with long-term therapy with subcutaneous deferoxamine is erratic compliance with therapy, which may decline as supervision of this regimen becomes increasingly the responsibility of the patient; objectively monitored compliance with deferoxamine is less than 70% in many older patients.258 Compliance with deferoxamine may be improved with intensive social and psychological support.259 260
IV deferoxamine.Regimens of IV ambulatory deferoxamine administered through implantable venous access ports reduce the local pain and irritation of subcutaneous infusions, and are associated with rapid reduction of body iron burden.102 Regimens of continuous IV ambulatory deferoxamine in which the infusion site is changed weekly by medical personnel require infusion site care and a weekly clinic visit, but remove the need for nightly self-administration and improve patient compliance.219
Bolus injections of subcutaneous deferoxamine.Very recently, studies of iron-loaded nonthalassemic191 and thalassemic patients261 patients have reported that deferoxamine administered by twice daily subcutaneous injections may be as effective as the same dose administered by subcutaneous infusion. Although bolus injections were administered in early clinical studies of deferoxamine, these reports represent the first attempts to evaluate the response to subcutaneous bolus injections, rather than infusions, of deferoxamine. If these early observations are confirmed, such a regimen may provide an alternative to prolonged infusions and freedom from infusion pumps.
Other forms of deferoxamine: Hydroxyethyl starch deferoxamine (HES-deferoxamine).Chemical attachment of deferoxamine to a hydroxyethyl starch polymer creates a high-molecular-weight chelator with affinity for iron identical to, but a vascular half-life 10 to 30 times longer than, that of standard deferoxamine.262,263 During a 4-hour IV infusion of HES-deferoxamine at doses equivalent to approximately 80 mg deferoxamine per kilogram body weight, no serious adverse clinical effects were observed in normal subjects.264 The efficacy and safety of a single infusion of this compound has now been assessed in a rising single dose study of eight iron-loaded patients.265 In patients with thalassemia major, approximately 50 or 85 mg of HES-deferoxamine per kilogram body weight induced urinary iron excretion equal to that achieved during a mean of 3 days of subcutaneous deferoxamine, with one patient excreting as much urinary iron after a single infusion of HES-deferoxamine as was achieved during 7 days of subcutaneous deferoxamine. A single infusion of HES-deferoxamine reduced nontransferrin-bound iron to zero or very low concentrations for 12 to 96 hours after infusion; nontransferrin-bound iron increased at the time point at which circulating chelator concentration began to decrease below the total plasma iron concentration. In one patient, urticaria prompted drug discontinuation; subsequent skin testing showed no allergy to starch, deferoxamine, or HES-deferoxamine. The efficacy and lack of toxicity of HES-deferoxamine in this single dose study in iron-loaded patients suggest that, if efficacy can be modified so that iron excretion after one infusion achieves that during 1 week of subcutaneous deferoxamine, this new compound might play a useful role in long-term reduction of body iron burden in selected patients with iron overload.
Other Indications for Chelating Therapy
Thalassemia “intermedia.”Iron loading secondary to increased gastrointestinal iron absorption in patients with thalassemia “intermedia” is less accelerated than that of transfusional iron overload in thalassemia major.266,267 Striking elevations of hepatic iron concentration, in parallel with modestly elevated levels of serum ferritin, have been observed in adult patients with thalassemia intermedia268; therefore, direct determination of body iron burden is indicated in any patient with thalassemia intermedia and an elevated serum ferritin concentration. Chelating therapy should be initiated if the hepatic iron concentration exceeds 7 mg per gram dry weight liver tissue, and hepatic iron concentration should assessed at frequent intervals during therapy. As detailed below, the orally active iron-chelating agent deferiprone has been shown to be rapidly effective in reducing body iron stores in thalassemia intermedia.192
Chelation therapy after BM transplantation (BMT) for thalassemia.Successful allogeneic BMT in thalassemia liberates patients from chronic transfusions269 but does not eliminate the necessity for iron-chelating therapy in all patients. Timely reduction of hepatic iron concentration is observed only in younger patients with low pretransplantation body iron burdens; parenchymal hepatic iron overload persists, up to 6 years after marrow transplantation, in most patients who do not receive posttransplant deferoxamine treatment.270 Short-term deferoxamine is safe and effective in the reduction of tissue iron in the “ex-thalassemic” patient,271 and should be initiated 1 year after successful marrow transplantation if the hepatic iron concentration exceeds 7 mg iron/gram liver tissue, dry weight, at that time.
Orally active iron chelators.The expense and inconvenience of deferoxamine has mandated a 20-year search for an orally active iron chelator, four of which have reached clinical trials in the past decade. The compounds N,N′-bis (2-hydroxybenzoyl) ethylenediamine N,N′-diacetic acid (HBED), the aryl hydrazone pyridoxal isonicotinoyl hydrazone (PIH), and the di-ethyl hydroxypyridinone CP94, have all been evaluated in short-term trials over the last 5 years,272-274 but are not under clinical development at this time. The orally active iron-chelating agent most extensively evaluated to date is 1,2-dimethyl-3-hydroxypyridin-4-one (deferiprone; L1), one of the 3-hydroxypyridin-4-one bidentate iron chelators patented in 1982 as an alternative to deferoxamine for the treatment of chronic iron overload.275 Deferiprone, a neutral molecule, forms a neutral 3:1 chelator:ferric iron complex at pH 7.4. The drug may mobilize iron from ferritin, hemosiderin, lactoferrin, and diferric transferrin.276,277 Animal studies have reported variable efficacy in rodents and rabbits,278-284 and efficiency of chelation apparently insufficient to maintain negative iron balance in iron-loaded primates.285 In transfused patients with thalassemia major, 75 mg of deferiprone per kilogram body weight induces urinary iron excretion approximately equivalent to that achieved with 30 to 40 mg of deferoxamine per kilogram,286-288 sufficient to induce net negative iron balance in many patients with thalassemia major. Because fecal iron excretion induced by deferiprone is much less than that by deferoxamine,286 289 the short-term efficacy of deferiprone is unquestionably inferior to that of deferoxamine.
Effectiveness of deferiprone in long-term trials of thalassemia.Although the earliest studies reported no sustained decrease in serum ferritin concentration over 1 to 15 months of deferiprone therapy,290-293 two trials subsequently reported statistically significant reductions in mean serum ferritin concentration in patients with thalassemia major, with the most substantial decreases observed in those whose prestudy ferritin concentrations exceeded 5,000 μg/L, and in whom treatment had been administered for at least 18 months.294,295 A recent study demonstrated that, over the short term, deferiprone may reduce or maintain the serum ferritin concentration to levels associated with cardiac disease free survival in deferoxamine-treated patients,93 using criteria derived from a prospective trial in deferoxamine-treated patients.91 As noted above, reliance on serum ferritin concentrations alone may lead to inaccurate assessment of body iron burden in individual patients, and direct assessment of changes in tissue iron is particularly crucial in the evaluation of any new chelator. These studies of serum ferritin supported, but did not establish, the efficacy of deferiprone in the reduction of body iron burden.
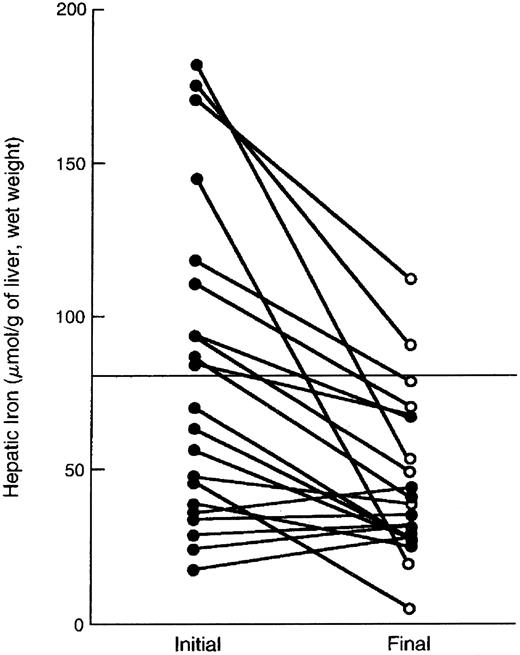
Reduction in hepatic iron stores during deferiprone therapy was first shown in a patient with thalassemia “intermedia,”192 followed by a study reporting deferiprone-induced reduction in hepatic iron concentration in patients with thalassemia major.93 This study demonstrated that deferiprone was able, over a mean of 3 years of therapy, to reduce or maintain hepatic storage iron at concentrations associated with prolonged survival free of the clinical complications of iron overload, using the criteria derived from a prospective trial in deferoxamine-treated patients of 80 μmol of iron per gram liver, wet weight (15 mg of iron per gram liver, dry weight).92 Overall, the mean hepatic iron concentration of this cohort declined over a mean period of 3 years. These patients constitute the only group worldwide to receive long-term deferiprone therapy in conjunction with repeated measurements of hepatic iron concentration. This trial was terminated by its sponsor, Apotex Pharmaceuticals (Weston, Ontario, Canada) in May 1996.
Relative effectiveness of deferiprone and deferoxamine.The relative effectiveness and safety of, and compliance with, deferiprone and deferoxamine were being compared in a prospective randomized trial begun in Canada in 1993.258 Patients stratified for hepatic iron concentration had been randomized to receive 75 mg deferiprone per kilogram per day, or 50 mg subcutaneous deferoxamine per kilogram per night. This trial was intended to provide information about the relative long-term effectiveness of deferiprone and deferoxamine, but was also terminated prematurely by Apotex Pharmaceuticals in May 1996.
Toxicity of Deferiprone
Animal studies.As detailed previously,288 deferiprone did not receive full formal toxicologic evaluation before being given to humans; permission to administer the drug in early studies in the United Kingdom, India, Europe, and Canada was granted on the basis of limited toxicity studies in rodents. Acute toxicity studies have estimated an LD50 of 1,000 mg/kg in mice,278 and one of approximately 650 mg/kg in rats.296 Subacute toxicity studies in non–iron-loaded animals reported anemia, leukopenia, and thrombocytopenia in mice,278,283 anemia and leukopenia in rats,297 and death in dogs298 at doses 2- and 10-fold times those administered to iron-loaded humans. Adrenal hypertrophy, gonadal and thymic atrophy, bone marrow atrophy and pancytopenia, growth retardation, and embryotoxicity have also been reported in animals.299
Human trials.The most common adverse effect associated with administration of deferiprone has been arthralgias, primarily of the large joints,294,295,300 the etiology of which remains elusive. The most serious adverse effect associated with the administration of deferiprone has been severe neutropenia or agranulocytosis, first reported in 1989.301 To date, this complication has been reported in 13 patients, of whom 10 have thalassemia major,301-305 as early as 6 weeks and up to 21 months after the initiation of deferiprone. No deaths have been reported as a result of this adverse effect. In five patients in whom rechallenge with deferiprone has been attempted after white blood cell counts returned to normal, a second decrease in neutrophil count has been observed.305 The mechanism of deferiprone-induced neutropenia is unknown. Although studies in animals and early reports in humans suggested that this effect might be related to administration of high doses of deferiprone, at least 7 patients have developed agranulocytosis during administration of the standard daily dose of 75 mg deferiprone per kilogram body weight; this adverse effect thus appears not to be dose-dependent, but idiosyncratic and unpredictable. A large trial of deferiprone in Italy and the United States is expected to provide an estimate of the incidence of this serious adverse effect, which is likely to limit the widespread use of deferiprone therapy.
Concerns regarding the adverse effects of deferiprone on immunologic function were raised in a case report describing fatal “systemic lupus erythematosus” in a patient receiving deferiprone in India,306,307 in studies reporting inhibition of human lymphocyte proliferation by deferiprone in vitro,308 and in studies describing thymic atrophy in rats.299 The significance of these reports remains unclear. The data from the case reports do not indicate a definite causal relationship between the symptom complex and treatment with deferiprone. Although deaths related to infection in deferiprone-treated patients in India have been attributed to immune dysfunction,309 these deaths were considered by the physicians responsible for the long-term treatment of the patients to be no different from those of other Indian patients, in whom pyogenic meningitis is a relatively common cause of death.310 Other adverse effects reported with deferiprone administration include dermatologic changes associated with decreases in serum zinc concentration resolving with oral zinc supplementation,311 nausea, and transient or sustained liver enzyme abnormalities.312
The licensing of deferiprone.Deferiprone was administered to humans before full animal toxicologic evaluation required by the United States Food and Drug Administration (FDA) had been obtained. The data from available animal toxicity studies and clinical trials were first reviewed by representatives of the FDA in 1991, at which time approval for an investigational new drug application for deferiprone was deferred. At a second review in 1993, representatives of the FDA judged that a prospective, randomized trial to compare therapy with deferiprone with deferoxamine, and a second prospective study to estimate the incidence of serious adverse effects of deferiprone in a large cohort of patients, would be required for the licensing of deferiprone in the United States. Both of these studies were supported in part by the Canadian pharmaceutical company Apotex Pharmaceuticals. The first has been terminated prematurely by Apotex, while the second was completed in September 1996. In 1995, deferiprone was licensed for sale in India.
SUMMARY
Iron-chelating therapy with deferoxamine in patients with thalassemia major has dramatically altered the prognosis of this previously fatal disease. The successes achieved with deferoxamine, as well as the limitations of this treatment, have stimulated the design of alternative strategies of iron-chelating therapy, including orally active iron chelators. The development of the most promising of these, deferiprone, has progressed rapidly over the last 5 years; data from several trials have provided direct and supportive evidence for its short-term efficacy. At the same time, the toxicity of this agent mandates a careful evaluation of the balance between risk and benefit of deferiprone in patients with thalassemia, in most of whom long-term deferoxamine is safe and efficacious therapy.
NOTE ADDED IN PROOF
Although support for both the long-term treatment cohort of deferiprone-treated patients93 and a randomized trial of deferiprone and deferoxamine258 was terminated prematurely by their corporate sponsor, APOTEX Pharmaceuticals (Weston, Canada) in 1996, follow-up of hepatic storage iron concentrations in both cohorts have provided information regarding the long-term effectiveness of deferiprone in thalassemia major. In the long-term treatment cohort of deferiprone-treated patients reported previously,93 hepatic iron concentrations are now above the threshold associated with increased risk of heart disease and early death in thalassemia major91 in one third of patients.313 In the randomized trial of deferiprone and deferoxamine,258 review of available hepatic iron concentrations in patients who had completed 2 years of study by August 1996 showed a mean increase in hepatic iron concentration of approximately 50% over baseline in patients treated with deferiprone, but no significant change in those treated with deferoxamine.314 These results, recently reported to the Canadian drug regulatory agency, Health Protection Branch, Ottawa, Canada, raise concerns that long-term therapy with deferiprone may not provide adequate control of body iron in a substantial proportion of patients with thalassemia major.
N.F.O. is a Scientist of the Medical Research Council of Canada.
Address reprint requests to Nancy F. Olivieri, MD, Director, Haemoglobinopathy Program, The Hospital for Sick Children, Room 9413, 555 University Ave, Toronto, Ontario, Canada M5G 1X8.