Abstract
Hepatocyte growth factor (HGF )/scatter factor (SF ) is the ligand for a tyrosine kinase cell surface receptor encoded by the MET protooncogene (c-MET). HGF/SF can induce proliferation and motility in epithelial cells and promotes invasion of carcinoma cells and NIH3T3 fibroblasts transfected with both HGF/SF and c-MET genes. Our results show that HGF/SF and c-MET also play a role in adhesion and invasion of human lymphoma cells. c-MET mRNA is expressed in hemopoietic cells, such as hemopoietic progenitor cells (CD34+ cells) in bone marrow (BM) and mobilized peripheral blood, immature B cells in cord blood and BM, and germinal center B-centroblasts. In normal peripheral blood B cells, which are c-MET−, c-MET expression was induced by PMA, ConA, HGF/SF, and Epstein-Barr virus (EBV) infection. Using immunohistochemistry, we detected c-MET on the cell surface of large activated centroblasts in lymph nodes from patients with B-non–Hodgkin's lymphoma and Hodgkin's disease. In the latter group, c-MET expression correlated well with the presence of EBV. Because HGF/SF and c-MET promote metastasis of carcinoma cells, we studied the effects of c-MET stimulation by HGF/SF of B-lymphoma cells on properties relevant for metastasis, ie, adhesion, migration, and invasion. HGF/SF stimulated adhesion of the c-MET+ B-cell lines to the extracellular matrix molecules fibronectin (FN) and collagen (CN) in a dose dependent manner. However, adhesion to laminin was not affected by HGF/SF. Adhesion to FN was mediated by β1-integrins α4β1 (VLA4) and α5β1 (VLA5) since blocking antibodies against β1- (CD29), α4- (CD49d), or α5- (CD49e) integrin subunits, completely reversed the effect of HGF/SF. Furthermore, HGF/SF induced adhesion was abrogated by addition of genistein, which blocks protein tyrosine kinases, including c-MET. Addition of HGF/SF resulted in a sixfold increase in migration of c-MET B-lymphoma cells through Matrigel, compared to medium alone. In rat fibroblast cultures, HGF/SF doubled the number of c-MET+ B-lymphoma cells that invaded the fibroblast monolayer. In these adhesion, migration and invasion assays HGF/SF had no effect on c-MET− cell lines. In conclusion, c-MET is expressed or can be induced on immature, activated, and certain malignant B cells. HGF/SF increased adhesion of c-MET+ B-lymphoma cells to FN and CN, mediated via β1-integrins α4β1 and α5β1 , and furthermore promoted migration and invasion.
THE MAIN barrier in dissemination of lymphoma cell is the peripheral blood (PB) vessel wall consisting of endothelial cells. The first step is adhesion to the endothelium followed by transendothelial migration. Normal lymphocytes can migrate from the lymph nodes to the PB and in response to inflammation into tissues and vice versa.1-3 For adhesion of lymphocytes to endothelium or extracellular matrix molecules, integrins play a major role. For optimal binding, the integrins on the lymphocytes have to be activated by chemoattractants that are secreted by and can be bound to endothelial cells.4,5 Chemoattractants can bind to G-protein coupled receptors present on lymphocytes, which results in activation of the integrins. Growth factors can act as chemoattractants. For instance, interleukin-8 (IL-8) is a chemoattractant that is produced by endothelial cells itself or by underlying inflammatory cells and subsequently transported to the vessel lumen.6,7 Other examples of chemoattractants are platelet-activating factor, complement product C5a, leukotriene B4, and the chemokines MIP-1 α and β.3 Recently, hepatocyte growth factor (HGF ), also known as scatter factor (SF), was identified as a chemoattractant for a subset of T cells.8
HGF/SF is produced by various cells of mesenchymal origin, but not by epithelial cells and has a pleiotropic function. HGF/SF was first identified as a major mediator of liver regeneration but later was shown to have mitogenic, but also morphogenic and motogenic effects on a variety of epithelial cells (eg, mammary, kidney, intestinal, and bronchial epithelial cells) as well as endothelial cells.9-15 The receptor for HGF/SF is encoded by the MET protooncogene (c-MET). The c-MET protein is a tyrosine kinase cell surface receptor and consists of an extracellular α- and a transmembrane β-chain.16 The β-chain contains the tyrosine kinase domain, as well as sites for tyrosine autophosphorylation.17 18
Based on transfection studies in NIH3T3 cells, a role has been suggested for HGF/SF in metastasis. Addition of human HGF/SF to NIH3T3 fibroblasts, which had been transfected with human c-MET induced changes in cell shape, migration in Boyden chambers, and invasion into collagen (CN) matrices in vitro.19 Rong et al20 showed that NIH3T3 cells produce murine HGF/SF. Transfection of these cells with the murine c-MET resulted in an autocrine activation loop and the cells became invasive in vitro and tumorigenic in vivo in a nude mouse model. Transfection of both the human c-MET and HGF/SF genes resulted in a similar invasive behavior.
Here, we describe that c-MET is expressed or can be induced on normal B cells present in bone marrow (BM), PB, and lymph nodes. In addition, the expression of c-MET in lymph node samples of non-Hodgkin's lymphoma (NHL) and Hodgkin's disease (HD) patients was documented. To determine whether c-MET and HGF/SF have a similar role in (malignant) B cells as in epithelial and in transfected NIH3T3 cells, we investigated whether HGF/SF affects adhesion of c-MET+ lymphoma cells to extracellular matrix molecules. HGF/SF promoted adhesion of malignant B cells to fibronectin (FN) and CN, but not to laminin. Adhesion of B cells to FN was mediated by α4β1 and α5β1 integrins through activation of tyrosine kinases. HGF/SF also enhanced migration of lymphoma cells through Matrigel and invasion into fibroblast monolayers.
MATERIALS AND METHODS
Cells
Normal BM cells were obtained by sternal aspiration of patients undergoing cardiac surgery with informed consent (approval by local ethical committee Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands). PB was obtained from healthy volunteers and from patients treated with chemotherapy and granulocyte colony-stimulating factor (5 to 10 μg/kg/d) subcutaneously (filgrastim; Amgen, Thousand Oaks, CA) for hematopoietic stem cell mobilization. Umbilical cord cells were obtained from the Slotervaart hospital (Amsterdam, The Netherlands) with informed consent. Mononuclear cells were isolated by gradient sedimentation (Lymphoprep; Nycomed, Oslo, Norway; density 1.077 g/mL; 20°C; 15 minutes at 2,200 rpm). B-cell lines (BJAB, Raji, Daudi, Ramos, Jiyoye [obtained from ATCC, Rockville, MD] and Epstein-Barr virus (EBV)-infected B-cell lines) were cultured in Iscove's modified Dulbecco's medium (IMDM) (GIBCO-BRL, Gaithersburg, MD), supplemented with 5% heat inactivated fetal calf serum (FCS) (BioWittaker, Brussels, Belgium), penicillin (100 U/mL; GIBCO-BRL) and streptomycin (100 μg/mL; GIBCO-BRL).
Epstein-Barr Virus (EBV) Infection of Peripheral B Lymphocytes
PB was collected from healthy volunteers. Mononuclear cells were isolated by gradient sedimentation using Lymphoprep (Nycomed; density 1.077 g/mL; 20°C; 15 minutes at 2,200 rpm). 2.5 × 106 cells were suspended in 1 mL RPMI (GIBCO-BRL, Gaithersburg, MD) supplemented with 5% FCS and incubated with 1 mL supernatant from the EBV secreting cell line B95-8 (ATCC). After 1 hour the cells were washed with culture medium and plated in a 96-well plate at a density of 5 × 104 cells/well in RPMI supplemented with 5% FCS. After 24 hours, cyclosporine (1 μg/mL; Sandoz Pharma BV, Uden, The Netherlands) was added to inhibit T-cell activation. Approximately 2 to 3 weeks later (CD19-expressing) B-cell lines were established.
Fluorescence-Activated Cell Sorting (FACS) and Analysis
For purification of different cell types from BM, PB or umbilical cord blood, mononuclear cells were labeled with CD3 (T cells), CD19 (B cells), CD16 + 56 (NK cells), CD14 (monocytes), CD15 (granulocytes), or CD34 (hematopoietic progenitor cells), and sorted with a FACS (FACS-STAR; Becton Dickinson, Leiden, The Netherlands). All antibodies were directly labeled with either fluorescein isothiocyanate (FITC) or phycoerythrin (PE) and purchased from Dako (Glostrup, Denmark), apart from CD34, which was obtained from Becton Dickinson (San Jose, CA; anti-HPCA-2 clone: 8G12). To facilitate sorting of CD34+ cells (± 1% to 2% in mononuclear cells from BM), immature cells were enriched by immunomagnetic depletion of T cells and monocytes. Mononuclear cells were incubated with a mixture of CD2 and CD14 at 4°C for 30 minutes. The cells were washed twice and incubated with immunomagnetic beads coated with goat anti-mouse Ig (ratio beads: cells = 3: 1; Dynal, Hamburg, Germany). After depletion, the remaining cells were incubated with FITC labeled CD34. The sorted cells (10,000 to 100,000) were collected in IMDM + 5% FCS and mRNA was isolated within 16 hours. In some experiments, B cells were stimulated with 0.1 μg/mL phorbol 12-myristate 13-acetate (PMA), ConA, IL-1α (R&D, Abingdon, UK), IL-4 (a gift from Dr H. Spits, Netherlands Cancer Institute), IL-6 (1,000 U/mL; a gift from Dr L.A. Aarden, CLB, The Netherlands), or HGF/SF (15 ng/mL; a gift from Dr T. Nakamura, Osaka, Japan) overnight.
For determination of the expression of β1-, α4-, and α5- integrin subunits, we incubated Raji, BJAB, and Daudi cells in the same conditions (same period of time in phosphate-buffered saline (PBS) and in the same medium with the same HGF/SF concentrations) as described for the adhesion assays (see below). The antibodies used were anti-β1 (CD29), anti-α4 (CD49d), or anti-α5 (CD49e); all purchased from Becton Dickinson. The secondary FITC-labeled antibodies (from Dako) were goat anti-mouse for the anti-α4 and goat anti-rat for anti-β1 and anti-α5 .
RNA Isolation and Synthesis of cDNA
RNA was isolated as previously described by Ziegler et al21 and Rapplee et al.22 Briefly, 103 to 106 cells were transferred into 100 μL of lysis buffer (4 mol/L GuSCN, 25 mmol/L sodium citrate, pH 5.0, 0.5% sodium lauroryl sarcosinate, 0.1 mol/L β-mercapto-ethanol) containing 20 μg glycogen (Boehringer Mannheim, Mannheim, Germany) as carrier. The mixture was thoroughly vortexed and layered on top of a 100 μL 5.7 mol/L cesium chloride cushion in 0.3 mL diethylpyrocarbonate (DEPC)-treated polycarbonate centrifuge tubes (Beckman, Palo Alto, CA). The tubes were centrifuged for 2.5 hours at 4°C at 55,000 rpm with a TLS-55 rotor in a Beckman TL-100 tabletop ultracentrifuge. The RNA pellet was resuspended in DEPC-treated H2O, precipitated with 0.1 volume of 3 mol/L potassium acetate and 2.5 volumes 100% ethanol for at least 2 hours at −20°C. After centrifugation, the pellet was washed in 70% ethanol, dried, and resuspended in 5 μL DEPC-treated H2O. First strand cDNA was synthesized by incubating RNA at 37°C for 1 hour in a final volume of 10 μL in reverse transcriptase (RT)-buffer (50 mmol/L Tris-HCl, pH 8.3, 75 mmol/L KCl, 3 mmol/L MgCl2 ) containing 100 U Moloney murine leukemia virus Reverse Transcriptase (M-MLV-RT, GIBCO-BRL), 0.5 mg oligo(dT) (Promega, Madison, WI), 15 U RNasin (Promega), 10 mmol/L dithiothreitol (DTT; GIBCO-BRL) and 1.0 mmol/L of each deoxyribonucleoside triphosphate (dNTP; Pharmacia Biotech, Uppsala, Sweden).
Polymerase Chain Reaction (PCR)
One tenth (1 μL) of the total cDNA obtained from the 103 to 106 cells was mixed with 1.25 U of Taq DNA polymerase (GIBCO-BRL), 1 mmol/L of each appropriate primer, 200 mmol/L of each dNTP (Pharmacia Biotech) in a buffer containing 10 mmol/L Tris-HCl, pH 8.3, 50 mmol/L KCl, 5 mmol/L MgCl2 , 0.01% (wt/vol) bovine serum albumin (BSA) in an end volume of 50 μL. The samples were overlaid with mineral oil and were amplified for 35 cycles at cycling temperatures of 94°C, 1 minute; 55°C, 1 minute; 72°C, 3 minutes for both the β-actin and the c-MET amplification.
For nested c-MET PCR, 35 more cycles were performed under the same conditions as described above with 10 μL of the PCR mixture obtained after the first 35 cycles.
P r i m e r s . β - a c t i n : 5 ′ - p r i m e r : 5 ′ G T G G G G C G C C C C A G G C ACCA 3′; 3′-primer: 5′ CTCCTTAATGTCACGCACGATTTC 3′.
c-MET (Taken from Ponzetto et al23 ): 5′-primer: 5′ CTAAATCCAGAGCTGGTCCAGG 3′; 3′-primer: 5′ GGGACCAAGCCTCTGGTTCTGATGC 3′.
Nested c-MET: 5′-primer: 5′ CAGTGCAGCATGTAGTGATTGGG 3′; 3′-primer: 5′ TCTGTCAGATAAGAAATTCC 3′.
Agarose Gel Electrophoresis and Hybridization of PCR Products
Twenty microliters of the PCR products were analyzed on 1% agarose gels. Fragments were visualized by UV illumination after staining with ethidium bromide. For hybridization the gels were dried in a gel dryer (Biorad, Veenendaal, The Netherlands), the DNA in the gels was denaturated (in 0.5 mol/L NaOH, 0.15 mol/L NaCl) for 30 minutes and neutralized (in 0.5 mol/L Tris, pH 8.0, 0.15 mol/L NaCl) for another 30 minutes. The gels were prehybridized in hybridization buffer (5× SSPE, 5× Denhardt, 0.5% SDS) for 1 hour at 56°C before the 32P-labeled (32P obtained from Amersham, Houten, The Netherlands) probe was added. Probes were labeled with a polynucleotide kinase kit (Boehringer Mannheim). Sequences of probes used: c-MET: 5′ GCAGTGCAGCATGTAGTGATTGG 3′; β-actine: 5′ GATGACCCAGATCATGTTTGAGAC 3′.
The gels were incubated overnight, washed three times in 6× SSC (1× SSC: 0.15 mol/L sodium chloride, 0.015 mol/L sodium citrate) containing 0.1% sodium dodecyl sulfate before autoradiography.
Immuno-histochemical Staining of Slides
Paraffin embedded tissue sections were deparaffinized and rehydrated. The sections were preincubated with 10% normal goat serum in PBS for 1 hour at room temperature (RT). Normal goat serum was removed and the sections were incubated overnight at 4°C with either C-28, the polyclonal antibody directed to the last 28C terminal aminoacids of c-MET (diluted 1:400 with PBS to a final concentration of 250 ng/mL; Santa Cruz), polyclonal control IgG (also used at 250 ng/mL; Southern Biotechnology Associates Inc, Birmingham, AL), or C-28 (250 ng/mL) previously mixed for 2 hours (RT) with a 10-fold higher concentration (2.5 μg/mL) of peptide to which C-28 was raised (Santa Cruz). Furthermore, sections were incubated with monoclonal antibodies (MoAb) directed to B cells (CD20, L26; Dako), T cells (CD3; Dako), CD30 (BerH2; Dako), EMA (115D8; The Netherlands Cancer Institute, Amsterdam). Sections were washed three times with PBS (5 minutes) after which the secondary antibody (biotinylated goat anti-rabbit [Dako, E432] for c-MET staining and biotinylated goat anti-mouse [Dako, E433] for B-cell staining) was added (1:1,000 diluted in 1% BSA in PBS) for 30 minutes at RT. Slides were washed with PBS (5 minutes) and incubated with streptavidine-biotin-peroxidase complex (StreptABComplex/HRP; Dako, K377) for 30 minutes at RT. Sections were washed with PBS (5 minutes) followed by 0.05 mol/L Tris/HCl solution (pH 7.4) and stained with DAB/H2O2 imidazol solution in the dark for 5 minutes at RT. The sections were rinsed with tap water for 5 minutes to stop the developing reaction, counterstained with hematoxylin and embedded in DPX (Klinipath; Zevenaar, The Netherlands).
We stained section of normal lymph nodes (N = 4), tonsils (N = 2), and spleens (N = 4), lymph nodes from 2 patients with infectious mononucleosis, and from patients with follicular center cell NHL (N = 11), large B-cell NHL (N = 11), large B-cell NHL with high expression of EMA, and CD30 (N = 6), Burkitt lymphoma (N = 3) and (HD) (N = 18).
EBV-RNA In Situ Hybridization
The presence of EBV in paraffin embedded tissue sections was determined as described by van Gorp et al,24 using a very sensitive RNA in situ hybridization against EBER 1 and 2, which are two EBV encoded RNAs that are expressed in a very high quantity in latently infected cells. After deparaffinization and rehydration, the slides were treated with proteinase K (3 g/mL) in PBS for 30 minutes at 37°C. Dehydration was followed by adding 1 to 2 drops (15 to 30 μL) of EBER oligonucleotides conjugated with FITC (undiluted; Dakopatts). The slides were hybridized for 2 hours at 37°C and immersed in PBS. After preincubation with 10% normal swine serum, a three-step peroxidase reaction was applied: the first step included mouse anti-FITC (Dakopatts); the second step, rabbit anti-mouse Ig; and the third step, swine anti-rabbit Ig. The latter two antibodies were conjugated to horseradish peroxidase (Dakopatts). Peroxidase was localized by 3,3 diaminobenzin (DAB; 60 mg/100 mL PBS; Sigma, Zwÿndrecht, The Netherlands) in H2O2 (0.03%) for approximately 5 seconds. The developing reaction was stopped by washing the slides in tap water for 5 minutes. Counterstaining and embedding of the sections were performed as described above.
Proliferation Assays
Proliferation of cell lines was tested using 3H-thymidine incorporation. A thousand cells were plated per well in a 96 wells plate (Greiner, Frickenhausen, Germany; round bottom) in 0.22 μ filtered culture medium (IMDM+ 5% FCS). After 1 day to 3 days at 37°C with increasing concentrations of HGF/SF (up to 100 ng/mL) the plates were pulsed with 3H-thymidine (0.5 μCi/well) for 4 hours and counted in a β-plate counter (1205 betaplate, Finland). Cell lines were also serum starved overnight before incubation with HGF/SF for 1 day after which 3H-thymidine was incorporated as described above. From cord blood, 50,000 CD19+ FACS sorted cells per well were incubated with HGF/SF (1,5 and 15 ng/mL) or PMA (0.1 μg/mL) for 24 hours immediately after cell sorting, followed by 3H-thymidine incorporation overnight.
Adhesion Assay
Adhesion assays were performed as described by Sonnenberg et al.25 96 wells plates (U-bottom plates were used, which allowed more smoothly washing of the wells than flat bottom plates, resulting in better triplicates; Greiner) were coated overnight at 4°C with 20 μg/mL of FN, CN IV, or laminin (all obtained from Sigma). Plates were washed three times with PBS and blocked with 1% BSA in PBS for at least 1 hour at room temperature. Exponentially growing cells (Raji, BJAB, Daudi, and Jiyoye) were labeled with Na512CrO4 (Amersham) for 30 minutes, washed three times, and resuspended in IMDM containing 0.35% BSA at a concentration of 1 × 106 cells/mL. The cells were preincubated with HGF/SF (5, 15, and 50 ng/mL) or PMA (0.1 μg/mL) for 10 minutes at 37°C. Thereafter, 100 μL of the cell suspension was plated in the coated wells. Cells were allowed to adhere to the extracellular matrix molecules for 45 minutes at 37°C after which the wells were gently washed in IMDM + 0.35% BSA at least 5 times until no more floating cells could be seen. Finally, the adhered cells were lysed with 0.2% SDS and counted in a gamma-counter (Packard, Downers Grove, IL). The percentage of adhered cells was calculated by dividing radioactivity in adhering cells by the radioactivity measured in 100 μL of the initial labeled cell suspension (set at 100%).
When evaluating the effect of genistein and blocking MoAbs on adhesion to FN, cells were preincubated with genistein (20 or 100 μmol/L; Sigma) or with the blocking antibodies anti-β1 (CD29), anti-α4 (CD49d) or anti-α5 (CD49e), and as control antibodies anti-α2 (CD49b) and anti-β2 (CD18), for 15 minutes at 37°C; 5% CO2 before HGF/SF or PMA were added. Genistein and (MoAbs) remained present during the rest of the assay. All five MoAbs were purchased from Becton Dickinson and used at a concentration of 0.1 μg/mL. To determine the effect of pertussis toxin on adhesion, cells were preincubated for 2.5 hours with pertussis toxin (200 or 500 ng/mL; List Biol Lab, Campbell, CA26) before labeling of the cells with 51chromium. Adhesion after these treatments was determined as described above.
Invasion Assays
Two different assays were used to measure invasiveness of cells:
Chemoattraction assay.Boyden chambers were used to assess invasiveness as described previously by Albini et al.27 6-well chemotaxis chambers were used (Becton Dickinson). Upper and lower wells were separated by Matrigel coated filters with 8 μm pores. After rehydration of the Matrigel in IMDM + 0.1% BSA, cells (2 × 105) were seeded in the upper wells in 1.5 mL IMDM containing 0.1% BSA. HGF/SF (15 ng/mL) or PMA (0.1 μg/mL) were added to the lower chambers in 2.6 mL of the same medium as present in the upper wells. The chambers were incubated overnight at 37°C. After 16 hours, the cells in the upper and lower chamber were transferred to tubes, centrifuged, resuspended in a small volume and counted. The filters were washed with PBS, fixed in 70% ethanol (10 minutes), stained with crystal blue (diluted 1:25 in water, 10 minutes) and the cells adhering to the filters were scored under a light microscope. Cells counted in the two compartments or adhering to the Matrigel were expressed as the percentage of the number of cells initially placed in the upper chamber.
Fibroblast invasion assay.Described by Verschuren et al,28 and modified by Collard et al.29 B lymphocytes (2.5 × 105 cells in 1 mL per well of 24-wells plates) were added to confluent rat embryo fibroblast monolayers in IMDM + 5% FCS without or with 5 or 15 ng/mL HGF/SF. After 4 hours the wells were washed with PBS containing 1 mmol/L MgCl2 and CaCl2 for at least 4 times with physical agitation until no floating cells could be detected anymore with a light microscope. Adhering cells and invading cells positioned between the fibroblasts were counted in five randomly distributed fields with an inverted microscope. From this the invading and adhering cells per well was calculated and expressed as a percentage of added cells (set at 100%).
Statistical Analysis
Results were reported as the mean ± standard deviation (SD) of triplicate samples from a representative of at least three experiments. Significance levels were determined by two-sided Student's t-test.
RESULTS
Expression of c-MET in Normal Hematopoietic Cells
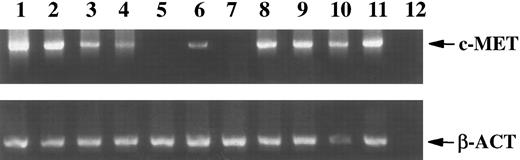
Mononuclear cells from peripheral blood (PBMC) and BM were tested for the presence of c-MET mRNA, using reverse transcriptase-polymerase chain reaction (RT-PCR). Both BM and PBMC express c-MET (Fig 1). The PCR signal faded rapidly when fewer cells were used. At least 10,000 mononuclear cells from BM or 1,000,000 cells from PB were required to obtain a PCR product, that was visible on an ethidium bromide stained gel after 35 PCR cycles. Specificity controls included hybridization of PCR products with a radioactive probe and/or performing a nested PCR. These extra steps increased the detection level approximately ten times. To identify the cell types that express the protooncogene, we separated peripheral blood mononuclear cells in T cells, B cells, NK cells, monocytes, and granulocytes with a fluorescence activated cell sorter. The c-MET product was not amplified from 10,000 cells of any of these cell populations, whereas the β-actin signal (positive control) was clearly visible (data not shown). Even when ten times more cells were analyzed (100,000) and a nested RT-PCR was performed, c-MET mRNA could not be detected. These results indicated that a rare cell population in PBMC and BM accounts for the c-MET signal. Further analysis of BM and leukapheresis material showed that the MET proto-oncogene is expressed in CD34+ cells (Fig 1). Immature B cells (CD19+CD20−) isolated from BM also expressed c-MET mRNA, in contrast to mature BM B cells (CD19+CD20+), which were c-MET negative, as were the B cells from PB. Consistent with this finding, CD19+ cells from umbilical cord (more immature B cells than those obtained from PB) were c-MET+ (Fig 1). c-MET mRNA expression was induced in freshly isolated PB B cells that were stimulated overnight with PMA, ConA, or HGF (Fig 1). IL-1α, IL-4, and IL-6 did not induce expression (results not shown). Infection with EBV also induced c-MET expression in B cells (Fig 1). In a B-cell line established by EBV infection of normal PB, c-MET was expressed for at least 3 months. After prolonged culture the EBV-B cells showed a heterogeneous expression of the c-MET gene.
Expression (RT-PCR) of c-MET and β-actin (β-act; used as a positive control) mRNA in 104 BM cells (lane 1), 106 PBMC (lane 2), and in 104 FACS STAR sorted CD34+ cells from BM (lane 3), immature B cells isolated from BM (CD19+CD20−, lane 4), mature B cells from BM (CD19+CD20+, lane 5), and B cells isolated from umbilical cord (CD19+, lane 6), PB B lymphocytes, either unstimulated (lane 7), stimulated with PMA (lane 8), ConA (lane 9), HGF/SF (lane 10), or infected with EBV (lane 11). Lane 12 is the water control for the PCR.
Expression (RT-PCR) of c-MET and β-actin (β-act; used as a positive control) mRNA in 104 BM cells (lane 1), 106 PBMC (lane 2), and in 104 FACS STAR sorted CD34+ cells from BM (lane 3), immature B cells isolated from BM (CD19+CD20−, lane 4), mature B cells from BM (CD19+CD20+, lane 5), and B cells isolated from umbilical cord (CD19+, lane 6), PB B lymphocytes, either unstimulated (lane 7), stimulated with PMA (lane 8), ConA (lane 9), HGF/SF (lane 10), or infected with EBV (lane 11). Lane 12 is the water control for the PCR.
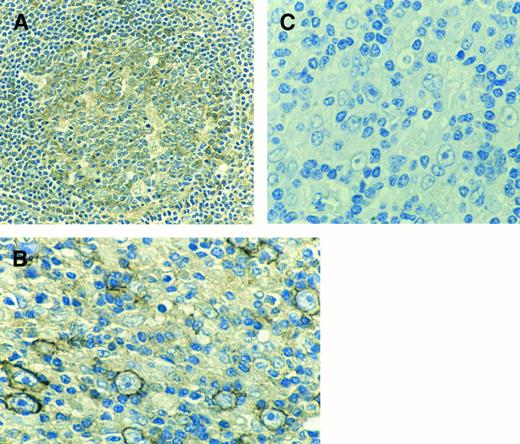
Similar results were obtained by staining cells in situ with a polyclonal antiserum (C-28) against the 28 C-terminal amino acids of the c-MET receptor. Sections from the same lymph nodes were stained with both C-28 and a MoAb against B cells (L-26). In normal lymph nodes as well as normal tonsils and spleens, B cells in the germinal centers (which contain activated B cells) and to a lesser extent B cells in the marginal zones of the secondary follicles were immunoreactive with the polyclonal antibody C-28 (Fig 2A). Control sections, incubated with polyclonal control IgG or with C-28 which was previously incubated with a 10-fold higher concentration of peptide to which the antibody was raised, did not stain (result not shown). Consistent with the finding that EBV-infection induces c-MET expression in B cells, B cells in lymph nodes from patients with infectious mononucleosis stained strongly with the C-28 (result not shown).
(A) Immunohistochemical staining of the germinal center of a secondary follicle in a spleen with a polyclonal antibody directed to the C-terminal part of the c-MET receptor. (B) Example of centroblasts reacting with C-28 (anti c-MET) of an EMA+ lymph node from a patient with NHL. (C) As a negative control C-28 was preincubated with a 10-fold higher concentration of the peptide to which the antibody was raised (blocking peptide).
(A) Immunohistochemical staining of the germinal center of a secondary follicle in a spleen with a polyclonal antibody directed to the C-terminal part of the c-MET receptor. (B) Example of centroblasts reacting with C-28 (anti c-MET) of an EMA+ lymph node from a patient with NHL. (C) As a negative control C-28 was preincubated with a 10-fold higher concentration of the peptide to which the antibody was raised (blocking peptide).
c-MET Expression in B-Lymphoma Cells
The studies on normal B cells showed a preferential staining pattern of immature B cells and activated B cells, especially cells infected by EBV. We investigated whether c-MET was also expressed in B cells in sections of lymph nodes from patients with NHL and HD. Different subtypes of B-NHL were tested. As EBV infection of B cells induced c-MET expression, the HD samples were of special interest because EBV was detected in 50% of the samples. Using immunohistochemistry, the localization of c-MET staining (with C-28 polyclonal antiserum) was compared with the distribution pattern of B and T cells.
Both in samples from patients with large B-cell NHL and follicular center cell NHL, the c-MET+ cells were identified as large activated centroblasts. In 8 of 11 follicular center cell NHL samples only centroblasts, but no centrocytes, stained with the C-28 polyclonal antibody. In the group of large B-cell lymphoma (all EBV−) 2 out of 11 cases were c-MET positive. However, in a subgroup of large B-cell lymphomas with highly activated malignant B cells, as indicated by high expression of EMA and CD30, there was strong staining of tumor cells in 5 out of 6 patients (Fig 2B). The c-MET staining was specific, since it was blocked by addition of the peptide against which the antibody was directed (Fig 2C). Only 1 out of 3 samples of Burkitt lymphoma (all EBV−) showed staining. In several samples, we also detected (activated) T cells that reacted with the c-MET antiserum.
Lymph node sections from patients with HD were tested for expression of both c-MET and EBV in all variants of Hodgkin cells. A strong correlation was found between the expression of the MET protooncogene and EBV in this group of patients. Six out of eight EBV+ samples from HD patients coexpressed the c-MET protein. Furthermore, none of the 10 EBV− samples from HD patients expressed c-MET (Table 1).
Several B-tumor cell lines were studied for c-MET expression using RT-PCR. Similar to the results in samples from NHL patients, c-MET was observed in part of the B-cell lines. Two Burkitt cell lines, BJAB and Raji, were c-MET+, whereas three others: Ramos, Daudi and Jiyoye, did not express c-MET. These c-MET positive and negative B-cell lines were used to study the function of c-MET and the effect of HGF/SF on c-MET positive B lymphocytes in several in vitro models.
Functional Role of c-MET and HGF/SF in B-Lymphoma Cells
Because both the c-MET gene and the HGF/SF gene play a role in dissemination of c-MET and HGF/SF transfected NIH3T3 and epithelial cells, we studied the effect of HGF/SF on proliferation, adhesion, and invasion of c-MET positive B-cell lines, ie, on properties relevant for metastasis formation.
Proliferation.CD19+ cells isolated from cord blood, which express c-MET, did respond to HGF/SF in proliferation. Addition of 15 ng/mL HGF/SF for 24 hours resulted in a fourfold increase in 3H-thymidine incorporation (overnight) compared to proliferation in medium without HGF/SF (data not shown). HGF/SF could not increase the proliferation of c-MET+, or as a control c-MET−, B-cell lines (data not shown). Thymidine incorporation of these highly proliferative B-cell lines was tested in the presence of various concentrations (up to 1,000 ng/mL) of HGF/SF with 5% FCS or without FCS for 1 or 3 days.
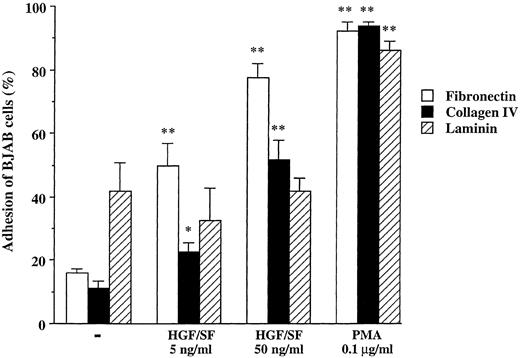
Adhesion.In contrast, we did observe a clear effect of HGF/SF on adhesion. Addition of HGF/SF to c-MET+ BJAB cells resulted in a dose-dependent increase in adhesion of these cells to both FN and CN (Fig 3). HGF/SF increased adhesion of BJAB cells to FN twice to four times. Whereas only 16% of the BJAB cells adhered to the FN-coated plates in medium without HGF/SF, addition of 15 or 50 ng/mL HGF increased adhesion approximately to 50% and 80%, respectively. PMA, used as a positive control, induced adhesion of 92%. Likewise, only 11% of BJAB cells adhered to CN and adhesion increased to 23% in 15 ng/mL HGF and to 52% in 50 ng/mL HGF (Fig 3). PMA induced adhesion was 94%. The HGF/SF effect was specific, in that HGF/SF did not induce adhesion to laminin, in contrast to PMA that caused almost 90% of cells to adhere to laminin. HGF/SF had a similar effect on the c-MET+ cell line Raji. HGF/SF increased adhesion to FN (from 43% without to 53% with HGF ) and CN, but not to laminin. Again, PMA strongly increased adherence to FN, CN, and laminin (data not shown). Adhesion of the c-MET− cell lines Jiyoye and Daudi to the three extracellular matrix molecules was not influenced by HGF/SF, whereas PMA increased adhesion to similar levels as observed with BJAB and Raji cells (data not shown).
Adhesion of BJAB cells to FN, CN, and laminin without or with HGF/SF (5 and 50 ng/mL) or PMA (0.1 μg/mL). Data represent the mean ± SD of triplicate samples from a representative of three experiments. **, *, Significant difference (**, P < .01; *, P < .05) between adhesion to same extracellular matrix molecule without stimuli and HGF/SF or PMA.
Adhesion of BJAB cells to FN, CN, and laminin without or with HGF/SF (5 and 50 ng/mL) or PMA (0.1 μg/mL). Data represent the mean ± SD of triplicate samples from a representative of three experiments. **, *, Significant difference (**, P < .01; *, P < .05) between adhesion to same extracellular matrix molecule without stimuli and HGF/SF or PMA.
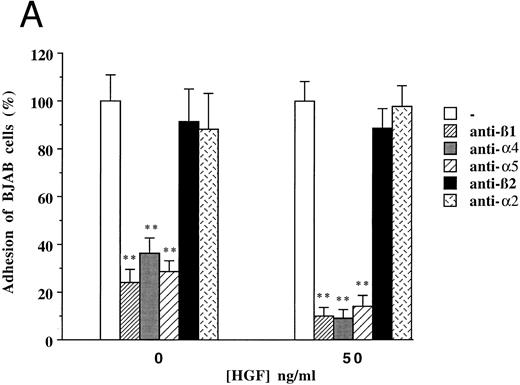
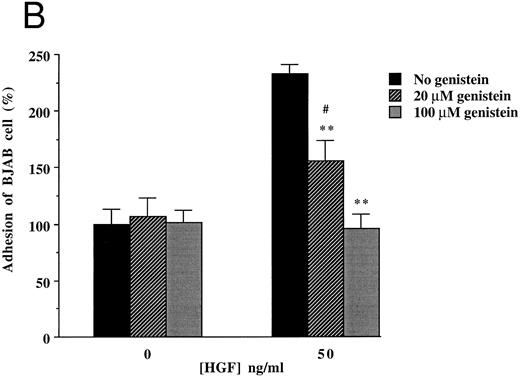
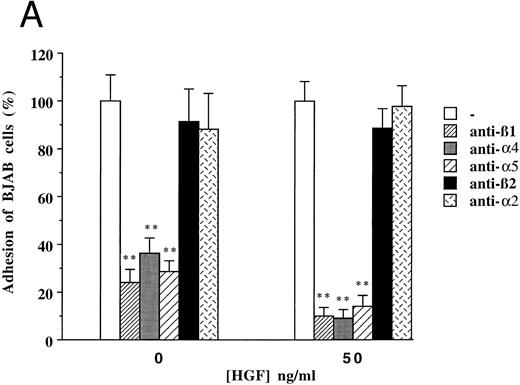
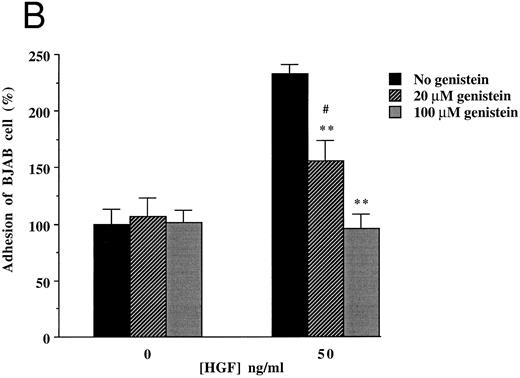
To clarify the mechanism by which HGF/SF induces increased adhesion of c-MET positive B-cell lines, we focused on (inhibition of ) adhesion of BJAB cells to FN. Since the integrins α4β1 (VLA4) and α5β1 (VLA5) are known to be FN receptors, we studied the role of these integrins in adhesion of the c-MET+ lymphoma cell line BJAB to FN using blocking antibodies. Antibodies against β1- (CD29), α4- (CD49d), and α5- (CD49e) integrin subunits completely inhibited adhesion of BJAB cells to FN (Fig 4A). Also HGF/SF independent adhesion of BJAB cells was strongly reduced by each of the three blocking MoAbs. This indicates that the integrins VLA4 and VLA5 are already present and active, but that their activity can be upregulated by HGF/SF, resulting in an increase in adhesion. FACS analysis revealed that stimulation with HGF/SF, using the same conditions as in the adhesion assay, does not upregulate one of the integrin subunits (β1 , α4 or α5 ) on BJAB or Raji cells (data not shown), suggesting that HGF/SF activates α4β1 and α5β1 integrins. Control antibodies, directed against α2- and β2-integrin subunits, did not affect adhesion of BJAB cells to FN (Fig 4A). Raji cells showed a very similar pattern of inhibition in adhesion to FN in response to addition of the blocking antibodies (data not shown). We observed that the tyrosine kinase inhibitor genistein blocked the HGF/SF induced adhesion of c-MET+ BJAB cells to FN (Fig 4B). Preincubation with 20 μmol/L genistein significantly inhibited HGF/SF induced adhesion and preincubation with 100 μmol/L genistein resulted in adhesion observed without addition of HGF/SF, whereas PMA induced adhesion was not affected (Fig 4B). This result was confirmed using Raji cells (data not shown). These data indicate that signals resulting from activating the protein tyrosine kinase c-MET by binding of HGF/SF are responsible for the increased adherence. Because effects of receptor tyrosine kinase ligands are often in part dependent on pertussis toxin-sensitive G-proteins, we tested the effect of pretreatment with 200 or 500 ng/mL pertussis toxin on adhesion. However, pertussis toxin did not influence HGF/SF stimulated adhesion of either BJAB or Raji cells to fibronectin (data not shown).
Effect of monoclonal blocking antibodies (A) and genistein (B) on adhesion of c-MET positive BJAB cells to FN. Data described in (A) and (B) represent the mean ± SD of triplicates from a representative of four experiments. ** In (A), significant difference (P < .01) between adhesion to FN without blocking antibodies and with blocking antibodies; ** in (B), Significant difference (P < .01) between adhesion to FN in the presence of HGF/SF without genistein and 20 μmol/L or 100 μmol/L genistein; # in (B), Significant difference (P < .05) between adhesion to FN in the presence of 20 μmol/L genistein without and with HGF/SF.
Effect of monoclonal blocking antibodies (A) and genistein (B) on adhesion of c-MET positive BJAB cells to FN. Data described in (A) and (B) represent the mean ± SD of triplicates from a representative of four experiments. ** In (A), significant difference (P < .01) between adhesion to FN without blocking antibodies and with blocking antibodies; ** in (B), Significant difference (P < .01) between adhesion to FN in the presence of HGF/SF without genistein and 20 μmol/L or 100 μmol/L genistein; # in (B), Significant difference (P < .05) between adhesion to FN in the presence of 20 μmol/L genistein without and with HGF/SF.
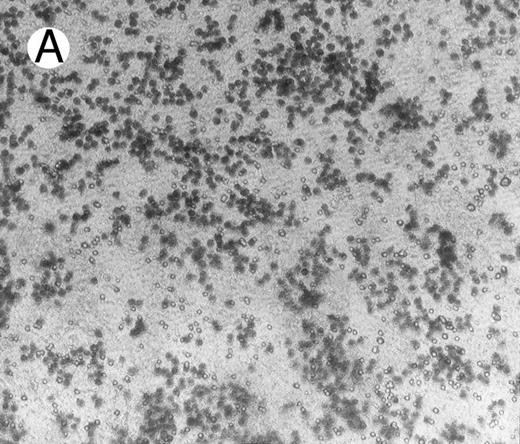
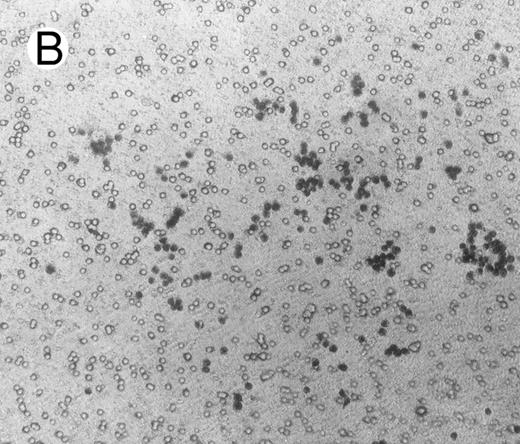
Migration and invasion.To study a possible effect of HGF/SF on migration of lymphoma cells, Boyden chambers were used with Matrigel-coated membranes (with pores of 8 μ) that separate the two compartments. c-MET positive and negative lymphoma cells were brought into the upper chambers. After 16 hours, cells that adhered to the Matrigel were stained and scored, and cells that migrated over the Matrigel-coated membrane into the lower chamber as well as cells still present in the uppper chamber were counted. Similar to the adhesion experiments, adhesion of c-MET positive cells to the Matrigel-coated membranes in Boyden chambers was highly stimulated by HGF/SF (approximately 7 times more cells adhered with HGF/SF; Fig 5). As the pores of 8 μ are smaller than the cells, migration from the upper to the lower chambers is an active process. After 16 hours a small proportion (1%) of the BJAB cells migrated through the Matrigel in HGF/SF free control medium. A clear effect was observed when HGF/SF was added, resulting in 6% of the BJAB cells migrating to the lower chamber (N = 3). As expected from the adhesion assay, PMA also highly increased adhesion to Matrigel, however, PMA did not induce migration of lymphoma cells. c-MET− Daudi cells did not migrate over the Matrigel coated membranes, irrespective to addition of HGF/SF or PMA. HGF/SF could also not increase adhesion of Daudi cells to Matrigel, whereas PMA did induce adhesion.
Adhesion of BJAB cells to Matrigel coated on membranes of Boyden chambers in the presence (A) or absence (B) of HGF/SF (15 ng/mL) for 16 hours.
Adhesion of BJAB cells to Matrigel coated on membranes of Boyden chambers in the presence (A) or absence (B) of HGF/SF (15 ng/mL) for 16 hours.
Another assay, using rat fibroblast monolayers, was used to study both the adhesion and invasion of lymphoma cells. Invading cells in this model migrate through the fibroblasts mimicking the invasion of tumor cells into normal tissues. We observed a clear increase of c-MET+ cell lines by HGF/SF in adhesion to and invasion in these rat fibroblast monolayers. Addition of 5 ng/mL HGF/SF doubled the number (from 1.8% to 4.4%) of adherent BJAB cells and in 15 ng/mL HGF/SF a fourfold increase (from 1.8% to 7.2%) in adhering cells was counted compared to the HGF/SF-free control (Table 2). BJAB cells were not invasive in rat fibroblast monolayers, irrespective of addition of HGF/SF. Raji cells (c-MET+) barely adhered to the monolayer, also in the presence of HGF/SF (Table 2). However, HGF/SF stimulated invasion of Raji cells. In the absence of HGF/SF, the Raji cells were already invasive, but in the highest concentration (15 ng/mL) of HGF/SF used, the number of invaded cells doubled (from 12% to 25%; Table 2). Daudi cells (c-MET−) did not adhere or invade, neither in the presence nor in the absence of HGF/SF (Table 2).
DISCUSSION
Several studies with epithelial cells have shown that c-MET and HGF/SF are involved in dissemination; HGF/SF disrupts intercellular junctions and stimulates the motility and invasiveness of various carcinoma cell types in vitro.30-33 Furthermore, NIH3T3 fibroblasts transfected with both HGF/SF and c-MET genes are tumorigenic in nude mice.20 We detected c-MET mRNA in early hematopoietic (CD34+) cells, early B cells and activated B cells. This pattern of mRNA expression was confirmed by detecting c-MET at protein level on activated B cells in germinal centers in lymph nodes. Large centroblasts from NHL or HD, which have their normal counterparts in activated B cells, also express c-MET. Induction of c-MET by various stimuli, including HGF/SF, has been described for epithelial cells.34 The expression pattern of c-MET is reminiscent of that of two other tyrosine kinase receptors, c-KIT and FLK-2/FLT-3 (fetal liver kinase-2/FMS-like-tyrosine kinase-3). These receptors are also expressed in early hematopoietic progenitor cells, and FLK-2 is known to be expressed on early B-lymphoid progenitors.35-38 Furthermore, in some cases of HD and NHL c-KIT and FLK-2 expression has been observed.39,40 c-MET expression has been detected in immature hematopoietic cells, especially in erythroid precursors, by Galimi et al41 and Jücker et al42 reported c-MET (over)expression in some cases of Burkitt's lymphoma and HD.
We investigated a possible role of c-MET and HGF/SF in B-cell lymphoma derived cell lines in properties relevant for metastasis, ie adhesion, migration, and invasion. HGF/SF increased adhesion of c-MET+ cell lines BJAB and Raji to the extracellular matrix molecules FN and CN in a dose dependent manner, but not to laminin. This indicates that mainly the FN receptors α4β1 (VLA4) and α5β1 (VLA5), but not the laminin receptor α6β1 (VLA6), are activated by HGF/SF.43 The mechanism that controls activity of integrins is poorly defined, but it has been observed that apart from the cytoplasmic domain of the β-chain also the cytoplasmic domain of the α-chain affects activation of the integrin, providing specific activation of integrins sharing the same β-chain.44-46 Experiments with blocking antibodies (to α4-, α5- and β1- integrin subunits) supported the hypothesis that VLA4 and VLA5 are the integrins activated on stimulation with HGF/SF. Surprisingly, all three antibodies diminished binding to FN almost completely. Because FN has different binding domains for VLA4 and VLA5, an additive effect was expected of the blocking capacities of anti-α4 and -α5 . A specific inhibition is suggested by the observation that control antibodies (to α2- or β2- integrin subunits) did not affect the binding of lymphoma cells to FN. Because saturating amounts of antibodies to α4- and α5- integrin subunits were used, steric hindrance caused by binding of one antibody could prevent adhesion of the unblocked integrin to FN. Since blocking antibodies, directed to the subunits of VLA4 and VLA5, also strongly decreased HGF/SF independent adhesion of c-MET+ B cells, a part of the integrins VLA4 and VLA5 are probably already active. However, our results indicate that HGF/SF activates more of these integrins, resulting in an enhanced adhesion. Similarly to our results with HGF/SF, stem cell factor (SCF ), the ligand of c-KIT, induces adhesion of hemopoietic cells to fibronectin.47-50 Adhesion can be inhibited by both tyrosine kinase inhibitor genistein and by antibodies directed to α4β1 and α5β1 . Mutations in SCF or c-KIT genes affect migration of hemopoietic stem cells, which can be explained by the observation that SCF enhances avidity of α4β1 and α5β1 integrins expressed on hemopoietic cells.49 50
HGF/SF induced adhesion is tyrosine phosphorylation dependent and probably G-protein independent,51,52 because genistein did inhibit adhesion in a dose dependent manner and pertussis toxin, which irreversibly inactivates certain G-proteins53 had no effect on adhesion induced by HGF/SF. In carcinoma cells, the HGF/SF induced motogenic response leads to a recruitment of integrins, cytoskeletal proteins, and focal adhesion kinase (FAK) to focal contacts. The β1 integrin subunit has been shown to bind to the cytoskeletal proteins α-actinin and talin, which in turn bind to vinculin allowing connection to the actin cytoskeleton.54 Recently, ezrin, a member of the protein family TERM that links the plasma membrane to the cytoskeleton, was found to be activated on HGF/SF stimulation.55-57 In epithelial cells, the recruitment of intracellular substrates to c-MET is followed by cell spreading, disruption of focal adhesion, and cell-cell contacts (modulation of cadherins) and finally cell motility.58-60 These steps are the result of HGF/SF induced phosphorylation of tyrosine residues within the carboxyl terminus of the β-chain of c-MET providing docking sites of multiple cytoplasmic transducers containing SH2 domains,61,62 including the p85 subunit of phosphatidylinositol 3-kinase (PI3-kinase), phospholipase Cγ, RasGAP, Grb2-Sos complex, and Src-related tyrosine kinases.63-66 For scattering of epithelial cells, besides these second messengers, an additional signal is required, which could be provided by positive signals resulting from FN receptors as has been indicated by experiments with MDCK cells.67
HGF/SF did not only affect adhesion but also promoted migration of c-MET positive BJAB cells, through Matrigel-coated membranes of Boyden chambers. Approximately six times more cells migrated from the upper to the lower chamber in the presence of HGF/SF, compared to control medium. The c-MET− Daudi cells did not adhere to or migrate through the Matrigel, irrespective of addition of HGF/SF. We also observed a comparable increase in adherent BJAB cells to the Matrigel after addition of HGF/SF as could be expected from our adhesion assays using purified FN and CN. Furthermore, a clear increase in both adhesion to or invasion in monolayer of rat fibroblasts was observed of the c-MET+ cell lines after addition of HGF/SF. Addition of HGF/SF resulted in a fourfold increase in the number of adherent BJAB cells to the monolayer and in a doubling of the number of invading Raji cells in the monolayer. These results are very similar to those reported for epithelial cells and transfected NIH3T3 cells. Addition of human HGF/SF to c-MET transfected NIH3T3 cells induced changes in cell shape, migration in Boyden chambers, and invasion into collagen matrices in vitro.19 Creation of an HGF/SF autocrine loop in transfected NIH3T3 cells or carcinoma cells, resulted in a similar invasive behavior.20,68 HGF/SF is a motility factor for many epithelial and carcinoma cells.11-14 30
Taken together all data, it is tempting to hypothesize that HGF/SF may modulate adhesion of lymphoma cells by activating certain β1-integrins on c-MET phosphorylation resulting in an increased adhesion to eg, FN. Apart from activating integrins, c-MET on lymphocytes might directly cause adhesion to immobilized HGF/SF for example on endothelium in tissues, which may be followed by migration through endothelial cells.69,70 Adhesion to endothelium by such a mechanism has been observed for a subset of T lymphocytes.70 HGF/SF can be bound to endothelial cells or extracellular matrix molecules via abundantly expressed proteoglycans and heparin, which has two distinct binding areas for HGF/SF and FN.63,71,72 Furthermore, HGF/SF may bind to a metastasis associated molecule CD44, from which many splice variants are known73 of which some contain heparan sulphate (exon v3) to which HGF/SF can bind.72 CD44 may increase the local concentration of HGF/SF around the c-MET+ lymphoma cells or HGF/SF bound to CD44 may dimerize and therefore activate c-MET,74-76 followed by adhesion and migration.
In conclusion, we found c-MET expression in hemopoietic precursor cells (CD34+), immature B cells, activated B cells, EBV-infected B cells, large activated centroblasts in various B-NHL and in some lymphoma cell lines. In this study we showed that HGF/SF promotes adhesion, migration and invasion of c-MET+ lymphoma cells. Furthermore, we showed that HGF/SF induced adhesion of lymphoma cells to FN probably via activating α4β1 and α5β1 integrins, which is a tyrosine phosphorylation dependent process.
ACKNOWLEDGMENT
We thank Dr E. Roos (Department of Cell Biology, Netherlands Cancer Institute, Amsterdam) and Drs A. Hekman (Department of Immunology, Netherlands Cancer Institute) for the very helpful discussions and the critical reading of this manuscript. Furthermore, we are grateful to E. Noteboom for FACS-sorting the cells and his help in preparing photographs.
Supported by the Dutch Cancer Society/KWF Grant No. 92-57.
Address reprint requests to Winald R. Gerritsen, MD, PhD, Department of Immunology/Medical Oncology, Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands.