Abstract
Expression of the CD4 antigen was observed on human fetal liver, fetal bone marrow (BM), and umbilical cord blood progenitors expressing high levels of CD34. Using clonal and liquid-culture assays, CD4+ CD34++ Lin− (lineage = CD3, CD8, CD10, CD14, CD15, CD16, CD19, CD20, and glycophorin A) fetal liver progenitors were found to have a greater proliferative potential than CD4− CD34++ Lin− progenitors, whereas the CD4− fraction was more enriched for erythroid progenitors. Both the CD4+ and the CD4− progenitor subpopulations also gave rise to multilineage engraftment upon transplantation into human fetal bone fragments, supportive of B-lymphoid and myeloid growth, or into human fetal thymic fragments, supportive of T-cell growth, implanted in scid/scid (SCID) mice. However, in SCID-hu mice transplanted with graded doses of donor cells ranging from 2.0 × 102 to 2.0 × 104 cells, BM reconstitution by the CD4+ fraction of CD34++ Lin− cells was more frequent than by the CD4− fraction when low numbers of cells were injected. These functional data strongly suggest that stem cells reside among CD4+ CD34++ Lin− fetal liver cells. This hypothesis was further supported by the observations that CD4+ CD34++ Lin− fetal liver cells were enriched for CDw90+ (Thy-1), CD117+ (kit), CD123+, HLA-DR+, CD7−, CD38−, CD45RA−, CD71−, CD115− (fms), and rhodamine 123dull cells, a phenotypic profile believed to represent fetal stem cells. Furthermore, all CD4+ CD34++ Lin− fetal liver cells also expressed CD13 and CD33.
CD4 IS A 56-kD glycoprotein, the expression of which is best known to define a subset of mature T cells.1 The CD4+ mature T cells usually interact with cells expressing class II major histocompatibility complex (MHC).2 The function of CD4 in these interactions is that of a coreceptor for class II molecules that augments the stability of the complex formed by the T-cell receptor (TCR) and the antigen presented by MHC. The requirement of CD4 expression on mature T cells for their function was, in part, shown in CD4-deficient mice that displayed deficient T-cell helper function.3 CD4 has also been shown to function as a receptor for the cytokine, lymphocyte chemoattractant factor, therefore suggesting a functional role for CD4 on cells that do not express TCR.4
CD4 expression has been observed on a number of human cell types that do not express TCR, including megakaryocytes,5 eosinophils,6 monocytes, and Langerhans cells.7 During intrathymic development in both mice and humans, CD4 is expressed by immature (TCR−) and mature (TCR+) thymocytes.8,9 In the human thymus, the existence of CD34+ CD4+ CD3− CD8− cells that are an intermediate stage between CD34+ CD3− CD4− CD8− (triple negative) thymocytes and CD34− CD3± CD4+ CD8+ (double positive [DP]) thymocytes has been recently shown.10,11 Furthermore, several studies in the mouse have documented the expression of CD4 on early multipotent progenitors in both the thymus and bone marrow (BM). Wu et al12 have shown the existence of a murine thymic CD4lo progenitor population that has lost myeloid and erythroid lineage potential but remains capable of T- and B-lymphoid development. Although the expression of CD4 on murine stem cells remains controversial,13,14 low levels of CD4 expression on murine BM progenitors15 and stem cells capable of long-term BM reconstitution have been observed.16-18
Low levels of CD4 expression on human adult BM progenitors, defined by their expression of CD34, have recently been reported.19,20 Louache et al20 have shown that CD4 is expressed on adult BM CD34+ cells with the phenotypic profile of primitive hematopoietic progenitors. Furthermore, these investigators observed that the CD34+ CD4+ population was also enriched for long-term culture-initiating cells (LTC-IC).20 These studies showed that CD4 expression is not only limited to mature T cells, but that CD4 is also expressed on hematopoietic progenitors at various stages of lineage commitment. In this study, we report the expression of low levels of CD4 by human fetal hematopoietic progenitors expressing high levels of CD34. Moreover, these CD4+ CD34++ cells were found to coexpress a pattern of cell surface antigens consistent with the phenotypic profile believed to represent fetal hematopoietic stem cells. Functionally, this population of CD4+ cells was shown to contain erythroid, myeloid, B-cell, and T-cell progenitors. The CD4+ cells also displayed an extensive in vitro and in vivo proliferative capacity that was greater than that observed of CD4− cells. These data show that cells isolated from the human fetal liver expressing low levels of CD4 appear to contain hematopoietic stem cells.
MATERIALS AND METHODS
Cytokines.Recombinant human c-kit ligand (KL) was purchased from R&D Systems, Inc (Minneapolis, MN) and was used at 20 ng/mL. Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF ), used at 20 ng/mL, was provided by the Schering-Plough Corp (Kenilworth, NJ). Recombinant human erythropoietin (EPO; Amgen, Thousand Oaks, CA) was used at 1 U/mL.
Antibodies.The following unconjugated, biotinylated, phycoerythrin (PE)-labeled, fluorescein isothiocyanate (FITC)-labeled, or tricolor (TC)-labeled polyclonal or monoclonal antibodies (MoAbs) were purchased from Becton Dickinson (San Jose, CA) or an otherwise stated vendor: mouse IgG1-PE, mouse IgG2a-PE, mouse IgG1-FITC, mouse IgG2a-FITC, mouse IgG1-TC (Caltag Laboratories, South San Francisco, CA), mouse IgG2a-TC (Caltag Laboratories), CD3 (SK7), CD3-PE (SK7), CD3-FITC (SK7), CD4 (SK3), CD4-FITC (SK3 + SK4), CD4-PE (SK3), CD7-FITC (4H9), CD8 (SK1), CD8-FITC (SK1), CD10-PE (5-1B4; Caltag Laboratories), CD13-PE (WM-47; DAKO, Carpinteria, CA), CD14 (MØP9), CD14-PE (MØP9), CD16-PE (B73.1), CD19 (4G7), CD19-PE (4G7), CD20 (L27), CD20-PE (L27), CD33-PE (P67.6), CD33-TC (251; Caltag Laboratories), CD34-biotin (8G12), CD34-PE (8G12), CD34-FITC (8G12), CD38-PE (HB-7), CD38-FITC (HIT2; Caltag Laboratories), CD45RO-PE (UCHL-1), CD45RA-FITC (L48), CD56 (MY31), CD56-PE (MY31), CD71-FITC (L01.1), CDw90-PE (5E10; PharMingen; San Diego, CA), CD115 (3-4A4-E4; Oncogene Science, Uniondale, NY), CD117-PE (95C3; Immunotech, Westbrook, ME), CD123-PE (9F5; PharMingen), HLA-DR-PE (L243), HLA-DR-TC (HL38; Caltag Laboratories), glycophorin A (GPA; 10F7MN; American Type Tissue Culture Collection [ATCC], Rockville, MD), HLA-A2-FITC (CR11351; kindly provided by Dr P. Parham, Stanford, CA), HLA-A3-FITC (GAP A3; ATCC), HLA-A11/A24-FITC (A11.1M; ATCC), and HLA-B7/B40-FITC (MB40.2; ATCC). Allophycocyanin (APC)-labeled streptavidin was also purchased from Becton Dickinson, whereas TC-labeled streptavidin and goat antirat IgG-PE were purchased from Caltag Laboratories.
Human hematopoietic tissues.Human fetal liver and fetal BM were obtained from Advanced Bioscience Resources (Alameda, CA) and were used with the approval of the Committee for the Protection of Human Subjects at our institute. The gestational age of the abortuses, ranging from 16 to 24 weeks, was approximated based on the foot length of the fetus.
Umbilical cord blood was obtained from normal full-term deliveries and was kindly provided by Dr U. Chitkara (Department of Labor and Delivery, Stanford University Medical School, Palo Alto, CA). After delivery of the infant and ligation of the umbilical cord, the umbilical cord blood was harvested, before the expulsion of the placenta, using a 60-mL syringe and transferred to a heparinized 50-mL tube.
Phenotypic analysis of hematopoietic tissues.The expression of cell surface antigens was determined by flow cytometry using a FACScan (Becton Dickinson). In all experiments in which fetal liver, fetal BM, or umbilical cord blood cells were labeled with MoAb, the cells were first preincubated for 10 minutes in a solution of phosphate-buffered saline (PBS) free of added Ca+2 and Mg+2 supplemented with 1 mmol/L ethylenediaminetetraacetic acid, 50 μg/mL gentamicin (GIBCO, Grand Island, NY), 0.5% human γ-globulins (Sigma Chemical Co, St Louis, MO), and 2% normal mouse serum followed by staining with MoAb for at least 30 minutes in the same solution. After staining, the cells were washed twice and resuspended in PBS containing 0.5% bovine serum albumin (BSA; Sigma Chemical Co) and 0.05% NaN3 followed by FACScan analysis. For some analyses, propidium iodide (PI; Sigma Chemical Co) was added at a concentration of 1 μg/mL to the final cell suspension to stain dead cells. A range of 5 × 103 to 4 × 104 events were collected per sample using a live-cell gate based on negative PI staining.
Rhodamine 123 (Rh123; Molecular Probes, Eugene, OR) staining was performed by incubating CD34++ Lin− cells, isolated by fluorescence-activated cell sorting (FACS), with 0.1 μg/mL Rh123 for 30 minutes at 37°C. Thereafter, the cells were washed and resuspended in medium without Rh123 for 30 to 120 minutes at 37°C to allow for Rh123 efflux. These cells were then washed at 4°C with PBS containing 0.5% BSA and 0.05% NaN3 and stained for CD4 according to the protocol described above.
Hematopoietic progenitor isolation.Light-density fetal liver (LDFL) cells were prepared by density centrifugation and depleted of GPA+ cells by immunomagnetic bead depletion as previously described.21 GPA− light-density fetal BM (LDFBM) cells and GPA− light-density umbilical cord blood (LDCB) cells were prepared in a similar manner. In preparation for cell sorting or for some phenotypic analysis, GPA− LDFL, LDFBM, or LDCB cells were depleted of lineage (Lin)-committed progenitors and mature hematopoietic cells by immunomagnetic bead depletion with a cocktail of MoAbs detailed in the Results. The MoAb-labeled cells were washed and resuspended in PBS containing 2% fetal calf serum (FCS) and 50 μg/mL gentamicin and FACS was performed under the conditions previously described.21
In vitro hematopoietic progenitor assays.Progenitors with myeloid potential were assayed in double-layered clonal cultures in 60 × 15 mm tissue culture dishes (Becton Dickinson).21 The culture medium consisted of Iscove's modified Dulbecco's medium (Sigma Chemical Co) supplemented with 20% FCS (JRH Biosciences, Lenexa, KS), 50 μg/mL gentamicin, and 7.5 × 10−5 mol/L α-thioglycerol (Sigma Chemical Co). The bottom layer of the cultures consisted of 2 mL of culture medium solidified with the addition of 0.5% SeaPlaque agarose, a low gelling temperature form of agarose (FMC BioProducts, Rockland, ME). The cytokine combination KL + GM-CSF, which has been shown to stimulate a high frequency as well as a broad spectrum of fetal hematopoietic progenitors,22 was added to the bottom layer of the cultures. Cells were seeded in a volume of 1 mL of culture medium supplemented with 0.36% agarose on top of the solidified bottom layer. The cultures were maintained at 37°C in a fully humidified atmosphere containing 5% CO2 in air. After 3 weeks, the cultures were analyzed for the growth of two types of progenitors based on colony size: low proliferative potential colony-forming cells (LPP-CFC) were defined as progenitors giving rise to colonies of ≥50 cells but smaller than colonies derived from high proliferative potential colony-forming cells (HPP-CFC). HPP-CFC were defined as progenitors giving rise to colonies ≥0.5 mm in diameter that were approximated to consist of 1 × 104 to 1 × 106 cells.
Erythroid progenitors were assayed in 1-mL clonal cultures stimulated by KL + EPO grown in 35 × 10 mm culture dishes (Becton Dickinson). The same culture medium as described above was used except for the substitution of 1.2% methyl cellulose (4,000 cp; Sigma Chemical Co) for agarose as the semisolid support matrix. Burst-forming units-erythroid (BFU-E) were defined as hemoglobinized colonies containing at least 50 cells and were enumerated after 3 weeks of growth in a fully humidified atmosphere containing 5% CO2 in air.
The proliferative potential of the purified progenitor subpopulations was further measured using the delta (Δ) assay as previously described.21 Briefly, sorted fetal liver cells were grown in liquid cultures stimulated by KL + GM-CSF for 7 days and then assayed for their potential to give rise to secondary HPP-CFC and LPP-CFC. The numbers of primary HPP-CFC and LPP-CFC were determined from cultures initiated in parallel with the liquid cultures. The data are presented as the fold increase (Δ) in progenitors during liquid culture. Furthermore, cells generated in liquid cultures were also analyzed for their expression of cell surface markers. In some experiments, the length of time in liquid culture was extended beyond 7 days before the cultures were harvested and the cell numbers were determined and phenotypic analysis was performed. Further details of the cytokines used for these long-term liquid cultures are described in the Results.
In vivo assays of human hematopoietic progenitors.T-cell progenitor activity was assessed by using SCID-hu mice established as described23 by combined fetal liver and fetal thymus implantation under the kidney capsule of CB.17 scid/scid (SCID) mice at least 8 weeks before progenitor transplantation. Fifty to 5.0 × 103 sorted CD4+ CD34++ Lin− LDFL cells or CD4− CD34++ Lin− LDFL cells were directly injected into human thymic grafts in a volume of 2 μL using a 10-μL Hamilton syringe 1 to 3 hours after whole body γ-irradiation with 2.5 Gy. Additionally, in some experiments, the sorted fetal liver progenitors were injected without prior irradiation of SCID-hu mice that were constructed with only fetal thymic tissue. The degree of donor cell reconstitution was analyzed by three-color FACScan analysis. Engraftment by donor cells was determined by detecting a human leukocyte antigen (HLA)-mismatch between donor and host thymocytes. BM-reconstituting ability, as well as B-cell lineage potential, of the sorted CD4+ CD34++ Lin− LDFL or CD4− CD34++ Lin− LDFL subpopulations was determined by injecting sorted cells directly into the human BM of SCID-hu mice implanted subcutaneously with human fetal bones at least 8 weeks before.24-26 The SCID-hu mice received 2.5 Gy γ-irradiation 1 to 3 hours before transplantation of the sorted fetal liver cells. At the indicated time points after transplantation, the level of donor cell engraftment, again determined by an HLA-mismatch between donor and host cells, was determined by FACScan analysis.25 26
Data presentation and statistical analysis.The data from multiple experiments were pooled and are presented as the mean ± 1 standard error of the mean (SEM). Statistical significance was determined on individual experiments (single tissues) using the two-tailed paired Student's t-test. Statistical significance of data pooled from multiple experiments was calculated using the nonparametric paired sign test. Differences in the data were considered significant when P ≤ .05.
RESULTS
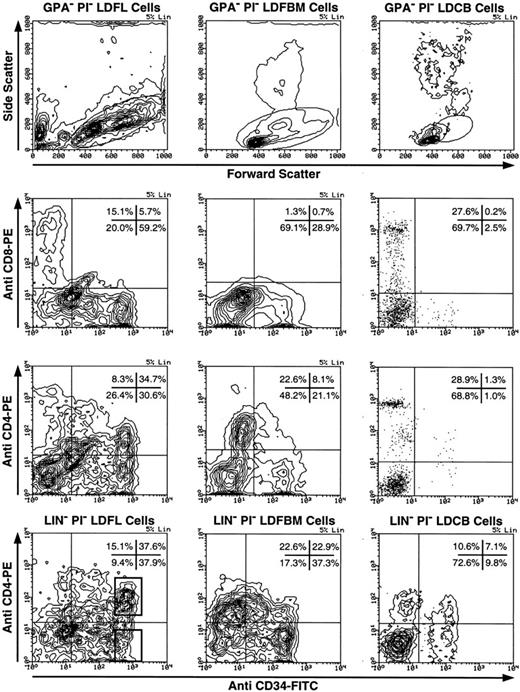
Phenotypic characterization of CD4+ human fetal hematopoietic progenitors.The expression of CD4 on CD34+ progenitor cells was investigated using light-density erythrocyte-depleted (GPA−) fetal liver, fetal BM, and umbilical cord blood cells (Fig 1). Low levels of CD4 expression were observed on CD34+ cells obtained from each of the three tissue types. In the representative experiment shown in Fig 1, CD34 was found to be expressed on 65% of GPA− LDFL cells, on 29% of GPA− LDFBM cells, and only on 2% to 3% of GPA− LDCB cells. The CD4 antigen expression was found on 53% of CD34+ GPA− LDFL cells, 28% of CD34+ GPA− LDFBM cells, and on approximately 57% of CD34+ GPA− LDCB cells. In control stainings, no clear expression of CD8, as detected by an MoAb of the same isotype as that of the CD4 MoAb, was observed on CD34+ cells. The levels of CD4 expression on CD34+ cells were lower than the levels of CD4 expressed by mature T cells, which were present in abundance among the CD34− GPA− fractions of LDFL and LDCB cells. The identification of the CD4++ CD34− LDFL cells as mature T cells was confirmed by the coexpression of CD3 observed in three-color stainings (data not shown). Cells of the monocytic lineage were most likely responsible for the low levels of CD4 expression on CD34− cells, because many of the CD4+ CD34− LDFL cells coexpressed CD11b, CD11c, CD13, CD14, CD15, CD18, and CD33 (data not shown). In contrast, very low levels of CD4 expression were observed on less than 5% of B-cell progenitors defined by their expression of CD34 and either CD10, CD19, or CD20 (data not shown). Further depletion of GPA− LDFL, LDFBM, or LDCB cells of Lin+ cells expressing CD3, CD8, CD14, CD19, CD20, and CD56 resulted in an enrichment of CD34+ cells expressing CD4 (Fig 1, bottom panels). After Lin depletion, the expression of low levels of CD4 (CD4+) on a subpopulation of cells expressing the highest levels of CD34 (CD34++) was clearly evident in each of the three hematopoietic tissues.
Expression of CD4 by CD34+ human fetal liver, fetal BM, and umbilical cord blood cells. LDFL, LDFBM, and LDCB cells were depleted of GPA+ cells and analyzed for the expression of CD34 in combination with CD8 or CD4. The analysis of antigen expression was limited to cells with a low side scatter, containing the bulk of the CD34+ cells, as defined by the gates shown in the top row. The expression of CD4 and CD34 on LDFL, LDFBM, and LDCB cells, depleted of Lin+ cells by magnetic bead depletion of CD3+, CD8+, CD14+, CD19+, CD20+, and CD56+ cells, is shown in the bottom row. CD4+ CD34++ Lin− and CD4− CD34++ Lin− LDFL cells are indicated in the bottom-left panel by regions representative of those used for the isolation of these cells by FACS in subsequent experiments. The percentage of events found in each quadrant is indicated. Contour plots were made using a linear density scale at the degree of resolution indicated in the top-right corner of each plot (% Lin). Dot plots are used to show data on cord blood cells containing a low percentage of CD34+ cells. These data are representative of at least three analyses per tissue type.
Expression of CD4 by CD34+ human fetal liver, fetal BM, and umbilical cord blood cells. LDFL, LDFBM, and LDCB cells were depleted of GPA+ cells and analyzed for the expression of CD34 in combination with CD8 or CD4. The analysis of antigen expression was limited to cells with a low side scatter, containing the bulk of the CD34+ cells, as defined by the gates shown in the top row. The expression of CD4 and CD34 on LDFL, LDFBM, and LDCB cells, depleted of Lin+ cells by magnetic bead depletion of CD3+, CD8+, CD14+, CD19+, CD20+, and CD56+ cells, is shown in the bottom row. CD4+ CD34++ Lin− and CD4− CD34++ Lin− LDFL cells are indicated in the bottom-left panel by regions representative of those used for the isolation of these cells by FACS in subsequent experiments. The percentage of events found in each quadrant is indicated. Contour plots were made using a linear density scale at the degree of resolution indicated in the top-right corner of each plot (% Lin). Dot plots are used to show data on cord blood cells containing a low percentage of CD34+ cells. These data are representative of at least three analyses per tissue type.
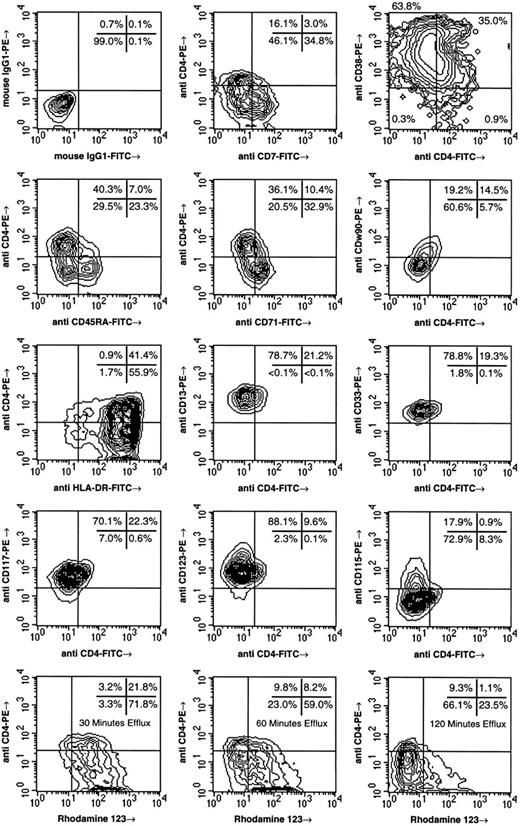
To characterize the phenotype of CD4+ CD34++ cells, fetal liver CD34++ Lin− cells were isolated by FACS and analyzed for the expression of CD4 in conjunction with various cell surface markers (Fig 2). Previous studies suggest that hematopoietic stem cells derived from adult and fetal tissues are among CD34++ cells expressing low to negative levels of CD7,27,28 CD38,21,29-31 CD45RA,21,31-33 and CD71.21,31,34 CD4 expression by CD34++ Lin− LDFL cells was found to be inversely correlated with the expression of CD7, CD38, CD45RA, and CD71. Moreover, the expression of CD4 by CD34++ Lin− LDFL cells correlated with the expression of CDw90 (Thy-1). The detection of CDw90 necessitated the use of a PE-labeled anti-CDw90 MoAb due to the weak expression of this antigen. Because higher levels of CD4 expression were also detected using PE-labeled as compared with FITC-labeled MoAbs, the population of CD34++ Lin− LDFL cells coexpressing CD4 and CDw90 is underestimated by the data shown in Fig 2. Most CD4+ cells were also found to express HLA-DR, although CD4 expression was also observed on the minority of CD34++ Lin− LDFL cells lacking the expression of HLA-DR. Additionally, all CD4+ CD34++ Lin− LDFL cells expressed low levels of CD13 and CD33. We have previously reported that CD13 and CD33, markers associated with myeloid cell differentiation, may be expressed on fetal hematopoietic stem cells.21 The growth factor receptors CD117 (c-kit) and CD123 (the α-chain of the interleukin-3 [IL-3] receptor) were both uniformly expressed on CD4+ CD34++ Lin− LDFL cells, whereas the expression of these receptors appeared to diverge on CD4− cells (Fig 2). Almost no expression of CD115, the receptor for macrophage colony-stimulating factor, was seen on CD4+ cells, but high levels of CD115 expression were seen on a subpopulation of CD4− cells. The heterogeneity of growth factor receptor expression on CD4− cells suggests that this population of cells is composed of functionally diverse progenitors.
Phenotypic analysis of CD4+ CD34++ Lin− LDFL cells. CD34++ Lin− LDFL cells were isolated by immunomagnetic bead depletion of GPA− LDFL cells after staining with FITC-labeled MoAb against CD3, CD8, CD10, CD14, CD15, CD16, CD19, and CD20. These Lin− cells were then stained for CD34 expression in a two-step protocol using anti-CD34-biotin followed by streptavidin-APC or streptavidin-TC. CD34++ Lin− PI− LDFL cells were isolated by FACS. The sorted cells were stained for CD4 and the other cell-surface markers indicated. Additionally, staining with CD4 and Rh123 was also performed, for which the incubation periods for Rh123 efflux are indicated. These data are shown using linear-density contour plots with one degree of smoothing. A 10% resolution was used for all plots except for the analyses of cytokine receptor expression and HLA-DR, which are shown at 5% resolution. However, the analysis of CD38 versus CD4 expression is shown on a 50% logarithmic-density contour plot to show the expression of CD4 on the rare subpopulation of CD34++ that does not express CD38. These data are compiled from the analyses of multiple tissues and are representative of at least two analyses per phenotype.
Phenotypic analysis of CD4+ CD34++ Lin− LDFL cells. CD34++ Lin− LDFL cells were isolated by immunomagnetic bead depletion of GPA− LDFL cells after staining with FITC-labeled MoAb against CD3, CD8, CD10, CD14, CD15, CD16, CD19, and CD20. These Lin− cells were then stained for CD34 expression in a two-step protocol using anti-CD34-biotin followed by streptavidin-APC or streptavidin-TC. CD34++ Lin− PI− LDFL cells were isolated by FACS. The sorted cells were stained for CD4 and the other cell-surface markers indicated. Additionally, staining with CD4 and Rh123 was also performed, for which the incubation periods for Rh123 efflux are indicated. These data are shown using linear-density contour plots with one degree of smoothing. A 10% resolution was used for all plots except for the analyses of cytokine receptor expression and HLA-DR, which are shown at 5% resolution. However, the analysis of CD38 versus CD4 expression is shown on a 50% logarithmic-density contour plot to show the expression of CD4 on the rare subpopulation of CD34++ that does not express CD38. These data are compiled from the analyses of multiple tissues and are representative of at least two analyses per phenotype.
Human stem cells have also been enriched on the basis of their low level of staining with the dye Rh123, which is in part due to the expression of the efflux pump P-glycoprotein on these cells.31,35 36 Sorted CD34++ Lin− LDFL cells were stained with Rh123, followed by 30 to 120 minutes of efflux. Both CD4+ and CD4− cells were found among those cells expressing the lowest levels of Rh123; however, the CD4+ fraction was enriched for Rh123dull cells (Fig 2).
Colony-forming activities of the CD4+ and CD4− subsets of CD34++ Lin− fetal liver cells.Functional studies were performed on the CD4+ and CD4− subsets of CD34++ Lin− LDFL cells, which were isolated by FACS using the criteria shown in Fig 1. Consistent with the results from phenotypic analyses (Fig 2) suggesting that CD4+ cells are enriched among the most primitive fetal liver progenitors, the frequency of early myeloid progenitors with a high proliferative capacity (HPP-CFC; Table 1) was found to be significantly greater among CD4+ cells than CD4− cells (paired sign test, P = .031; n = 6 experiments). The frequencies of LPP-CFC detected among CD4+ and CD4− progenitors were similar. We have previously reported that the most primitive HPP-CFC, those progenitors with the greatest proliferative capacity, are enriched among CD34++ CD38− Lin− LDFL cells,21 although in the presence of KL + GM-CSF large colony formation can also be detected in cultures of the more mature CD34++ CD38+ Lin− LDFL cells.22 Thus, to investigate the expression of CD4 among fetal liver progenitors expressing negative to low levels of CD38, CD34++ CD38− Lin− LDFL cells were isolated based on their expression of CD4. The frequency of HPP-CFC among CD4− CD34++ CD38− Lin− LDFL cells was found to be significantly less than among CD4+ CD34++ CD38− Lin− LDFL cells. In three analyses of cells stained for FACS, the percentage of CD4+ cells among CD34++ CD38− Lin− LDFL cells was found to range from 60% to 87%, which is consistent with the phenotypic analysis shown in Fig 2. Thus, the majority of the most primitive HPP-CFC, those residing among CD34++ CD38− Lin− LDFL cells, express CD4.
Both sorted cell populations contained BFU-E detected in cultures stimulated by EPO + KL (Table 1). However, significantly greater numbers of BFU-E were observed among CD4− CD34++ Lin− LDFL cells than among CD4+ CD34++ Lin− LDFL cells. Because the culture conditions used support the growth of very few erythroid progenitors found among primitive CD34++ CD38− Lin− LDFL progenitors, these data reflect the growth of more mature erythroid progenitors that express CD38 and respond to EPO + KL (M.O.M., M.-G.R., and R.N., manuscript in preparation). Thus, among the more mature CD34++ CD38+ Lin− LDFL cells, erythroid progenitors are concentrated in the CD4− fraction. In contrast, the predominance of HPP-CFC in the CD4+ fraction of CD34++ Lin− LDFL cells suggests that this population is enriched for primitive progenitors.
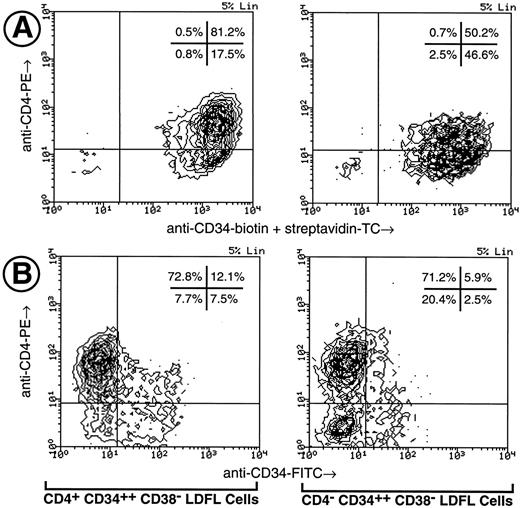
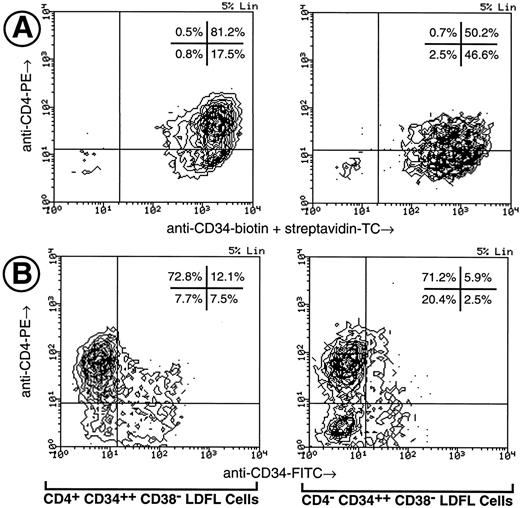
Ex vivo expansion potential of the CD4+ and CD4− subsets of CD34++ fetal liver progenitors.The growth in liquid cultures of CD4+ CD34++ CD38− Lin− and CD4− CD34++ CD38− Lin− LDFL cells showed marked differences in these two progenitor subpopulations. After 4 days of culture in the presence of KL + GM-CSF + EPO, both the cultured CD4+ and CD4− progenitors were observed to express CD4 and CD34 (Fig 3A). The mean intensity of CD34 fluorescence was twofold higher on the cultured CD4+ progenitors (1,835) than on the cultured CD4− progenitors (912). After 7 days of growth, higher percentages of cells expressing higher levels of CD34 continued to be observed in cultures of CD4+ progenitors, relative to cultures of CD4− progenitors (Fig 3B). Therefore, the kinetics of the loss of CD34 expression clearly distinguish the CD4+ progenitors from the CD4− progenitors. It is also of interest to note that the CD4− CD34++ CD38− Lin− LDFL cells could become CD4+ CD34+ cells in vitro.
Analysis of CD4 and CD34 expression on cultured CD4+ CD34++ CD38− Lin− and CD4− CD34++ CD38− Lin− LDFL cells. (A) Fetal liver cells were prepared for FACS by the method described in the legend to Table 1, with the exception that streptavidin-TC was substituted for streptavidin-APC. After 4 days of culture in KL + GM-CSF + EPO, the cultured cells were restained using the same MoAbs as used for cell sorting and analyzed using a FACScan. (B) CD4+ CD34++ CD38− Lin− and CD4− CD34++ CD38− Lin− LDFL cells, isolated by the identical procedure used in Table 1, were reanalyzed after 7 days of culture in KL + GM-CSF. CD34 expression was clearly observed to be greater on the cultured CD4+ cells than on the cultured CD4− cells.
Analysis of CD4 and CD34 expression on cultured CD4+ CD34++ CD38− Lin− and CD4− CD34++ CD38− Lin− LDFL cells. (A) Fetal liver cells were prepared for FACS by the method described in the legend to Table 1, with the exception that streptavidin-TC was substituted for streptavidin-APC. After 4 days of culture in KL + GM-CSF + EPO, the cultured cells were restained using the same MoAbs as used for cell sorting and analyzed using a FACScan. (B) CD4+ CD34++ CD38− Lin− and CD4− CD34++ CD38− Lin− LDFL cells, isolated by the identical procedure used in Table 1, were reanalyzed after 7 days of culture in KL + GM-CSF. CD34 expression was clearly observed to be greater on the cultured CD4+ cells than on the cultured CD4− cells.
In Δ-assays, the cellularity of cultures of CD4+ CD34++ CD38− Lin− LDFL cells increased 1,000-fold over 7 days, whereas the cellularity of cultures of CD4− CD34++ CD38− Lin− LDFL cells increased only 730-fold. The cultures of CD4+ CD34++ CD38− Lin− LDFL cells gave rise to a 60-fold increase in HPP-CFC and a 430-fold increase in LPP-CFC, in contrast to the 9.6-fold increase in HPP-CFC and 130-fold increase in LPP-CFC measured from cultures of CD4− CD34++ CD38− Lin− LDFL cells (P = .042 and .001, respectively). The ability of CD4+ progenitors to proliferate to a greater degree than CD4− progenitors was confirmed in 13 experiments in cultures maintained for up to 21 days in the presence of various growth stimuli.
Both CD4+ and CD4− fetal liver progenitors can reconstitute lymphoid and myeloid lineages in SCID-hu mice.Multilineage potential as well as the ability of progenitors to maintain long-term hematopoiesis were investigated using SCID-hu mice, which provide an in vivo model system for human hematopoiesis.23-26 The T-cell lineage potential of both the CD4+ and CD4− subsets of fetal liver progenitors was shown by the ability of 5.0 × 101 to 2.0 × 103 of these cells to give rise to T cells upon injection into human fetal thymic tissue implanted into SCID mice (Table 2). Immature CD4+ and CD8+ DP thymocytes of donor origin were observed in all tissues engrafted with either CD4− CD34++ Lin− or CD4+ CD34++ Lin− LDFL cells (Table 2 and Fig 4). Thus, precluding the possibility that engraftment was a consequence of contaminating single-positive (SP) T cells of fetal liver origin. Furthermore, in all cases of engraftment, CD4+ SP and CD8+ SP thymocytes with the donor HLA phenotype were observed. Interestingly, in most cases, the frequency of CD4+ SP and CD8+ SP cells was similar, in contrast to fresh fetal thymic tissue in which the number of CD4+ SP thymocytes exceeds the number of CD8+ SP thymocytes by twofold.27 In some transplants, as few as 50 cells of each fraction reconstituted thymic T lymphopoiesis for greater than 2 months. These results show that T-cell progenitors are present at high frequencies among both the CD4+ and the CD4− fractions of CD34++ Lin− LDFL cells.
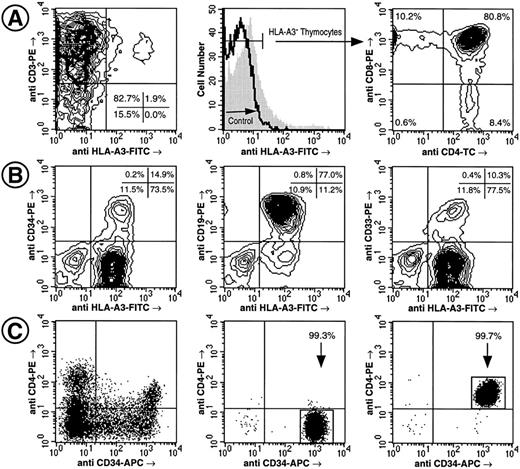
Engraftment of human fetal thymic and BM tissue implanted in SCID mice by CD4− CD34++ Lin− LDFL and CD4+ CD34++ Lin− LDFL progenitors. Generation of CD3+, CD4 SP, and CD8 SP and DP thymocytes by 50 HLA-A3− CD4+ CD34++ Lin− LDFL cells injected into an HLA-A3+ thymic implant 95 days before analysis. Note the presence of a small (<1.9%) fraction of CD3+ HLA-A3+ host cells (A). Generation of CD34+, CD19+, and CD33+ cells by 200 HLA-A3+ CD4+ CD34++ Lin− LDFL cells injected into human fetal bone fragments (HLA-A3−) 125 days before analysis (B). The data are shown using 3% (A) and 2% (B) linear-density contour plots with 1 degree of smoothing. Analysis of CD4 and CD34 expression on Lin− PI− LDFL cells before (C, left) and after FACS (C, center and right). Ten thousand PI− events were collected for the sort reanalysis shown. These sorted cells are donor B in Table 2 and donor D in Table 3.
Engraftment of human fetal thymic and BM tissue implanted in SCID mice by CD4− CD34++ Lin− LDFL and CD4+ CD34++ Lin− LDFL progenitors. Generation of CD3+, CD4 SP, and CD8 SP and DP thymocytes by 50 HLA-A3− CD4+ CD34++ Lin− LDFL cells injected into an HLA-A3+ thymic implant 95 days before analysis. Note the presence of a small (<1.9%) fraction of CD3+ HLA-A3+ host cells (A). Generation of CD34+, CD19+, and CD33+ cells by 200 HLA-A3+ CD4+ CD34++ Lin− LDFL cells injected into human fetal bone fragments (HLA-A3−) 125 days before analysis (B). The data are shown using 3% (A) and 2% (B) linear-density contour plots with 1 degree of smoothing. Analysis of CD4 and CD34 expression on Lin− PI− LDFL cells before (C, left) and after FACS (C, center and right). Ten thousand PI− events were collected for the sort reanalysis shown. These sorted cells are donor B in Table 2 and donor D in Table 3.
The potential of CD4+ CD34++ Lin− and CD4− CD34++ Lin− LDFL cells to reconstitute BM hematopoiesis when injected into bone grafts implanted in SCID mice was investigated (Table 3). Full engraftment was defined by the presence of CD34+, CD19+, and CD33+ cells of donor HLA origin, as shown in Fig 4. Both CD4+ and CD4− fetal liver cells were observed to contain progenitors with myeloid and/or B-lymphoid potential. Furthermore, both the CD4+ and the CD4− subpopulations had considerable proliferative potential when transplanted into human bone fragments. A similar frequency of donor cell engraftment was observed in the human BM of SCID-hu mice injected with either 2.0 × 104 CD4+ or CD4− cells and analyzed 143 days after transplantation (Table 3, donor A). The discrepancy between these in vivo results and the stark differences in the proliferative capacities between CD4+ and CD4− cells in vitro lead us to hypothesize that the high number of cells injected into the SCID-hu mice may have interfered with the ability to discern any differences in proliferative potential between CD4+ and CD4− cells. Indeed, with the injection of a 10-fold fewer number of cells, a difference between the CD4+ and the CD4− fetal liver subsets was observed. Progenitor (CD34), B-lymphoid (CD19), and myeloid (CD33) engraftment, measured at about 3 months after the injection of 2.0 × 103 CD4+ cells, was observed in 3 of 4 SCID-hu mice receiving injections of donor B cells and in all 4 mice receiving injections of donor C cells, whereas injection of 2.0 × 103 CD4− cells from donor B gave rise to full engraftment in only 3 of 7 mice and no reconstitution was observed with CD4− cells derived from donor C. CD4+ cells were again found to be superior in reconstituting human hematopoiesis when only 2.0 × 102 cells were injected. Reanalysis of 10,000 of these sorted CD4+ and CD4− cells (Fig 4C) indicates that no contamination by the counterpart population occured with the injection of only 2.0 × 102 cells. Approximately 4 months after transplantation, full reconstitution was observed for 3 of 6 mice receiving injections of CD4+ cells, whereas full engraftment was not observed in the 7 mice receiving transplants of CD4− cells (Table 3, donor D). Taken together, these data show that CD4+ progenitors have, on average, a greater proliferative capacity than do CD4− progenitors, although both populations of cells can reconstitute multilineage hematopoiesis in vivo.
DISCUSSION
The CD4 molecule has been shown to be expressed by mature cells and progenitors of various hematopoietic lineages.1,5,7,19 We have found CD4 to be expressed by a subset of human hematopoietic progenitors obtained from umbilical cord blood, fetal BM, and fetal liver. These observations are in agreement with recent reports showing low levels of CD4 expression on human CD34+ progenitors isolated from adult BM.19 20
Although CD4 expression was detected on progenitors expressing both low and high levels of CD34, we focused our study on progenitors expressing the highest levels of CD34. These CD34++ cells have been shown by various means to contain primitive hematopoietic progenitors, including stem cells.21,25,29,31 In the human fetal liver, fetal BM, and umbilical cord blood, the highest levels of CD4 expression on progenitor cells was on those cells also expressing the highest levels of CD34. However, the levels of CD4 expression on CD34++ cells were lower than those found on CD4+ mature T cells. The phenotypic profile of CD4+ CD34++ Lin− LDFL cells was similar to the profile suggested to represent fetal and adult pluripotent stem cells: CDw90+ (Thy-1),21,31,37 CD117+ (c-kit),21,31,38-41 CD7−,27,28 CD38−,21,29-31 CD45RA−,21,32-34 CD71− (transferrin receptor),21,31,34 and Rh123dull.31,35,36 Indeed, the expression of CD4 on CD34++ Lin− LDFL cells was found to be highest on cells with the lowest expression of CD7, CD38, CD45RA, and CD71. These observations are similar to those of Louache et al,20 who observed that CD4+ adult BM cells are CD38−/low, CD71low, and CDw90+. In summary, these data show the existence of a subpopulation of CD34++ fetal liver cells that is CD4+, CD13+, CD33+, CDw90+, CD117+, CD123+, CD7−, CD38−, CD45RA−, CD71−, CD115−, and Rh123dull. Based on the phenotypic similarity of these cells to those previously described to be candidate stem cells and on the functional characteristics of CD4+ CD34++ Lin− LDFL cells, it is highly likely that this cell population contains fetal hematopoietic stem cells.
However, a number of differences between the expression of cell surface markers on adult and fetal stem cells do appear to exist. Our previous studies on the expression of CD13 and CD33 by fetal liver progenitors suggested that these myeloid-associated antigens are expressed by fetal stem cells,21 in contrast to adult stem cells.42-44 The expression of CD33 by CD5+ CD2+ fetal thymocytes with T-cell and natural killer cell progenitor activity also indicated that CD33 expression is not limited to myeloid cells.45,46 The results reported here confirm this supposition, because all CD4+ CD34++ Lin− LDFL cells expressed CD13 and CD33 and these cells were found to give rise to T, B, and myeloid cells in vivo. Although the expression of CD117 by early fetal progenitors is consistent with the distribution of this cytokine receptor on adult progenitors, the expression of the α-chain of the IL-3 receptor (CD123) on CD4+ CD34++ Lin− LDFL cells is in contrast with the observation that candidate adult stem cells are refractory to IL-3.47 However, these antithetical findings are likely to represent functional differences between adult and fetal stem cells, because CD34++ CD38− Lin− LDFL cells were shown to be responsive to IL-3.22 The expression of HLA-DR by hematopoietic stem cells may also change during human ontogeny. The CD4+ and CD4− subpopulations of CD34++ Lin− LDFL cells differed only slightly in their expression of HLA-DR, with both subpopulations containing a minor fraction of HLA-DR− cells and a major fraction of HLA-DR+ cells. We have previously failed to observe any difference between the in vitro proliferative potential of HLA-DR− and HLA-DR+ fetal liver progenitors,21 whereas others have suggested that the fetal progenitors with the greatest proliferative potential reside among the HLA-DR+ fraction of fetal BM48 or umbilical cord blood cells.49 Conversely, numerous studies of adult hematopoietic progenitors favor the hypothesis that HLA-DR is not expressed by stem cells.50-52 Interestingly, in agreement with our findings on fetal liver cells, CD4+ adult BM progenitors were shown to be predominantly HLA-DR+.20 The observation that CD4 expression is conserved during human ontogeny suggests that this molecule plays an important role in hematopoiesis.
Hematopoietic stem cells are defined by their pluripotentiality and their extensive proliferative capacity. The results of in vitro and in vivo assays of these two functions indicated that most of the stem cell activity found in the human fetal liver was contained in the CD4+ subset. In vitro clonal assays showed that CD4+ CD34++ Lin− LDFL cells are enriched for HPP-CFC activity relative to CD4− CD34++ Lin− LDFL cells. Likewise, with the further enrichment of primitive progenitors by the isolation of CD34++ CD38− Lin− cells,21 greater HPP-CFC activity was in the subset of these cells expressing CD4. More importantly, the Δ-assay showed that the CD4+ fraction consistently generated significantly greater numbers of secondary HPP-CFC than were generated by the CD4− fraction, a finding similar to the observed enrichment of LTC-IC among CD4+ adult BM progenitors.20 Erythroid potential was also observed in both the CD4+ and the CD4− subpopulations of CD34++ Lin− LDFL cells. However, similar to the results using adult BM progenitors,19 20 greater numbers of BFU-E were measured in the CD4− fraction of fetal liver progenitors, suggesting a loss of CD4 during erythropoiesis.
Further evidence for the enrichment of stem cells among CD4+ cells was obtained using the SCID-hu mouse model. The incidence of BM engraftment in SCID-hu mice at least 3 months after the transplantation of CD4+ CD34++ Lin− LDFL cells was more frequent than observed with CD4− CD34++ Lin− LDFL cells. This difference became more evident when low numbers of cells were transplanted. Transplantation of 2 × 102 CD4+ progenitors led to 50% engraftment of progenitors, myeloid cells, and B cells, whereas no engraftment was observed with CD4− progenitors. The injection of 2 × 103 CD4+ progenitors resulted in an 88% rate of engraftment, and a 30% rate of engraftment was obtained with the transplantation of the same number of CD4− progenitors. However, when 2 × 104 cells were injected, a functional difference between the CD4+ and the CD4− progenitors was not apparent (71% v 60% engraftment, respectively). It is possible that a contamination of the CD4− cells by CD4+ cells may contribute to this reconstitution, because only 2.0 × 102 CD4+ cells were sufficient for reconstitution. Alternatively, the transplantation of a large number of CD4− cells may reduce the degree of proliferation these cells undergo in reconstituting the human bone fragments, thereby extending the lifetime of these grafts. These data show that human fetal liver cells are capable of reconstituting human fetal BM hematopoiesis in the in vivo environment of the SCID-hu mouse and that the BM-reconstituting cells are enriched among the fraction of CD4+ CD34++ Lin− LDFL cells.
Both populations of CD4+ CD34++ Lin− LDFL and CD4− CD34++ Lin− LDFL progenitors gave rise to multilineage differentiation in vivo. Multilineage engraftment in bone-implanted SCID mice was indicated by the expression of CD33 (myeloid) and CD19 (B lymphoid). T-cell progenitor activity, indicated by the generation of DP and SP thymocytes, was observed in both the CD4+ and the CD4− subpopulations of CD34++ Lin− LDFL cells using SCID-hu mice implanted with human thymic tissue. These results are consistent with our previously reported observation that 1 × 104 fetal liver progenitors, isolated by FACS based on the expression of CD34 and the lack of expression of a panel of lineage markers including CD4, could give rise to multilineage hematopoiesis in human fetal thymic organ cultures. These CD4− CD34+/++ Lin− fetal liver cells were observed to give rise to myeloid, dendritic, B, natural killer, and T cells during culture in depleted fetal thymic fragments.10,27 45 A caveat with experiments measuring thymic or BM reconstitution is the inability to distinguish the multilineage engraftment due to pluripotent stem cells from that due to a mixture of lineage-restricted progenitors. Therefore, further studies are required to determine if cells in both the CD4+ and the CD4− fractions of CD34++ Lin− fetal liver progenitors are capable of pluripotential differentiation.
In agreement with our findings, the expression of low levels of CD4 on murine stem cells has been shown,16,17 although two more recent reports have suggested that the most primitive murine stem cells reside among CD4− BM or fetal liver cells.13,14 Surprisingly, despite the expression of CD4 on murine hematopoietic progenitors13,14,16-18 and T-cell progenitors,53,54 mice lacking functional CD4 genes were not found to be grossly deficient in their lympho-hematopoietic capabilities.3 The functional significance of CD4 expression on cells other than mature CD4 SP T cells therefore remains to be determined. The expression of CD4 by human fetal hematopoietic progenitors does have implications towards our understanding of the biology of human immunodeficiency virus (HIV). Because CD4 is one of the receptors for HIV,55,56 our data imply that fetal hematopoietic stem cells may be infected by HIV in utero. This possibility is supported by the observation that adult CD4+ hematopoietic progenitors bound HIV gp120 via the CD4 antigen.19,20 Some studies have also found adult hematopoietic progenitors to be infected by HIV, although there is disagreement as to the incidence of these infections and the role of HIV-infected progenitors in the hematologic abnormalities observed in HIV-infected individuals.57-60 The discovery of human fetal hematopoietic tissues as a source of primitive CD4+ progenitors makes the role of these cells in normal and abnormal hematopoiesis amenable to further study.
ACKNOWLEDGMENT
We are indebted to E. Callas, J. Cupp, V. Hong, and D. Polakoff for help with cell sorting and to S. Antonenko and N. Carballido-Perrig for help in the construction and care of SCID-hu mice. We also thank A. Bárcena for her critical review of this manuscript.
DNAX Research Institute is supported by the Schering-Plough Corp.
Address reprint requests to Reiko Namikawa, MD, PhD, DNAX Research Institute of Molecular and Cellular Biology, 901 California Ave, Palo Alto, CA 94304-1104.