Abstract
We examined regulation of the human erythropoietin (Epo) gene through the GATA sequence in the Epo promoter and showed that Hep3B and HepG2 cells express human GATA-2 (hGATA-2) mRNA and protein. Nuclear extracts of QT6 cells transfected with hGATA-1, 2, or 3 transcription factors showed specific binding to the GATA element in the human Epo gene promoter by gel mobility shift assay. Transient transfection of Hep3B cells with hGATA-1, 2, or 3 showed that each of these transcription factors significantly decreased the level of expression of Epo mRNA as assessed by a competitive polymerase chain reaction. Transient transfection of Hep3B cells with hGATA-1, 2, and 3 and an Epo-reporter gene (growth hormone [GH]) construct showed significant inhibition of the Epo promoter. Antisense oligonucleotide for hGATA-2 transcription factor significantly increased the Epo protein in Hep3B cells under 1% O2 for 24 hours incubation. Furthermore, transient transfection of Hep3B cells with hGATA-1, 2, and 3 and an Epo-reporter gene (luciferase) construct also showed significant inhibition of the Epo promoter. However, transfection of the mutated GATA sequence of the Epo-luciferase gene with hGATA-1, 2, and 3 interfere with the inhibition of the Epo promoter. We conclude that the hGATA-1, 2, and 3 transcription factors specifically bind to the GATA element in the human Epo gene promoter and negatively regulate Epo gene expression.
ERYTHROPOIETIN (Epo) is produced in the kidney and fetal liver in response to hypoxia,1 as well as CoCl2 .2 However, little is understood about the intracellular pathway by which hypoxia leads to an increase in Epo expression. Goldberg et al3 have shown that in the human hepatoma cell lines Hep3B and HepG2 , Epo protein and mRNA can be induced in response to hypoxia or CoCl2 . They have proposed that the cells have an oxygen-sensing mechanism in which a ligand-dependent conformational change in a heme protein in response to either hypoxia or cobalt results in an induction of Epo expression.4
Positive and negative regulatory elements in the Epo gene were studied using synthetic oligonucleotides. These oligonucleotides were designed to control Epo transcription by means of an antigene strategy.5 We recently used this method to show that CACCC elements at −60 bp from the CAP site are positive regulatory sites of the Epo gene, whereas the GATA element at −30 bp is a negative regulatory element for transcription of the Epo gene5 (Fig 1A). Furthermore, we showed that transcription factors in nuclear extracts from Hep3B cells specifically bind to CACCC elements or the GATA element.5
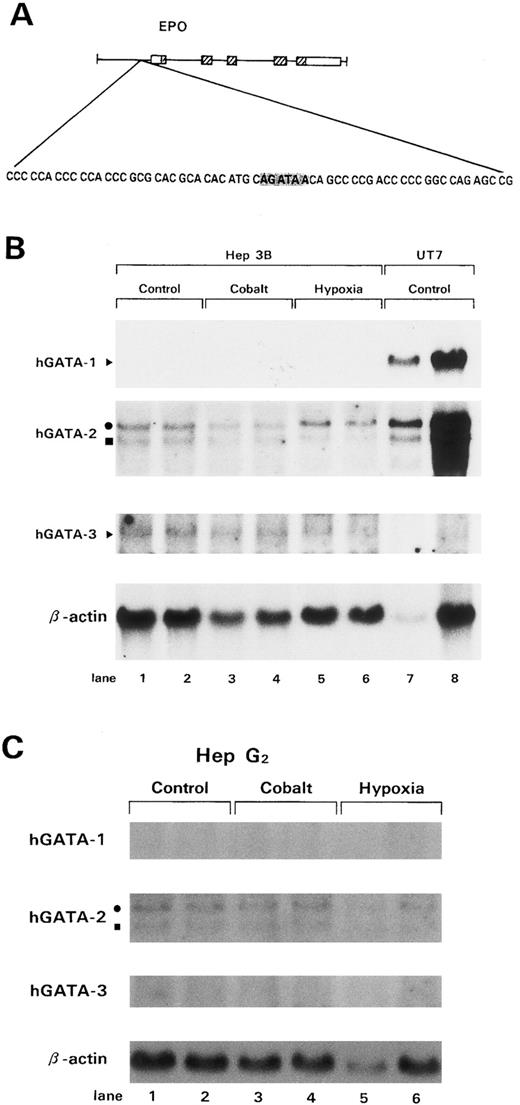
hGATA-specific mRNA expression. (A) GATA-binding site in the Epo 5′ promoter region (shaded region). (B) Northern blot analysis of Hep3B cells. Northern blot analysis was performed using 20 μg total RNA from control Hep3B cells (lanes 1 and 2), Hep3B cells treated with 50 μmol/L CoCl2 for 24 hours (lanes 3 and 4), and Hep3B cells treated with 1% O2 for 24 hours (lanes 5 and 6). UT7 cells (lanes 7 and 8) were used as a positive control for hGATA-1 and 2. The filter was hybridized to a probe of hGATA-1, -2, and -3 cDNA and then stripped and rehybridized to a β-actin cDNA probe. (C) Northern blot analysis of HepG2 cells. Northern blot analysis was performed using 20 μg total RNA from control HepG2 cells (lanes 1 and 2), HepG2 cells treated with 50 μmol/L CoCl2 for 24 hours (lanes 3 and 4), and HepG2 cells treated with 1% O2 for 24 hours (lanes 5 and 6). The filter was hybridized to a probe of hGATA-1, -2, and -3 cDNA, respectively, and then stripped and rehybridized to a β-actin cDNA probe.
hGATA-specific mRNA expression. (A) GATA-binding site in the Epo 5′ promoter region (shaded region). (B) Northern blot analysis of Hep3B cells. Northern blot analysis was performed using 20 μg total RNA from control Hep3B cells (lanes 1 and 2), Hep3B cells treated with 50 μmol/L CoCl2 for 24 hours (lanes 3 and 4), and Hep3B cells treated with 1% O2 for 24 hours (lanes 5 and 6). UT7 cells (lanes 7 and 8) were used as a positive control for hGATA-1 and 2. The filter was hybridized to a probe of hGATA-1, -2, and -3 cDNA and then stripped and rehybridized to a β-actin cDNA probe. (C) Northern blot analysis of HepG2 cells. Northern blot analysis was performed using 20 μg total RNA from control HepG2 cells (lanes 1 and 2), HepG2 cells treated with 50 μmol/L CoCl2 for 24 hours (lanes 3 and 4), and HepG2 cells treated with 1% O2 for 24 hours (lanes 5 and 6). The filter was hybridized to a probe of hGATA-1, -2, and -3 cDNA, respectively, and then stripped and rehybridized to a β-actin cDNA probe.
Recently, it has been hypothesized that the TATA-binding protein of TFIID interacts with the core promoter of the GATA motif in the Epo gene, thereby competing with the GATA protein.6 However, the significance of the GATA protein in the regulation of Epo transcription is not understood.
In the present study, we examined transcription factors that regulate the human Epo gene, especially through the GATA-specific sequence. We showed that Hep3B and HepG2 cells express human GATA-2 (hGATA-2) mRNA and protein by Northern blotting analysis and immunohistochemical staining, respectively. QT6 cells that do not express GATA transcription factors were transfected with hGATA-1, 2, and 3 transcription factor constructs, and binding of these hGATA transcription factors to the GATA element in the human Epo gene promoter was assessed by gel mobility-shift assay. The effects of hGATA transcription factors on the level of Epo mRNA in Hep3B cells was assessed by measuring Epo mRNA by competitive polymerase chain reaction (PCR) after transient transfection of hGATA-1, 2, and 3 expression plasmids into Hep3B cells. The effects of hGATA transcription factors on the activity of Epo promoter were determined on Epo-reporter genes (GH and luciferase) and also on mutated GATA sequence of Epo-luciferase gene after transient transfection of hGATA-1, 2, and 3 expression plasmids into Hep3B cells. Furthermore, antisense oligonucleotides for hGATA-1, 2, and 3 transcription factors were incubated with the Hep3B cells under 1% O2 for 24 hours, and the supernatant was assayed for Epo protein by enzyme immunoassay (EIA). We conclude that the hGATA-1, 2, and 3 transcription factors specifically bind to the GATA element of the human Epo gene promoter and negatively regulate Epo gene expression. Moreover, in Hep3B and HepG2 cells, the hGATA-2 transcription factor is a strong negative regulator of the Epo gene.
MATERIALS AND METHODS
Cell Culture and RNA Preparation
The Hep3B, HepG2 and QT6 cell lines were obtained from the American Type Culture Collection (Rockville, MD). These cells were cultured in Dulbecco's modified Eagle's medium (Life Technologies, Inc, Gaithersburg, MD), supplemented with penicillin (100 U/mL), streptomycin (100 μg/mL), and 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT) in 25-cm2 tissue culture flasks. Cells were maintained in a humidified 5% Co2 , 95% air incubator at 37°C. One day before experimentation, cells were harvested and plated at a density of 3 × 106 cells/25-cm2 flask. Cells were grown in the presence or absence of 50 μmol/L CoCl2 or hypoxia (1% oxygen), as previously described.5 After 24 hours of incubation with 50 μmol/L CoCl2 or 1% oxygen, extracts from stimulated or unstimulated cells were prepared. Total cellular RNA was harvested by conventional methods.7
RNA Blot Analysis
Probes were labeled with [α-32P]deoxycytidine triphosphate by random priming8 and used in RNA blot hybridization.8 Formaldehyde gels for RNA electrophoresis were prepared as described.8 RNA blot hybridization was performed using 20 μg total RNA from Hep3B or HepG2 cells. Filters were hybridized to probes of hGATA-1, 2, or 3 cDNA. The same filter was stripped and rehybridized to a β-actin cDNA probe to determine the level of RNA in each lane.
Immunohistochemical Analysis
Immunohistochemical staining was performed as previously described.9 UT7/Epo, Jurkat, HD3, MSB1, Hep3B, and HepG2 cells were loaded on glass slides using a Cytospin (Life Sciences International [Europe LTD], Cheshire Runcorn, UK), fixed in 1% paraformaldehyde for 5 minutes and then fixed in cold acetone for 5 minutes. After incubation in 2% sheep serum, specimens were reacted with specific anti–hGATA-1, -2, -3 monoclonal antibodies overnight at 4°C as previously described.9 After washing in phosphate-buffered saline (PBS), samples were incubated with a horseradish peroxidase-conjugated F(ab′)2 fragment of antimouse IgG (1:100 dilution; Amersham, Bucks, UK) overnight at 4°C. Diaminobenzidine was used as the chromogen. Nuclei were counterstained with methylgreen for 1 hour. The chromogen diaminobenzidine stains antibody-reactive structures brown, whereas the counterstain details the position of the nuclei in all cells. The fixation and photographic procedure used shows only the nuclei in these stained cells.
Transfection
Electroporation of hGATA transcription factors into Hep3B cells were performed as previously described.10 A total of 3 to 10 × 106 cells were electroporated in 1 mL of 20 mmol/L HEPES buffer (pH, 7.05) with 137 mmol/L NaCl, 5 mmol/L KCL, 0.7 mmol/L Na2HPO4 , 6 mmol/L dextrose containing 20 μg or 40 μg of vector DNA (closed circular rather than linearized), and 500 μg carrier salmon sperm DNA at a voltage of 250 V and a capacitance 960 μF (Bio-Rad, Hercules, CA). The time constant of the shock was approximately 12 to 14 milliseconds. The competitive PCR for Epo mRNA was performed 24 hours after transfection. GH was assayed in the media after a 4-day incubation, because production of GH was linear during a 4-day period, as previously described, and the cell pellet was assayed for chloramphenicol acetyltransferase (CAT).10 To correct for variations in transfection efficiency, these results were normalized for expression of CAT. The values of GH/CAT in a 1% O2 incubator were normalized to the values with 21% O2 . The results in the presence of hGATA-transcription factors were then normalized to the results without hGATA-transcription factors. The effect of hGATA transcription factors on the activity of Epo promoter were expressed by normalizing the results in cells with the XGH construct alone.
DNA Binding Assays
Nuclear extracts were prepared by published methods.11 Protein concentrations were determined by a Bio-Rad assay (Bio-Rad, Hercules, CA) with bovine serum albumin standards. Sense strand oligonucleotides were (CATGCAGATAACAGCCCCGACCC CCGGCCA) end-labeled with T4 polynucleotide kinase (Toyobo, Tokyo, Japan) and annealed to a fourfold excess of the corresponding unlabeled antisense oligonucleotide. A probe (2.0 ng) was used in each binding reaction. The binding buffer consisted of 10 mmol/L Tris-HCl (pH 7.5), 1 mmol/L EDTA, 4% Ficoll, 1 mmol/L dithiothreitol, and 75 mmol/L KCl. An equimolar mixture of 1.5 μg poly[d(I-C)] and poly[d(A-T)] (Sigma, St Louis, MO) was used as nonspecific competitor. Binding reactions (25 μL) were incubated for 15 minutes at 4°C and electrophoresed on 5% nondenaturing polyacrylamide gels in 0.25× TBE buffer (22 mmol/L Tris borate, 22 mmol/L boric acid, 0.5 mmol/L EDTA) at room temperature at 150 V for 1.5 hours as previously described.12 Gels were vacuum-dried and autoradiographed with intensifying screens at −80°C for 2 to 24 hours.
Competitive PCR
Epo map with position of oligonucleotide primers.Epo-A (5′-GTCGGGCAGCAGGCCGTAGAAGTCTGGCAG-3′) and Epo-B (5′-AGATGTCATTGCTGGCACTGGAGTGTCCAT-3′) flanking primers were each 30 bp; had 67% and 50% G + C contents, respectively; and lacked 3′ complementarity between primer pairs as previously described.5
Reverse transcription of RNA into cDNA.Epo mRNA was reverse-transcribed from total RNA and was coamplified with a competitive template by competitive PCR.5 After initial denaturation of the RNA at 65°C to eliminate possible secondary structure, 5 μg of RNA from Hep3B cells was reverse-transcribed in a reaction mixture containing 20 nmol of each deoxynucleotide triphosphate (dNTP; Amersham), antisense (Epo-B) primer (40 pmol), 1 U of RNasin (Boehringer Mannheim, Marburg, Germany), and 16 U of avian myeloblastosis virus reverse transcriptase (RT; Life Sciences, Inc, St Petersburg, FL) in a 40-μL total volume of (1×) PCR buffer for 1 hour at 37°C. The transcription reaction was terminated by incubating the samples at 65°C for 10 minutes. The cDNAs were immediately applied for competitive PCR. The 10-fold PCR buffer contained 500 mmol/L Tris-HCl (pH, 8.2), 15 mmol/L MgCl2 , 500 mmol/L KCl, and 0.01% (wt/vol) gelatin. Each reaction mixture contained dNTPs (200 μmol/L final concentration in each), 0.2 μmol/L each primer, and 5 U/mL of Taq polymerase (Cetus, Norwalk, CT) in 1× PCR buffer in a final volume of 100 μL. In all experiments, the presence of possible contaminants was checked by control reactions in which amplification was performed on samples in (1) the absence of RT and (2) lysis buffer alone. Samples were amplified by 60 cycles at 94°C for 1 minute, 62°C for 2 minutes, and 72°C for 3 minutes, containing various amounts of Epo genomic DNA competed against a fixed volume (10 μL) of Epo cDNA. An aliquot of each reaction mixture was subjected to electrophoresis on 1% agarose, 2% NuSieve gels. Gels were stained with ethidium bromide, photographed, and analyzed by densitometry (Immunomedica Co, Ltd, Sizuoka, Japan).
RT-PCR
RT-PCR analysis was performed to examine the transcription of hGATA transcription factors.13 The following primers were used: hGATA-1 sense (5′) GATCCTGCTCTGGTGTCCTCC (3′) and antisense (5′) ACAGTTGAGCAATGGGTACACC (3′), nucleotides 116 to 136 and 298 to 276, respectively, of the hGATA-1 sequence14; hGATA-2 sense (5′) CCCTAAGCAGCGCAGCAAGAC (3′) and antisense (5′) GATGAGTGGTCGGTTCTGGCC (3′), nucleotides 1012 to 1032 and 1174 to 1154, respectively, of the hGATA-2 sequence15; and hGATA-3 sense (5′) GTACAGCTCCGGACTCTTCCC (3′) and antisense (5′) CTGCTCTCCTGGCTGCAGACA (3′), nucleotides 887 to 907 and 1146 to 1126, respectively, of the hGATA-3 sequence.16
Plasmid Vectors
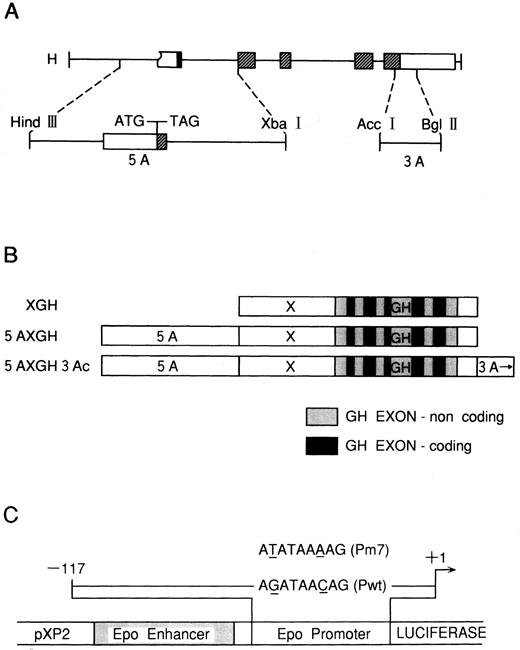
The GH gene was used as a reporter gene in a construct in which expression of GH is driven by the mouse metallothionein-I promoter (XGH). We constructed a vector, 5AXGH, which harbors a 1,192-bp HindIII-Xba I fragment, extending from 378 bp upstream of the cap site through the first exon of Epo (contains only 13 bp of coding sequence) and entire first intron, which is inserted into the polylinker upstream of XGH (Fig 2A and B). To obviate the problem of false initiation of translation from the Epo ATG start codon, this site was mutated to TAG by preparation of a gapped heteroduplex and a 15-bp mutant primer. Mutant colonies were selected by Grunstein hybridization and verified by dideoxy sequencing. A second vector, 5AXGH3Ac, contains an additional a 255-bp Acc I-Bgl II fragment that extends 67 bp upstream from the Epo termination codon and covers much of the 3′ noncoding region that is homologous with the mouse Epo gene (Fig 2A and B).10 hGATA-1, -2, and -3 transcription factor expression plasmids have been as previously described.12 We used the reporter plasmid pEP Luc described by Blanchard et al,17 in which the 126-bp 3′ Epo enhancer [120 to 245 bp 3′ of the poly(A) addition site] and the 117-bp minimal Epo promoter (from bp 117 to +1 relative to the transcription initiation site) were placed upstream of the firely luciferase gene18 in PXP219 (V2-Ewt-Pwt-PXP2 ). In the mutant construct, the GATA sequence in the Epo promoter was mutated to TATA (AGATAACAG → ATATAAAAG) (V3-Ewt-Pm7-PXP2 ).
(A) Human Epo gene. The five exons are shown as rectangles. Coding portions are hatched. The two fragments used in this study, Epo 5A and 3A, are shown. (B) Constructs prepared from the fragments shown in (A) and inserted into HGH-PUC12 plasmids. The coding and noncoding portions of the GH exons are shown by solid and shaded areas, respectively. (C) Diagrams of the reporter construct and the wild-type (Pwt) and the mutated GATA (Pm7) used in this study. The pEP Luc reporter construct is shown in the middle. Shown above it is the 117-bp insert from the Epo promoter. The mutation is shown as an underline. (Data from Galson et al.28 )
(A) Human Epo gene. The five exons are shown as rectangles. Coding portions are hatched. The two fragments used in this study, Epo 5A and 3A, are shown. (B) Constructs prepared from the fragments shown in (A) and inserted into HGH-PUC12 plasmids. The coding and noncoding portions of the GH exons are shown by solid and shaded areas, respectively. (C) Diagrams of the reporter construct and the wild-type (Pwt) and the mutated GATA (Pm7) used in this study. The pEP Luc reporter construct is shown in the middle. Shown above it is the 117-bp insert from the Epo promoter. The mutation is shown as an underline. (Data from Galson et al.28 )
Antisense Oligonucleotides
The following sense and antisense S-oligonucleotides for hGATA-1, -2 and -3 were synthesized:14-16 Sense for hGATA-1, TCCCCAGAGGCTCCATGGAGTTCCCTGGCC; antisense for hGATA-1, GGCCAGGGAACTCCATGGAGCCTCTGGGGA; sense for hGATA-2, CCGCCCGGCCGGCCATGGAGGTGGCGCCCG; antisense for hGATA-2, CGGGCGCCACCTCCATGGCCGGCCGGGCGG; sense for hGATA-3, GCACAGCCGAGGCCATGGAGGTGACGGCGG; and antisense for hGATA-3, CCGCCGTCACCTCCATGGCCTCGGCTGTGC. Synthetic oligonucleotides were incubated with Hep3B cells in the presence of hypoxia (1% O2 ) for 24 hours at 37°C in serum-free conditions. After incubation, supernatant was assayed for Epo protein by enzyme immunoassay (EIA).
Assays
The radioimmunoassay (RIA) for human growth hormone was performed with a kit produced by Nichols Institute Diagnostics (San Juan Capistrano, CA). Cell were rinsed with PBS and lysed on the 10-cm dish with 800 μL of cell lysis buffer (PicaGene, Tokyo, Japan). Luciferase activity in 10 μL of extract was assayed by luminometer for 10 seconds. Each measurement of relative light units was corrected by subtraction of the background. Hypoxic inducibility was defined as the ratio of corrected relative light units of the hypoxic (1% O2 ) dish to corrected relative units of the normoxic (21% O2 ) dish. Chlorampehnicol acetyltransferase (CAT) was measured as described by Neumann et al.20 Epo protein was measured by EIA as previously described.21
RESULTS
Hep3B and HepG2 Cells Express hGATA-2 mRNA and Protein
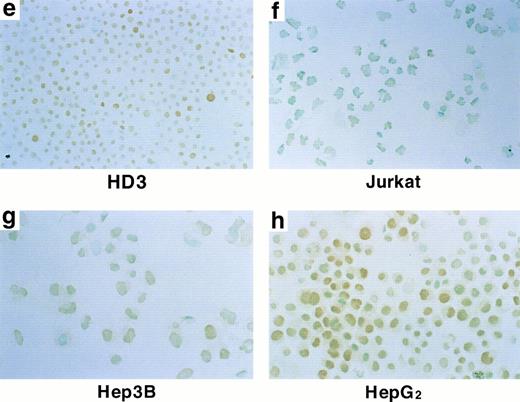
Human hepatoma cell lines Hep3B and HepG2 can be induced to produce large amounts of biologically active and immunologically identifiable Epo in response to hypoxia, as well as cobalt.1 2 Furthermore, on such stimulation, markedly increased levels of Epo mRNA have been observed. Therefore, the expression of hGATA transcription factors was examined in Hep3B and HepG2 cells by Northern blotting analysis. Hep3B cells express hGATA-2 and -3 transcription factors (Fig 1B), whereas HepG2 cells express hGATA-2 (Fig 1C). To determine whether Hep3B and HepG2 cells express hGATA factor proteins, immunohistochemical staining was performed. UT7/Epo, HD3, and MSB1 cells were used as positive controls for hGATA-1, -2, and -3 transcription factors, respectively (Fig 3a, e, and i). and Jurkat cells were used as a negative control for hGATA-1 and -2 (Fig 3b and f ). hGATA-2 was abundantly expressed within the nucleus of Hep3B and HepG2 cells (Fig 3g and h). Magnified photographs showed that Hep3B and HepG2 cells were positive staining (Fig 4g and h). However, hGATA-1 and -3 were not expressed (Fig 3c, d, j, and k), suggesting that hGATA-2 negatively regulates the Epo gene in Hep3B and HepG2 cells.
Expression of the hGATA proteins. (A) Immunohistochemical analysis of hGATA-1 expression in four cell lines (UT7/Epo [a], Jurkat [b], Hep3B [c], and HepG2 [d]). hGATA-1 was abundantly expressed within the nucleus of UT7/Epo (a), whereas expression was not detected in control cells (Jurkat [b]). Hep3B (c) and HepG2 (d) showed undetectable staining. (B) Immunohistochemical analysis for hGATA-2 in four cell lines (HD3 [e], Jurkat [f], Hep3B [g], and HepG2 [h]). hGATA-2 was abundantly expressed within the nucleus of HD3 (e), whereas expression was not detected in control cells (Jurkat [f]). Hep3B (g) and HepG2 (h) showed positive staining. (C) Immunohistochemical analysis for hGATA-3 in three cell lines (MSB1 [i], Hep3B [j], and HepG2 [k]). hGATA-3 was expressed within the nucleus of MSB1 (i), whereas expression was not detected in Hep3B (j) and HepG2 (k).
Expression of the hGATA proteins. (A) Immunohistochemical analysis of hGATA-1 expression in four cell lines (UT7/Epo [a], Jurkat [b], Hep3B [c], and HepG2 [d]). hGATA-1 was abundantly expressed within the nucleus of UT7/Epo (a), whereas expression was not detected in control cells (Jurkat [b]). Hep3B (c) and HepG2 (d) showed undetectable staining. (B) Immunohistochemical analysis for hGATA-2 in four cell lines (HD3 [e], Jurkat [f], Hep3B [g], and HepG2 [h]). hGATA-2 was abundantly expressed within the nucleus of HD3 (e), whereas expression was not detected in control cells (Jurkat [f]). Hep3B (g) and HepG2 (h) showed positive staining. (C) Immunohistochemical analysis for hGATA-3 in three cell lines (MSB1 [i], Hep3B [j], and HepG2 [k]). hGATA-3 was expressed within the nucleus of MSB1 (i), whereas expression was not detected in Hep3B (j) and HepG2 (k).
hGATA-1, -2 and -3 Transcription Factors Specifically Bind to the GATA Element in the Human Epo Gene Promoter
Constructs of hGATA-1, -2, and -3 transcription factors were transfected into QT6 cells by CaPO4 precipitation. Binding of proteins from nuclear extracts of QT6 cells transfected with hGATA transcription factors was assessed by a gel mobility shift assay (Fig 5). DNA-protein interactions in nuclear extracts of cells transfected with hGATA-1, -2 and -3 and in Hep3B cells (Fig 5A circle, triangle) were identified as retarded complexes using the GATA element as a probe. The addition of nonradiolabeled GATA element oligonucleotide showed that these DNA-protein interactions were specific (circle, triangle in Fig 5A and B). Furthermore, 150-fold molar excess of unlabeled irrelevant oligonucleotide and of one of the mutant GATA oligonucleotides were not able to compete away these complexes. To further clarify that these retarded bands show GATA transcription factors, mutated probes of GATA element (AGATAA → AGCGAA, or CGCGAT) have been used as probes. These results showed no retarded bands (data not shown). RT-PCR was performed on QT6 cells transfected with hGATA transcription factors and showed that hGATA-1, -2, and -3 transcription factors were expressed in each transfection (data not shown). These experiments showed that hGATA-1, -2, and -3 transcription factors specifically bind to the GATA element of the human Epo gene promoter.
Gel mobility-shift assay in transfected QT6 cells with GATA transcription factors. (A) Gel mobility-shift assays were performed using 2.5 μg of protein from QT6 cells (lanes 2 and 3), hGATA-1–transfected QT6 cells (lane 4), hGATA-1–transfected QT6 cells under 1% O2 for 24 hours (lane 5), hGATA-2–transfected QT6 cells (lane 6), hGATA-2–transfected QT6 cells under 1% O2 for 24 hours (lane 7), hGATA-3–transfected QT6 cells (lane 8), hGATA-3–transfected QT6 cells under 1% O2 for 24 hours (lane 9), Hep3B cells (lane 10), and Hep3B cells under 1% O2 for 24 hours (lane 11). The position of the complex is noted with a triangle (upper band) and a circle. (B) Gel mobility-shift assay was repeated using competitor for GATA element. A total of 300 ng (6 μL; 150-fold molar excess) of competitor DNA was added to each reaction mixture.
Gel mobility-shift assay in transfected QT6 cells with GATA transcription factors. (A) Gel mobility-shift assays were performed using 2.5 μg of protein from QT6 cells (lanes 2 and 3), hGATA-1–transfected QT6 cells (lane 4), hGATA-1–transfected QT6 cells under 1% O2 for 24 hours (lane 5), hGATA-2–transfected QT6 cells (lane 6), hGATA-2–transfected QT6 cells under 1% O2 for 24 hours (lane 7), hGATA-3–transfected QT6 cells (lane 8), hGATA-3–transfected QT6 cells under 1% O2 for 24 hours (lane 9), Hep3B cells (lane 10), and Hep3B cells under 1% O2 for 24 hours (lane 11). The position of the complex is noted with a triangle (upper band) and a circle. (B) Gel mobility-shift assay was repeated using competitor for GATA element. A total of 300 ng (6 μL; 150-fold molar excess) of competitor DNA was added to each reaction mixture.
hGATA-1, -2 and -3 Transcription Factors Decrease Expression of Epo mRNA in Hep3B Cells
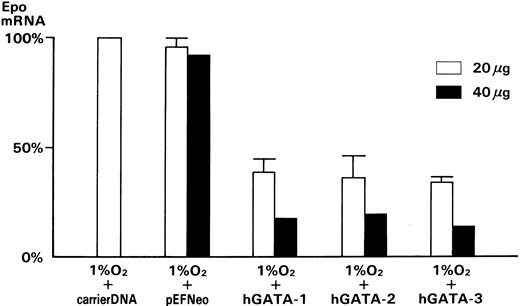
To examine the effect of hGATA transcription factors on Epo mRNA in Hep3B cells, hGATA-1, -2, or -3 expression plasmids were transfected into Hep3B cells by electroporation. The level of Epo mRNA was then measured in the transfected Hep3B cells by competitive PCR. We determined that 80 fg of Epo mRNA/μg of RNA was at the limit of dectability in our assay. The measured change in the amount of Epo mRNA following CoCl2 treatment or hypoxia was a 20-fold or 100-fold increase, respectively. These results showed that 20 μg of hGATA-1, -2, and -3 transcription factors decreased the expression level of Epo mRNA, to 38.3% ± 6.4%, 36.1% ± 9.8% and 33.8% ± 2.4%, respectively as compared with Epo mRNA levels after incubation in 1% O2 with transfection of carrier DNA only (Fig 6, □). A total of 40 μg of hGATA-1, -2, and -3 transcription factors further decreased the expression levels of Epo mRNA to 18.2%, 19.5%, and 14.5% respectively as compared with Epo mRNA level after incubation in 1% O2 with transfection of carrier DNA only (Fig 6, ▪). pEFNeo, which has vector only without hGATA transcription factors, showed 95.8% ± 3.7% (20 μg), 92.5% (40 μg), respectively as compared with Epo mRNA level after incubation in 1% O2 with transfection of carrier DNA only (Fig 6). We performed RT-PCR and Northern blotting analysis on Hep3B cells transfected with hGATA transcription factors and demonstrated that hGATA-1, -2, and -3 factors were expressed in each transfection (data not shown).
Effect of the GATA transcription factors on Epo mRNA expression in Hep3B cells. The level of Epo mRNA was measured by competitive PCR as described in the experimental procedures. These results were normalized to the level of Epo mRNA in Hep3B cells incubated in 1% O2 with transfection of carrier DNA only: (□), 20 μg of DNA (n = 4); (▪), 40 μg of DNA (n = 1).
Effect of the GATA transcription factors on Epo mRNA expression in Hep3B cells. The level of Epo mRNA was measured by competitive PCR as described in the experimental procedures. These results were normalized to the level of Epo mRNA in Hep3B cells incubated in 1% O2 with transfection of carrier DNA only: (□), 20 μg of DNA (n = 4); (▪), 40 μg of DNA (n = 1).
hGATA-1, -2, and -3 Transcription Factors Inhibit the Activity of the Epo Promoter
To further clarify the negative regulation of human Epo gene expression by hGATA transcription factors, cis-elements of the Epo gene fused with a reporter gene (GH) were transfected into Hep3B cells by electroporation. These constructs were made as described previously10 (Fig 2A and B). In Fig 2, 5A is an 1,192-bp HindIII-Xba I fragment that extends from 378 bp upstream of the Epo CAP site through the first exon and the entire first intron. To obviate the problem of false initiation from the Epo ATG start codon, this site was mutated to TAG by site-directed mutagenesis. In Fig 2, 3A is a 255-bp Acc I-Bgl II fragment that extends 67 bp upstream from the Epo termination codon and covers much of the 3′ noncoding region and is homologous with the mouse Epo gene (Fig 2A). We used GH as a reporter gene with the mouse metallothionein I promoter, XGH; and 5AXGH has 5A upstream of the mouse metallothionein I promoter, whereas 5AXGH3Ac has 3A in the correct orientation downstream of the GH gene (Fig 2B). Hep3B cells were transfected with each of Epo GH constructs, hGATA transcription factors, as well as RSVCAT as an internal standard. At the end of a 4-day incubation with 1% O2 or 21% O2 (control), GH was measured in the cell media, and the cell pellet was assayed for CAT. The CAT normalized results for each construct under normoxic and hypoxic conditions with or without hGATA overexpression are shown in Table 1. These results are summarized in Table 2 and showed that the hGATA-1 transcription factor significantly inhibited the activity of the Epo promoter in the presence of 5A or of 5A and 3Ac to 41.2% ± 20.2% and 17.8% ± 7.5%, respectively (Table 2). The hGATA-2 transcription factor also inhibited the activity of the Epo promoter in the presence of 5A or of 5A and 3Ac to 26.3% ± 9.7% and 34.6% ± 20.0%, respectively (Table 2). Furthermore, the hGATA-3 transcription factor inhibited the activity of the Epo promoter in the presence of 5A or of 5A and 3Ac to 34.4% ± 21.3% and 30.0% ± 21.4%, respectively (Table 2).
Antisense Oligonucleotide for hGATA-2 Transcription Factor Increases Epo Protein in Hep3B Cells
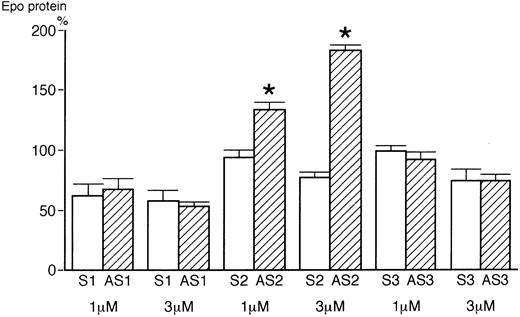
To further clarify that hGATA-2 negatively regulates Epo gene in Hep3B cells, sense and antisense oligonucleotides for hGATA-1, -2, and -3 transcription factors were added to Hep3B cells under 1% O2 for 24 hours without serum. After incubation, the supernatant was assayed for Epo protein by EIA. 1 μmol/L of sense and antisense for hGATA-1 showed 63.0% ± 9.0% and 67.4% ± 9.3% compared with the control (Hep3B cells without oligonucleotide under 1% oxygen) (Fig 7). A total of 3 μmol/L of those for hGATA-1 showed 59.3% ± 7.0%, 53.7% ± 3.5%. A total of 1 μmol/L or 3 μmol/L of sense and antisense for hGATA-3 showed 99.5% ± 2.6%, 93.6% ± 2.6%, 74.2% ± 9.8% and 74.9% ± 5.6%. These results showed no significant difference. However, antisense for hGATA-2 showed 133.5% ± 7.0% (1 μmol/L) and 183.8% ± 4.2% (3 μmol/L), on the other hand sense for hGATA-2 showed 94.4% ± 5.3% (1 μmol/L) and 77.0% ± 4.4% (3 μmol/L). These results clearly showed that hGATA-2 transcription factor negatively regulated the Epo gene in Hep3B cells.
Effect of sense and antisense oligonucleotides for hGATA transcription factors on Hep3B cells. 1 μmol/L or 3 μmol/L of sense and antisense oligonucleotides for hGATA-1, -2, and -3 transcription factors were added to Hep3B cells under 1% O2 for 24 hours without serum. After incubation, the supernatant was assayed for Epo protein by EIA. These results were normalized to the level of Epo protein in Hep3B cells incubated in 1% O2 without oligonucleotide. Bars represent the mean ± 1 SD of four separate experiments. *P < .001 compared with sense (by the Student's t-test).
Effect of sense and antisense oligonucleotides for hGATA transcription factors on Hep3B cells. 1 μmol/L or 3 μmol/L of sense and antisense oligonucleotides for hGATA-1, -2, and -3 transcription factors were added to Hep3B cells under 1% O2 for 24 hours without serum. After incubation, the supernatant was assayed for Epo protein by EIA. These results were normalized to the level of Epo protein in Hep3B cells incubated in 1% O2 without oligonucleotide. Bars represent the mean ± 1 SD of four separate experiments. *P < .001 compared with sense (by the Student's t-test).
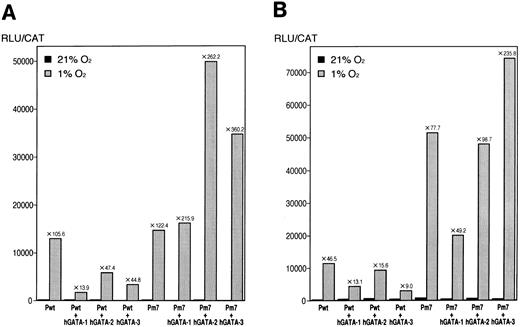
Effect of hGATA-1, hGATA-2, and hGATA-3 transcription factors on induction of the wild-type and the mutated GATA of the Epo promoter with luciferase reporter in Hep3B cells. (A) Experiment no. 1. (B) Experiment no. 2. The results of total relative light units per CAT units are shown. Numbers above the bars indicate the hypoxic induction.
Effect of hGATA-1, hGATA-2, and hGATA-3 transcription factors on induction of the wild-type and the mutated GATA of the Epo promoter with luciferase reporter in Hep3B cells. (A) Experiment no. 1. (B) Experiment no. 2. The results of total relative light units per CAT units are shown. Numbers above the bars indicate the hypoxic induction.
Mutation of the GATA Sequence of the Epo Promoter Interferes With the Inhibition of the Epo Promoter Activity by hGATA-1, -2, and -3 Transcription Factors
To understand the regulation mechanism of the Epo promoter by hGATA transcription factors, reporter constructs (luciferase) that contain a mutated GATA sequence were prepared. Pwt (V2-Ewt-Pwt-PXP2 ) was wild-type plasmid in which the 126-bp 3′ Epo enhancer and the 117-bp Epo promoter were placed upstream of the luciferase gene PXP2 . To investigate the effect of GATA transcription factors, GATA sequence of the Epo promoter in Pwt was mutated (AGATAACAG → ATATAAAAG) and named as Pm7 (V3-Ewt-Pm7-PXP2 ). Therefore, GATA transcription factors cannot bind to this mutant; however, TFIID can bind to this TATA. Transient transfection of Pwt showed 105.6-fold (Exp 1) and 46.5-fold (Exp 2) induction by hypoxia, as shown in Table 3. hGATA-1 transcription factor significantly inhibited the hypoxic induction to 13.9-fold (Exp 1) and 13.1-fold (Exp 2). Compared with Pwt only, hGATA-1 inhibited Epo promoter activity to 13.2% (Exp 1) and 28.2% (Exp 2). As shown in Table 3, hGATA-2 and hGATA-3 transcription factors also inhibited hypoxic induction to 47.4-fold (hGATA-2: Exp 1, 44.9% compared with Pwt), 15.6-fold (hGATA-2: Exp 2, 33.5% compared with Pwt), 44.8-fold (hGATA-3: Exp 1, 42.4% compared with Pwt), and 9.0-fold (hGATA-3: Exp 2, 19.4% compared with Pwt), respectively. These results coincide with the Epo-GH constructs and are summarized as shown in Fig 8. However, transient transfection of the mutated GATA (Pm7) showed 122.4-fold (Exp 1) and 77.7-fold (Exp 2) induction by hypoxia. The hypoxic inductions of them are increased compared with wild-type, Pwt. Furthermore, hGATA-1, -2, and also -3 transcription factors significantly interfered with the inhibition of Epo promoter activity, 215.9-fold (hGATA-1, Exp 1), 49.2-fold (hGATA-1, Exp 2), 262.2-fold (hGATA-2, Exp 1), 98.7-fold (hGATA-2, Exp 2), 360.2-fold (hGATA-3, Exp 1), and 235.8-fold (hGATA-3, Exp 2) induction by hypoxia. These results are also summarized, as shown in Fig 8. These results clearly showed that hGATA transcription factors bind to the GATA sequence of the Epo promoter, inhibit the activity of the Epo promoter, and, finally, regulate Epo gene expression, negatively.
DISCUSSION
A comparison of the sequences of mouse and human Epo genes provides insight into candidate regulatory cis elements. An equivalent degree of homology exists in three stretches of noncoding sequence: (1) the 140-bp region upstream of the transcription start site, (2) two segments within the first intron, and (3) a fragment extending 100 to 220 bp downstream of the translation termination codon. We previously have shown, using GH reporter gene constructs and Hep3B cells, the presence of promoter and enhancer elements within the 5′-flanking region, the first intron, and the 3′-flanking region of the human Epo gene that are responsive to both hypoxia and cobalt treatment.10 However, the effects of these regions were weaker than expected, suggesting that these regulatory regions include both positive and negative regulatory elements.
To investigate positive and negative regulatory elements within the Epo gene in more detail, synthetic oligonucleotides were designed to control Epo transcription by means of an antigene strategy. By this method, we recently showed that the CACCC elements at −60 bp from the CAP site are positive regulatory elements of the Epo gene, whereas the GATA element at −30 bp is a negative regulatory element.5
A hypoxia-inducible enhancer was defined in the 3′-flanking sequence of the Epo gene.17,22-28 This hypoxia-inducible enhancer is functionally tripartite, with the first two sites essential for hypoxia inducibility and a third site functioning to amplify the induction signal.25 A hypoxia induced DNA-binding protein (HIF-1), that binds specifically to site of the hypoxia-inducible enhancer, was identified.25 The promoter also contains an element that responds to hypoxia and cooperates with the enhancer element to faithfully reproduce the transcriptional induction seen in vivo.17 The regions of the promoter and enhancer that are essential for the hypoxic induction of Epo transcription contain steroid receptor response elements.17
One of the orphan nuclear receptors, hepatic nuclear factor-4 (HNF-4) and EAR3/COUP-TF1, bound specifically to the steroid receptor response elements in the Epo promoter and enhancer.28 HNF-4 enhances hypoxic induction, and EAR3/COUP-TF1 negatively regulates hypoxic induction by competing with HNF-4 for binding to the Epo gene.26 The relative levels of HNF-4 and the COUP family members may control the fine tuning of Epo expression in response to hypoxia.
Similar fine tuning of Epo production at the GATA box-containing promoter of Epo gene has also been proposed.6 Aird et al6 investigated the function of GATA box-containing promoters in vitro and showed that the TATA-binding protein of TFIID was required for initiation of transcription from these GATA box-containing promoters. They showed that initiation of in vitro transcription from GATA box-containing core promoters, including that of the mouse Epo gene, can be inhibited by GATA protein.6 However, thus far, there have been no data showing that GATA transcription factors inhibit Epo gene expression. The DNA-binding protein GATA has been found to be a positive-acting transcription factor known to regulate most or all erythroid cell-specific genes.29,30 However, the mouse albumin gene enhancer has been shown to contain a GATA element that acts as a negative regulatory element.31 This study clearly demonstrates that expression of hGATA-1, -2, and -3 transcription factors significantly inhibits Epo mRNA expression in Hep3B cells. This decrease in expression may be due to inhibition of Epo promoter activity by hGATA-1, -2, and -3 transcription factors. In Hep3B and HepG2 cells, which regulate Epo protein and mRNA expression in response to hypoxia and CoCl2 , the hGATA-2 transcription factor may bind the GATA element in the human Epo gene promoter, negatively regulating Epo gene expression. Because Hep3B cells already express hGATA-2, this additional decrease in Epo expression after transient transfection of the hGATA-2 plasmid may be caused by an increase in hGATA-2 expression. The results of transient transfection of Epo-GH and also Epo-luciferase significantly demonstrated that the Epo promoter activity was inhibited by the overexpression of hGATA transcription factors. The transient transfection of the mutated GATA of the Epo promoter with luciferase construct clearly showed that the inhibition of the Epo promoter activity by hGATA transcription factors was interfered (Table 3), furthermore, hGATA transcription factors stimulate the Epo promoter activity in the transient transfection of the mutated GATA. Because TFIID can bind to the mutated GATA (TATA) sequence more tightly than the GATA sequence, it is tempting to speculate that hGATA transcription factors may bind TFIID or another associated molecule. Further study of the overexpression of mutant forms of GATA transcription factors that either are unable to bind to DNA or lose their capacity to transduce a transcriptional signal needs to be clarified.
ACKNOWLEDGMENT
We thank H. Franklin Bunn, M.A. Goldberg, J. Fandrey, W. Jelkmann, and J.D. Engel for helpful suggestions and discussions, and H. Motohashi and H. Harigae for immunohistchemical staining. We thank Deborah L. Galson for providing Pwt and Pm7. We thank Motoko Yoshida for preparing this manuscript, Kyoko Kubo and Fumie Saotome for expert technical assistance.
Supported by grants-in-aid for scientific research from the Ministry of Education, Science and Culture of Japan, the Uehara Memorial Foundation, Tokyo, Japan; the Yamanouchi Foundation, Tokyo, Japan; the Ichiro Kanehara Foundation, Tokyo, Japan; and the Chugai Foundation, Tokyo, Japan.
Address reprint requests to Shigehiko Imagawa, MD, PhD, Division of Hematology, Department of Medicine, Jichi Medical School, Minamikawachi-machi, Tochigi-ken, 329-04, Japan.

![Fig. 3. Expression of the hGATA proteins. (A) Immunohistochemical analysis of hGATA-1 expression in four cell lines (UT7/Epo [a], Jurkat [b], Hep3B [c], and HepG2 [d]). hGATA-1 was abundantly expressed within the nucleus of UT7/Epo (a), whereas expression was not detected in control cells (Jurkat [b]). Hep3B (c) and HepG2 (d) showed undetectable staining. (B) Immunohistochemical analysis for hGATA-2 in four cell lines (HD3 [e], Jurkat [f], Hep3B [g], and HepG2 [h]). hGATA-2 was abundantly expressed within the nucleus of HD3 (e), whereas expression was not detected in control cells (Jurkat [f]). Hep3B (g) and HepG2 (h) showed positive staining. (C) Immunohistochemical analysis for hGATA-3 in three cell lines (MSB1 [i], Hep3B [j], and HepG2 [k]). hGATA-3 was expressed within the nucleus of MSB1 (i), whereas expression was not detected in Hep3B (j) and HepG2 (k).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1430/4/m_bl_0005f3.jpeg?Expires=1769573667&Signature=5FRFnLn1BKWYQc1FsH75m7gcFIKYkTaBLtgpTT6C~yf7OtnsNMdCysXKes9ApAQs5IKTQdfvpcWvDsScu9WsaOdRBjj122K9O1OmDtAyG4w1Si6WeTTghrjf2gl-A8iBLGkGX9BDTqyqqMENviy04wxSXuUqvIX5VR95XPmGUP0ejGygLhAGilUrsG00h4nlOICHN1-9WcyCMSf5N0q1nG9JMey5Sx~cd-ldRr4qBqkRB~QWpeFc4NRzPBmnsNQxuk8AEFwzOPwGGVbhyE85A0~vJghFwOfOEBxEN43cPC-Wk3qP5m~odNTZIo5h3JS37MOAhgBm46ffZfOlCgpWZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)