Abstract
To understand the regulatory mechanism of erythropoietin (EPO) receptor (EPOR) gene expression, the effect of EPO on the steady-state level of EPOR mRNA was examined using the human EPO-dependent cell line UT-7 as a model system. We found that the treatment of UT-7 cells with EPO resulted in a transient decrease of the EPOR mRNA level. This transient downregulation was also induced by stimulation with granulocyte-macrophage colony-stimulating factor (GM-CSF ), another stimulator of UT-7 cell growth. These results raised the possibility that EPOR gene expression is in part related to cell growth. Moreover, it was found that EPO-induced downregulation of EPOR mRNA level was preceded by a transient downregulation of GATA-1 mRNA. To examine the relationship between the expression of EPOR, GATA-1, and GATA-2 mRNA levels and the cell cycle, logarithmically growing UT-7 cells were centrifugically fractionated according to the cell-cycle phase. Both EPOR and GATA-1 mRNA levels, but not the GATA-2 mRNA level, concomitantly decreased at the G0/G1 phase and increased at the S and G2/M phases. An electrophoretic mobility shift assay (EMSA) showed that in EPO-stimulated UT-7 cells, the dynamic changes in EPOR gene expression paralleled the GATA-1 DNA-binding activity to the oligonucleotide probe containing a GATA-binding site located at the promoter region of the EPOR gene. These findings suggest that the regulation of EPOR mRNA level is mainly associated with GATA-1 gene expression in UT-7 cells undergoing proliferation, and that these serial events are under the control of, or related to, the cell cycle.
ERYTHROPOIETIN (EPO) is a principle regulator of proliferation and differentiation of erythroid progenitor cells.1 EPO mediates these effects through binding to a specific cell-surface receptor (erythropoietin receptor; EPOR). The cDNA coding for EPOR has been cloned, and structural analysis showed that EPOR is a member of the cytokine receptor superfamily.2,3 The structure of the EPOR gene has also been elucidated; homologies to the consensus binding motifs of several transcription factors, such as myb, ets, Sp1, and GATA, are found in the regulatory regions of the EPOR gene.4 A GATA factor and Sp1 have been shown to bind specifically to the EPOR promoter in vitro.5,6 However, the mechanism by which EPOR gene expression is regulated during hematopoietic cell proliferation and differentiation has not yet been elucidated.
Because UT-7 cells express high levels of EPOR mRNA, they represent a potentially useful model for investigating the regulation of EPOR gene expression.7 We previously found that exposing UT-7 cells to phorbol myristate acetate (PMA) resulted in a loss of response to EPO.7 With exposure to PMA, UT-7 cells showed an 85% decrease in the number of EPO-binding sites and a greater than 95% decrease in the level of EPOR mRNA. Analyses of the rate of transcription of the EPOR mRNA and the half-time of EPOR mRNA disappearance in PMA-untreated and -treated UT-7 cells demonstrated that the PMA-induced downregulation of EPOR mRNA occurred mainly at the posttranscriptional level; the stability of EPOR mRNA in PMA-treated UT-7 cells was markedly decreased as compared with that in PMA-untreated control UT-7 cells.7 In addition, the EPOR gene is reportedly regulated at the transcriptional level under the condition of growth-factor starvation and during erythroid differentiation.8,9 In the murine interleukin-3 (IL-3)–dependent cell line, 32D and its sublines, the expression of EPOR protein is also posttranslationally modified.10 Thus, EPOR expression appears to be regulated by multiple mechanisms.
To further understand the regulatory mechanism of EPOR gene expression, we examined the changes in EPOR mRNA level by EPO stimulation using UT-7 cells. Since EPO is an essential factor for the growth of UT-7 cells, its effect on EPOR gene expression in these cells is considered to mimic the regulatory process through which it normally regulates EPOR gene expression in cells undergoing proliferation. We show here that in logarithmically growing UT-7 cells, the EPOR mRNA level is regulated in a cell-cycle–dependent manner. This is the first report to demonstrate that expression of a cytokine receptor is closely linked to the cell cycle.
MATERIALS AND METHODS
Cell culture.UT-7 cells were maintained in Iscove's modified Dulbecco's medium (IMDM; GIBCO, Grand Island, NY) supplemented with fetal bovine serum (FBS; 10% vol/vol; Hyclone Laboratories, Logan, UT) and granulocyte-macrophage colony-stimulating factor (GM-CSF; 1 ng/mL).11 The cells were passaged twice a week.
Hematopoietic growth factors and reagents.Recombinant human EPO, with a specific activity of 1.7 × 105 U/mg, was a gift of Snow Brand Milk Products (Tochigi, Japan). Recombinant human GM-CSF, with a specific activity of 1.0 × 1010 U/mg, was provided by Hoechst Japan (Tokyo, Japan). Plasmid DNA for human ribosomal genomic DNA was provided by the Japanese Cancer Research Resources Bank. Human EPOR cDNA was recently cloned from UT-7.12 Human GATA-1 and GATA-2 cDNAs were provided by Dr S.H. Orkin. Antimurine GATA-1 polyclonal and antichicken GATA-2 monoclonal antibodies were prepared according to methods described previously.13 Both antibodies were found to be cross-reactive to and specific for their human counterparts. Moreover, when tested by electrophoretic mobility shift assay (EMSA), human GATA-1 and GATA-2 proteins were supershifted by binding to each antibody (manuscript in preparation). Antimurine GATA-1 monoclonal antibody was prepared as described previously.13
RNA preparation and RNA blot hybridization.UT-7 cells (1 × 106 cells/mL) were incubated at 37°C in IMDM supplemented with 10% FBS in the presence or absence of 10 U of EPO/mL or 10 ng of GM-CSF/mL for the indicated period. Total cellular RNA was prepared from cells using guanidinium isothiocyanate as described.14 Cell pellets were lysed and vigorously homogenized in a solution that contained 4 mol/L guanidinium isothiocyanate, 25 mmol/L sodium citrate, 200 mmol/L sodium acetate (pH 4.0), 0.5% sarcosyl, and 0.1 mol/L 2-mercaptoethanol. RNA was then phenol-extracted and precipitated in isopropyl alcohol. The RNAs (20 μg/sample) were run on a formaldehyde/agarose gel and transferred to nylon membranes (Zeta-probe; Bio-Rad, Richmond, CA) in 10× standard sodium citrate (SSC). The membranes were hybridized to human EPOR cDNA or GATA-1 cDNA fragments, or a bovine β-actin cDNA fragment. These fragments were labeled with α-[32P]dCTP by the random priming method. Membranes were then washed as described and autoradiographed using Kodak XAR-5 films (Eastman Kodak, Rochester, NY) with intensifying screens at −70°C. The EPOR mRNA content obtained by RNA blot hybridization was quantitated by densitometry (CS-9000; Shimadzu, Kyoto, Japan).
Colorimetric MTT assay.Cell growth was estimated by a modified colorimetric MTT assay of Mosmann.15 Briefly, 20 μL of a sterilized 5 mg/mL MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide; Sigma, St Louis, MO) solution was added to each culture well containing a final volume of 100 μL of cell suspension. After 5 hours of incubation, 100 μL of 10% sodium dodecyl sulfate solution was added to the wells and mixed thoroughly. Each culture well of the plate was examined using a microplate reader (model 3550; Bio-Rad) at a wavelength of 595 nm.
EMSA.The protein concentrations of all extracts were determined by the Bradford assay (Bio-Rad). Each reaction contained (in 20 μL) 20 μg of nuclear extract, 10 mmol/L HEPES (pH 7.9), 50 mmol/L KCL, 2 mmol/L MgCl2 , 4% Ficoll, 1 mmol/L EDTA, 1 mmol/L dithiothreitol (DDT), 0.5 μg poly (dI-dC), and 1 pmol 32 P-labeled, double-stranded oligonucleotide probe that contained the preferred sequence for DNA-binding by GATA protein (upper strand: 5′-CAGCCTGGGTGAGATAAGTGCCT-GGCGGGA-3′ ) or the mutant oligonucleotide (upper strand: 5′-CAGCCTGGGTGAGCGAAGTGCCTGGCGGA-3′ ). After a 15-minute incubation at room temperature, the assay mixture was loaded onto a 5% polyacrylamide gel and electrophoresed at 180 V for 2 to 3 hours. For supershift assay, the nuclear extract was preincubated with an excess of the indicated antibodies and then mixed with labeled probe. Where indicated, an approximately 150-fold molar excess of unlabeled competitor oligonucleotides was included in the binding reaction. EMSA with oligonucleotides corresponding to the Sp1 consensus sequence (upper strand: 5′-ATTCGATCGGG-GCGGGGCGAG-3′ ) was performed to show the amount of protein loaded on the gel.
Elutriations.Counterflow centrifugal elutriations were performed using the SRR6Y elutriation system and rotor equipped with a 4.5-mL chamber (Hitachi Koki, Tokyo, Japan).16 Log phase-growing UT-7 cells were resuspended at 1 to 2 × 108 cells in 50 mL of culture medium and injected into the elutriation system at 4°C using an initial flow rate of 12 mL/min and a rotor speed of 2,000 rpm. The flow rate was incrementally increased, and cell fractions were collected serially as follows: fraction 1, 200 mL at 15 mL/min; fraction 2, 200 mL at 20 mL/min; fraction 3, 200 mL at 25 mL/min; fraction 4, 200 mL at 30 mL/min; fraction 5, 200 mL at 35 mL/min; fraction 6, 200 mL at 40 mL/min; and fraction 7, 200 mL at 45 mL/min.
Cell-cycle analysis.Cell-cycle analysis was performed by staining DNA with propidium iodide in preparation for flow cytometry with the FACScan/CellFIT system (Becton-Dickinson, San Jose, CA). Cell-cycle analysis of the cells fractioned by the elutriation system was performed and the results were as follows: fraction 1: G0/G1 phase, 80%; fraction 2: G0/G1 , 73%; fraction 3: S phase, 48%; fraction 4: S phase, 76%; fraction 5: S phase, 55%; fraction 6: S phase, 61.9%; and fraction 7: G2 /M phase, 55%.
RESULTS
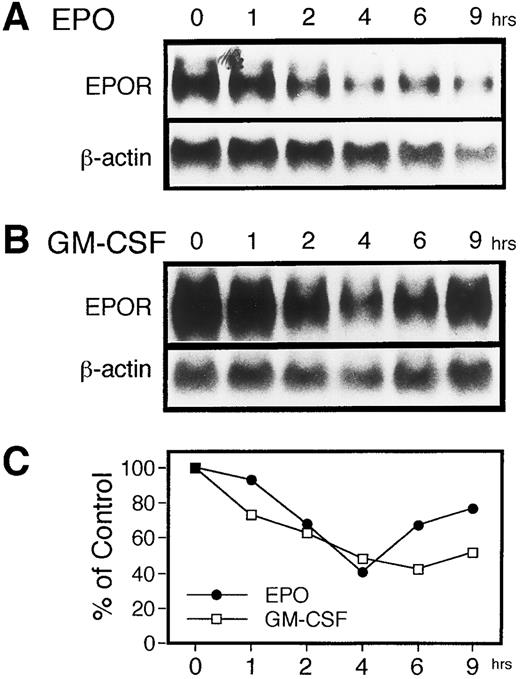
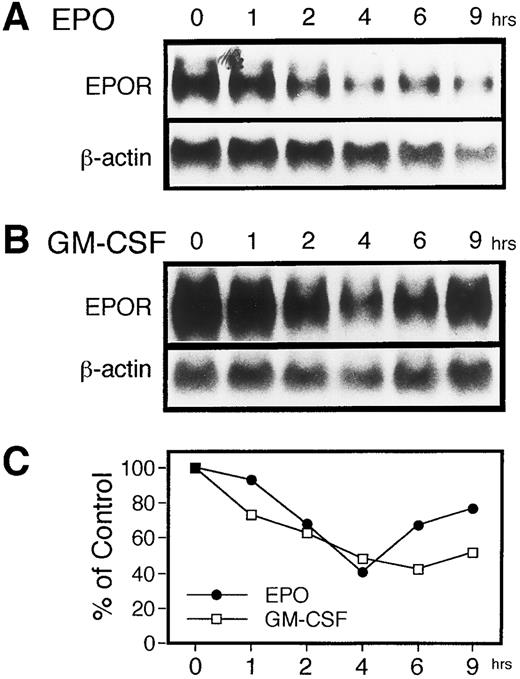
We first examined changes in the level of EPOR mRNA after adding EPO to the culture medium. UT-7 cells were cultured for 16 hours in IMDM medium supplemented with 10% FCS, but not with any hematopoietic growth factors, and then 10 U/mL of EPO was added to the culture medium. The expression of EPOR mRNA in UT-7 cells was determined by RNA blot hybridization analysis. Total RNA was isolated from the UT-7 cells at various time points as indicated in Fig 1. After EPO was added to the starved cells, a transient reduction was observed in the EPOR mRNA level. The magnitude reached the lowest level at 4 hours and gradually recovered after 6 hours (Fig 1A and C). We previously observed that prolonged exposure of UT-7 cells to EPO diminishes the steady-state level of EPOR mRNA in the cells, and the UT-7 cells eventually expressed high levels of erythroid properties.17 This process is irreversible and a subline, UT-7/EPO, the growth of which depends exclusively on the presence of EPO, was finally isolated. However, it seems unlikely that the downregulation of EPOR mRNA level brought by a transient exposure to EPO is related to the long-term promotion of the erythroid development of the UT-7 cells. Indeed, β-globin mRNA was not detected throughout the observation period on the UT-7 cells treated with EPO (data not shown).
EPO as well as GM-CSF induces a transient reduction in the level of EPOR mRNA in UT-7 cells. The effects of EPO and GM-CSF on the expression of EPOR mRNA in UT-7 cells was examined by RNA blot hybridization analysis. Logarithmically growing cells were deprived of growth factors for 16 hours in IMDM containing 10% FBS and then stimulated with 10 U EPO/mL (A) or 10 ng GM-CSF/mL (B). The total cellular RNA was isolated from the cells at the time points indicated. RNAs (20 μg/lane) were electrophoresed on a formaldehyde/agarose gel. The RNAs were then transferred to a nylon membrane and hybridized to 32P-labeled human EPOR cDNA as a probe. (C) Densitometric analysis of the data presented in (A) and (B). The amounts of the mRNAs were quantitated and normalized to β-actin mRNA levels.
EPO as well as GM-CSF induces a transient reduction in the level of EPOR mRNA in UT-7 cells. The effects of EPO and GM-CSF on the expression of EPOR mRNA in UT-7 cells was examined by RNA blot hybridization analysis. Logarithmically growing cells were deprived of growth factors for 16 hours in IMDM containing 10% FBS and then stimulated with 10 U EPO/mL (A) or 10 ng GM-CSF/mL (B). The total cellular RNA was isolated from the cells at the time points indicated. RNAs (20 μg/lane) were electrophoresed on a formaldehyde/agarose gel. The RNAs were then transferred to a nylon membrane and hybridized to 32P-labeled human EPOR cDNA as a probe. (C) Densitometric analysis of the data presented in (A) and (B). The amounts of the mRNAs were quantitated and normalized to β-actin mRNA levels.
To investigate whether EPO-induced downregulation of EPOR mRNA is associated with proliferation rather than erythroid differentiation, we added GM-CSF, another stimulator of UT-7 cell growth, to the UT-7 cells starved of growth factors. The UT-7 cells were harvested at various time points after the addition of GM-CSF, when total RNA was isolated. As shown in Fig 1B and C, hybridization analysis of the RNAs showed that the EPOR mRNA level declined 2 to 6 hours after GM-CSF treatment and gradually recovered thereafter. Similar to EPO stimulation, the maximal decrease of the EPOR mRNA level was observed at 4 to 6 hours of incubation time with GM-CSF, presumably resulting in the downregulation of EPO-binding sites.18 These observations raised the possibility that the downregulation of the EPOR mRNA level is not specific for EPO, but is somehow related to the growth of UT-7 cells.
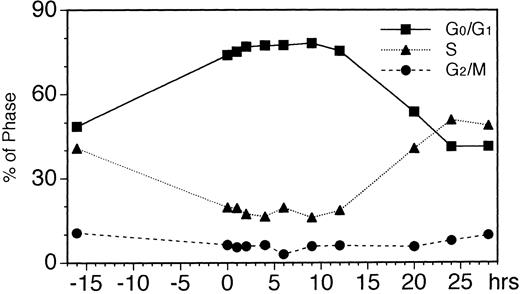
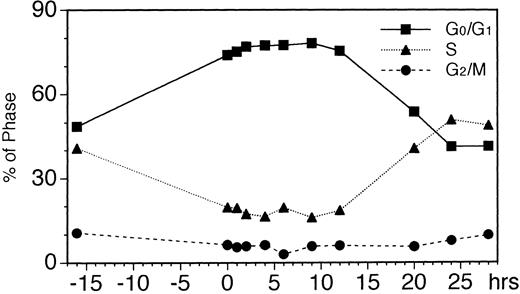
To further investigate the mechanism of EPO-induced downregulation of EPOR mRNA level, we analyzed the cell cycle of UT-7 cells stimulated with EPO for the times indicated in Fig 2. When maintained with GM-CSF, 50% of the log phase-growing UT-7 cells were in the G0/G1 phase and 40% of the cells were in the S phase. By 16 hours of growth-factor starvation, about 70% of the cells were in the G0/G1 phase and 20% of the cells were in the S phase, and even after stimulation with EPO for 4 to 9 hours, the ratio of these populations remained unchanged. After 12 to 24 hours of exposure to EPO, the cells in S phase increased and resumed their cycling activity (Fig 2).
Cell-cycle analyses of EPO-stimulated UT-7 cells. Logarithmically growing cells were deprived of growth factors for 16 hours in IMDM containing 10% FBS and then stimulated with EPO (10 U/mL). At the indicated times after stimulation, cells were stained with propidium iodide and analyzed by flow cytometry.
Cell-cycle analyses of EPO-stimulated UT-7 cells. Logarithmically growing cells were deprived of growth factors for 16 hours in IMDM containing 10% FBS and then stimulated with EPO (10 U/mL). At the indicated times after stimulation, cells were stained with propidium iodide and analyzed by flow cytometry.
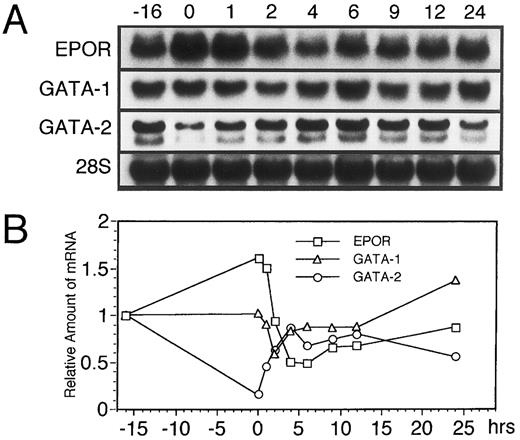
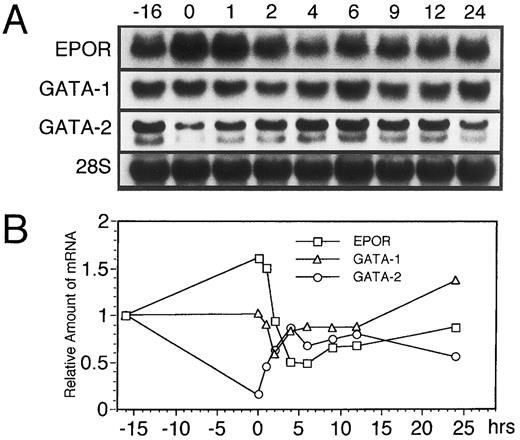
Since GATA-1 transcription factor is reportedly important for the transcriptional activation of the EPOR gene,5,6 we examined whether the dynamic changes in EPOR mRNA level are accompanied by changes in the GATA-1 mRNA level. As shown in Fig 3A and B, the transient downregulation of GATA-1 mRNA was seen at 2 hours after EPO addition. This dynamic change preceded the downregulation of EPOR mRNA, while GATA-2 mRNA was transiently upregulated, with a maximal increase at 9 to 12 hours' incubation with EPO. Moreover, we found that the increase in EPOR and GATA-1 mRNA levels observed at 9 to 24 hours after EPO stimulation was consistent with the increase in the ratio of the S phase (Fig 2). This supports our hypothesis that the expression of the EPOR gene is related to the cell cycle and suggests that this regulation is controlled under the GATA-1 expression.
EPO induces a transient reduction in the level of GATA-1 mRNA followed by downregulation of EPOR mRNA in UT-7 cells. The effect of EPO on the expression of both EPOR, GATA-1, and GATA-2 mRNAs in UT-7 cells was examined by RNA blot hybridization analysis. Logarithmically growing cells were deprived of growth factors for 16 hours in IMDM containing 10% FBS and then stimulated with 10U EPO/mL (time 0). (A) Northern blot analysis. The total cellular RNA was isolated from the cells at the time points indicated. The RNAs (20 μg/lane) were electrophoresed on a formaldehyde/agarose gel. RNAs were then transferred to a nylon membrane and hybridized to 32P-labeled probes. The amount of RNA loaded in each lane was assessed by the intensity of the 28S ribosomal RNA bands. (B) Densitometric analysis of the data presented in (A). The amounts of the mRNAs were quantitated and normalized to 28S ribosomal RNA levels.
EPO induces a transient reduction in the level of GATA-1 mRNA followed by downregulation of EPOR mRNA in UT-7 cells. The effect of EPO on the expression of both EPOR, GATA-1, and GATA-2 mRNAs in UT-7 cells was examined by RNA blot hybridization analysis. Logarithmically growing cells were deprived of growth factors for 16 hours in IMDM containing 10% FBS and then stimulated with 10U EPO/mL (time 0). (A) Northern blot analysis. The total cellular RNA was isolated from the cells at the time points indicated. The RNAs (20 μg/lane) were electrophoresed on a formaldehyde/agarose gel. RNAs were then transferred to a nylon membrane and hybridized to 32P-labeled probes. The amount of RNA loaded in each lane was assessed by the intensity of the 28S ribosomal RNA bands. (B) Densitometric analysis of the data presented in (A). The amounts of the mRNAs were quantitated and normalized to 28S ribosomal RNA levels.
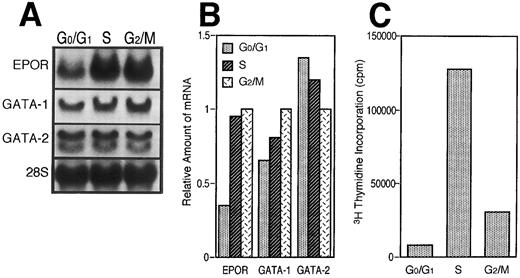
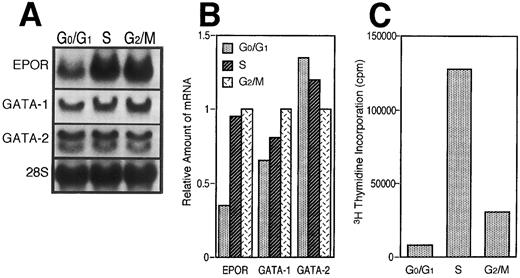
To further confirm our hypothesis, we examined the expression of EPOR, GATA-1, and GATA-2 mRNAs in the cells fractionated according to the cell cycle, G0/G1 , S, or G2/M phase, respectively. Log phase-growing UT-7 cells were harvested and prepared for counterflow centrifugal elutriations. We chose fraction 1 as the representative population of G0/G1 phase, fraction 4 as that of S phase, and fraction 7 as that of G2/M phase by DNA distribution patterns (see the Materials and Methods). As shown in Fig 4A and B, both the EPOR and GATA-1 mRNA levels concomitantly decreased at the G0/G1 phase and increased relatively at the S and G2/M phases. In contrast, the GATA-2 mRNA level increased at the G0/G1 phase and decreased relatively at the S and G2/M phases. As shown in Fig 4C, the data from the 3H-thymidine incorporation assay support the hypothesis that the fractionated cell population is certainly in each cell cycle.
EPOR and GATA-1 gene expression depends on the cell-cycle event. (A) Northern blot analysis of EPOR, GATA-1, and GATA-2 mRNA levels. Log phase-growing UT-7 cells were fractionated according to cell-cycle phase by centrifugal elutriator. Then each RNA was isolated from the fractionated cells. (B) Densitometric analysis of the data presented in (A). The amount of RNA loaded in each lane was assessed by the intensity of the 28S ribosomal RNA bands. The data show the relative ratio of the amount of mRNA in G0/G1 or S phase to that of G2/M phase. (C) 3H-thymidine incorporation into the cells used for Northern blot analysis above.
EPOR and GATA-1 gene expression depends on the cell-cycle event. (A) Northern blot analysis of EPOR, GATA-1, and GATA-2 mRNA levels. Log phase-growing UT-7 cells were fractionated according to cell-cycle phase by centrifugal elutriator. Then each RNA was isolated from the fractionated cells. (B) Densitometric analysis of the data presented in (A). The amount of RNA loaded in each lane was assessed by the intensity of the 28S ribosomal RNA bands. The data show the relative ratio of the amount of mRNA in G0/G1 or S phase to that of G2/M phase. (C) 3H-thymidine incorporation into the cells used for Northern blot analysis above.
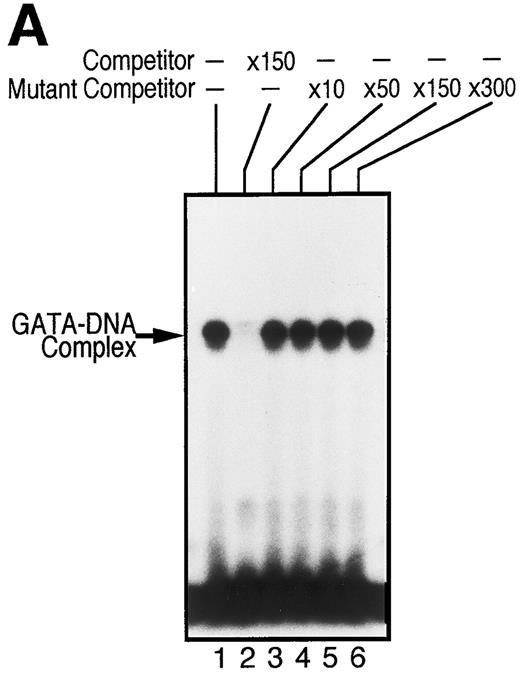
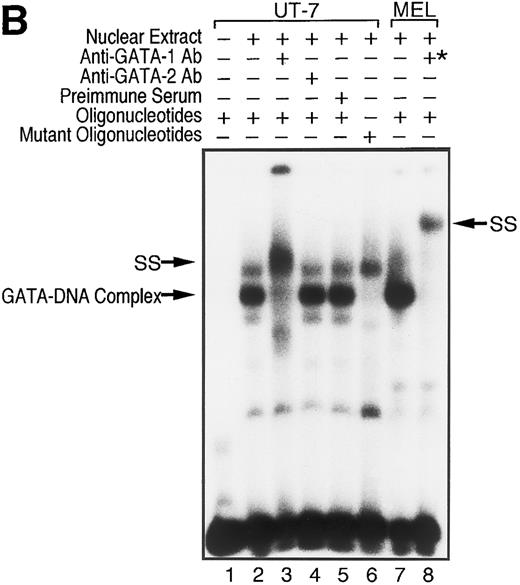
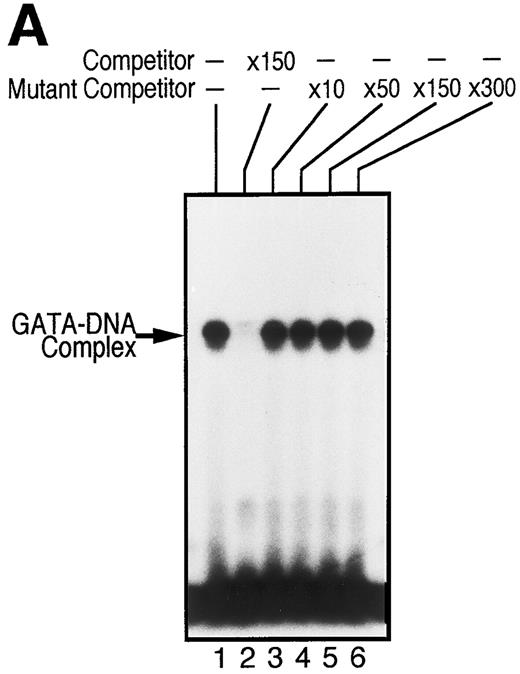
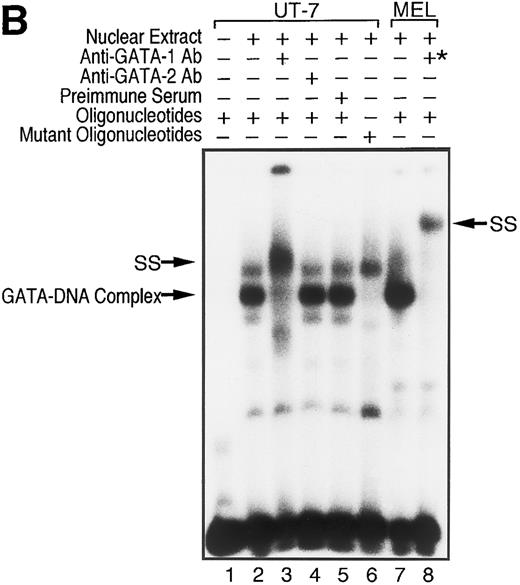
To examine whether GATA-1 is involved in EPO-induced downregulation of EPOR mRNA level, we evaluated the DNA-binding activity of GATA-1 in EPO-stimulated UT-7 cells. EMSA was performed with a radiolabeled oligonucleotide probe that contained the GATA-binding consensus sequence (A/T)GATA(A/G) located at the EPOR promoter region. As shown in Fig 5A, incubation of this probe with nuclear extract from UT-7 cells generated a protein-DNA complex. We also observed that the complex disappeared with the addition of unlabeled oligonucleotide probe containing the GATA sequence, but not by unlabeled oligonucleotide probe containing the mutated GATA sequence, GCGA. This strongly supports the hypothesis that the GATA-binding protein specifically binds to the GATA sequence located at the promoter of the EPOR gene.5,6 Moreover, as shown in Fig 5B, the mobility complex was supershifted by the preincubation of nuclear extract with an anti–GATA-1 antibody, but not with an anti–GATA-2 antibody, which can specifically recognize human GATA-2 protein, indicating that GATA-1 protein directly and specifically binds to the GATA-binding site of the EPOR promoter region and regulates the transcription of the EPOR gene, and that GATA-2 protein may not be involved in the activation of the EPOR promoter.
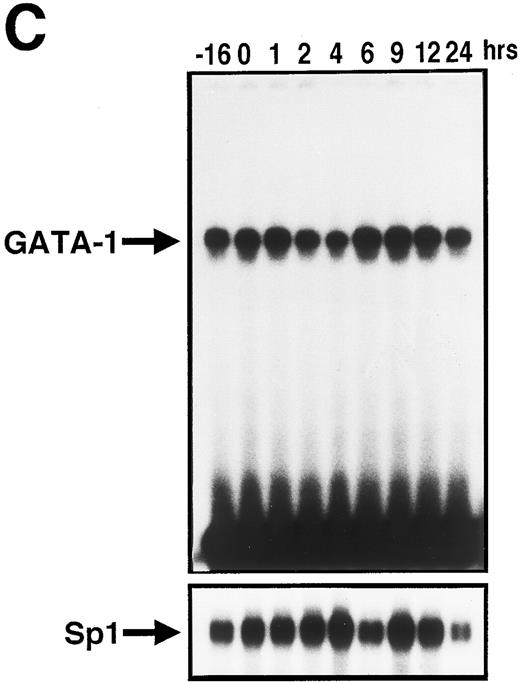
GATA-1 is directly involved in EPO-induced downregulation of EPOR mRNA level. (A) Specific recognition of GATA-protein to the oligonucleotide probe containing GATA-binding consensus sequence (A/T)GATA(A/G) located at the EPOR promoter region. Nuclear extracts from UT-7 cells were incubated with labeled wild-type oligonucleotide probe, and EMSA was performed as described in the Materials and Methods. Unlabeled wild-type or mutant oligonucleotides were added at an excess of the indicated fold. (B) Specific binding of GATA-1 protein to GATA-binding consensus sequence located at the EPOR promoter region. Nuclear extracts prepared from UT-7 cells were preincubated with an excess of antimurine GATA-1 polyclonal antibody, antichicken GATA-2 monoclonal antibody, or preimmune serum, then incubated with a double-stranded 32P-labeled oligonucleotide probe containing the GATA-binding consensus sequence or the mutant oligonucleotide probe, and EMSA was performed as described in the Materials and Methods. The GATA-1 protein extracted from MEL cells was used as a positive control and supershifted by preincubation with antimurine GATA-1 monoclonal antibody (*). Supershifted complexes are indicated (SS). (C) Time course of specific GATA-1–binding activity detected in EPO-stimulated UT-7 cells. GATA-1–binding activity induced by EPO treatment was analyzed by EMSA method. A double-stranded 32P-labeled wild-type oligonucleotide probe was incubated with nuclear extracts from the UT-7 cells stimulated with 10 U EPO/mL for the indicated times. Unlabeled competitor DNA was added at 150-fold excess. EMSA with the oligonucleotides corresponding to Sp1 consensus sequence (upper strand: 5′-ATTCGATCGGGGCGGGGCGAG-3′ ) was simultaneously performed to show the amount of protein loaded on the gel.
GATA-1 is directly involved in EPO-induced downregulation of EPOR mRNA level. (A) Specific recognition of GATA-protein to the oligonucleotide probe containing GATA-binding consensus sequence (A/T)GATA(A/G) located at the EPOR promoter region. Nuclear extracts from UT-7 cells were incubated with labeled wild-type oligonucleotide probe, and EMSA was performed as described in the Materials and Methods. Unlabeled wild-type or mutant oligonucleotides were added at an excess of the indicated fold. (B) Specific binding of GATA-1 protein to GATA-binding consensus sequence located at the EPOR promoter region. Nuclear extracts prepared from UT-7 cells were preincubated with an excess of antimurine GATA-1 polyclonal antibody, antichicken GATA-2 monoclonal antibody, or preimmune serum, then incubated with a double-stranded 32P-labeled oligonucleotide probe containing the GATA-binding consensus sequence or the mutant oligonucleotide probe, and EMSA was performed as described in the Materials and Methods. The GATA-1 protein extracted from MEL cells was used as a positive control and supershifted by preincubation with antimurine GATA-1 monoclonal antibody (*). Supershifted complexes are indicated (SS). (C) Time course of specific GATA-1–binding activity detected in EPO-stimulated UT-7 cells. GATA-1–binding activity induced by EPO treatment was analyzed by EMSA method. A double-stranded 32P-labeled wild-type oligonucleotide probe was incubated with nuclear extracts from the UT-7 cells stimulated with 10 U EPO/mL for the indicated times. Unlabeled competitor DNA was added at 150-fold excess. EMSA with the oligonucleotides corresponding to Sp1 consensus sequence (upper strand: 5′-ATTCGATCGGGGCGGGGCGAG-3′ ) was simultaneously performed to show the amount of protein loaded on the gel.
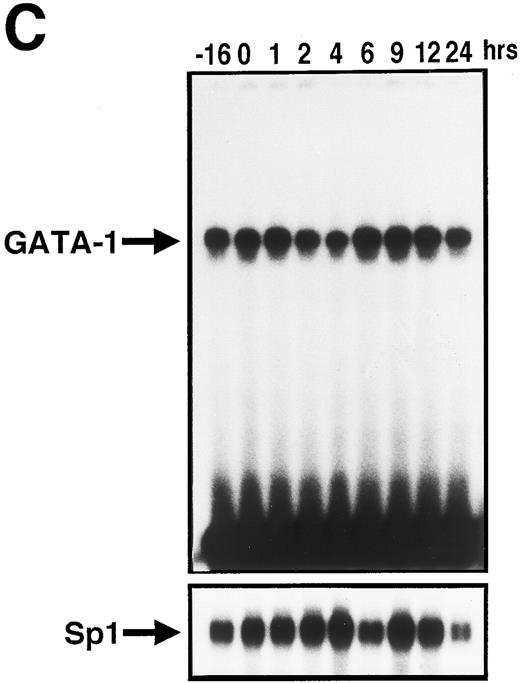
We then prepared the nuclear extracts from UT-7 cells stimulated with EPO for the indicated times and performed the EMSA assay. As shown in Fig 5C, consistent with the kinetics of GATA-1 mRNA level, GATA-1 DNA-binding activity to the oligonucleotide probe was transiently reduced. The magnitude reached the lowest level at 2 to 4 hours, overshot at 6 to 12 hours, and recovered to the basal level (at the starting point of starvation: −16 hours) at 24 hours. This indicates that the dynamic changes in EPOR mRNA level parallel the GATA-1 DNA-binding activity to the EPOR gene promoter.
DISCUSSION
We have shown here that the treatment of UT-7 cells with EPO resulted in a transient decrease of the steady-state level of EPOR mRNA. This decrease was preceded by a transient downregulation of GATA-1 mRNA level. Consistent with this, EMSA showed that there was a transient reduction of GATA DNA-binding activity to the oligonucleotide probe encompassing the GATA-binding site located at the promoter region of the EPOR gene. Moreover, EMSA with specific antibodies against GATA proteins showed that the GATA protein, which can bind to the EPOR promoter region, is identical to GATA-1. Taken together with the previous report that the transcription rate of EPOR gene decreased 3 hours after the growth factors (GM-CSF for UT-7 and EPO for UT-7/EPO) were added to the growth factor-deprived cells,8 our data suggest that a transient reduction of EPO mRNA induced by EPO is transcriptionally regulated by GATA-1.
In addition, we have demonstrated that the GATA-1 gene expression is regulated through the cell-cycle event. Peschle et al previously reported that when the early progenitor cells enter into the cell cycle, GATA-1 protein is initially expressed.19 Once the progenitor cells were committed to granulocyte-macrophage lineage, GATA-1 expression was downregulated.19 However, when the progenitor cells had committed to erythroid lineage, the level of GATA-1 expression increased.19 These results suggest that GATA-1 is involved in not only the commitment to erythroid lineage, but also the entrance of quiescent progenitors into the cell cycle. Taken together with our results, there is little doubt that GATA-1 gene expression is in part dependent on the cell cycle. As a result, EPOR gene expression would be at least in part regulated in a cell-cycle–dependent manner through the GATA-1 protein.
Our data show that GATA-1 protein specifically recognizes the GATA-binding site located at the promoter region of the EPOR gene in UT-7 cells, although all the different members of the family bind in vitro with similar affinities to DNA sequences characterized by a GATA core consensus sequence (A/T)GATA(A/G).20,21 Considering that UT-7 endogenously expresses GATA-1 and GATA-2 at the mRNA, our findings suggest that a third molecule outside the GATA family may be involved in the determination of the specificity of the GATA protein-binding activity. Recently, it was reported that the phosphorylation of GATA-2 is caused by mitogen-activated protein (MAP) kinase.22 Although the GATA-2–binding activity to its binding site did not change in vitro,22 it is also possible that the DNA-binding activity of GATA-1 is modified by some molecules, including MAP kinase in vivo, leading to the specific binding of GATA-1 to the EPOR promoter. A transfection experiment with GATA-2 cDNA showed that GATA-2 can also transactivate the EPOR promoter specifically through the GATA sequence in NIH 3T3, which originally lacks GATA-2 mRNA, suggesting that exogenously overexpressed GATA-2 proteins can bind to the promoter region of the EPOR gene. Indeed, there was a significant negative correlation between GATA-2 and EPOR mRNA levels. Although this concept is speculative at present, GATA-2 may also play some role in EPOR gene expression as a negative regulator, as in the case of EPO gene regulation.23 However, anti–GATA-2 antibody did not recognize GATA proteins that bind to the oligonucleotides corresponding to the GATA-binding site located at the promoter region of the EPOR gene, indicating that GATA-2 is not directly associated with the activation of the EPOR promoter in UT-7. Although we cannot rule out the possibility that this discrepancy is due to the different cell lines used for these experiments, or that the competition between GATA-1 and GATA-2 proteins for binding to GATA consensus sequence at the EPOR promoter occurs, our data strongly suggest that GATA-1, rather than another GATA-binding protein including GATA-2, is activated and actually transactivates the EPOR promoter in vivo.
It is noteworthy that the density of EPOR mRNA at time zero (starting point of stimulation with EPO) was much stronger than that at −16 hours (starting point of starvation of growth factors). Growth-factor starvation for 16 hours increased the expression of EPOR mRNA by 1.6-fold without significant changes in the GATA-1 mRNA level (Fig 3A and B). In addition, several repeated EMSAs showed that the GATA-1–binding activity to the promoter region of the EPOR gene did not show a significant change even after 16 hours starvation of growth factors (Fig 5C). These findings suggest that the elevation of EPOR mRNA level at the resting phase induced by growth-factor starvation is dependent not on GATA-1, but on other transcriptional factor(s). This is in contrast with the previous report that upregulation of GATA-1 mRNA level was observed after growth-factor starvation.8 Indeed, the promoter region of the EPOR gene has homologies to the consensus binding motifs of such transcription factors as myb, ets, and Sp1, besides the GATA-1–binding motif.4 Moreover, it was found that a potential AP-2–binding site is located between the Sp1 and GATA-1–binding sites and a repetitive element is located upstream of the EPOR promoter.24,25 Because these consensus sequences can exert negative effects on the transcription of EPOR gene in vitro,24,25 regression of transcription factors binding to these motifs may also be involved in the upregulation of EPOR mRNA level after growth-factor starvation. Alternatively, the elevated stability of EPOR mRNA may be involved in upregulation of EPOR mRNA. Thus, our data suggest that EPOR gene expression is regulated at least in part by other mechanisms independent of GATA-1.
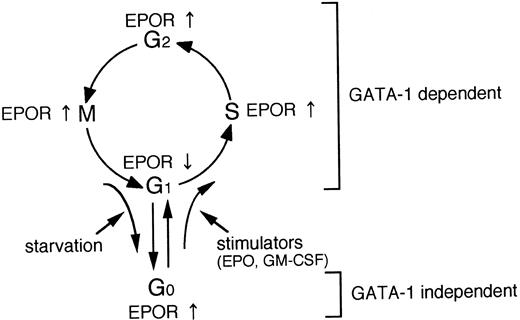
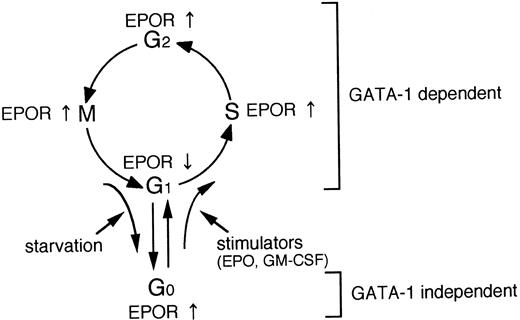
A schematic illustration of the relationship between EPOR and GATA-1 gene expression and cell cycle.
A schematic illustration of the relationship between EPOR and GATA-1 gene expression and cell cycle.
There was a significant difference in the EPOR mRNA level between time zero and 4 hours after adding EPO, but none in the ratio of G0/G1 to S phase between them. It is possible that the UT-7 cells express a large amount of EPOR at the resting stage when the cells were starved of growth factors, and that once the cells enter the cell cycle from the resting phase, EPOR is transiently downregulated through the G1 phase. If so, the marked difference in EPOR mRNA levels between the samples harvested at time zero and 4 hours after the addition of EPO may be explained by the difference of its expression between the resting phase (G0 phase) and the early cell-cycling phase (G1 phase), although the ratio of G0/G1 to S phase is similar at the two time points. However, this problem is still open to question because at present we cannot divide the cells in the G0/G1 phase into those in the G0 and G1 phases. In addition, we cannot completely rule out the possibility that upregulation of EPOR mRNA is a result of factor deprivation, but not of entry into the resting phase. If another method besides factor deprivation were available to induce the cells to enter the resting phase, we could address this question.
We present a schematic illustration in Fig 6. EPOR gene expression is regulated in the process of not only erythroid differentiation, but also cell proliferation, presumably mediated through the cell-cycle–dependent GATA-1 gene expression. In addition, EPOR gene expression appears to be at least in part regulated by other mechanisms independent of GATA-1 in the quiescent state (G0 phase) induced by growth-factor starvation.26 27
ACKNOWLEDGMENT
We thank Dr S.H. Orkin (Harvard Medical School) for his generous gift of the human GATA-1 and GATA-2 cDNAs and the Japanese Cancer Research Resources Band for human ribosomal DNA gene. We also thank T. Ando for her technical assistance and M. Yoshida for manuscript preparation.
Supported in part by Grants-in-Aid for Cancer Research and Scientific Research from the Ministry of Education, Science and Culture, Japan.
Address reprint requests to Norio Komatsu, MD, PhD, Division of Hematology, Department of Medicine, Jichi Medical School, Minamikawachi-machi, Tochigi-ken, 329-04, Japan.