Abstract
Adhesive interactions between CD34+ hematopoietic progenitor cells (HPC) and bone marrow stroma are crucial for normal hematopoiesis, yet their molecular bases are still poorly elucidated. We have investigated whether cell surface proteoglycan CD44 can mediate adhesion of human CD34+ HPC to immobilized hyaluronan (HA), an abundant glycosaminoglycan of the bone marrow extracellular matrix. Our data show that, although CD34+ cells strongly express CD44, only 13.3% ± 1.1% spontaneously adheres to HA. Short-term methylcellulose assay showed that HA-adherent CD34+ cells comprised granulo-monocytic and erythroid committed progenitors (19.6% ± 2.5% and 7.3% ± 1.0% of the input, respectively). More primitive progenitors, such as pre–colony-forming units, also adhered to HA. Moreover, we found that CD44-mediated adhesion of CD34+ cells to HA could be enhanced by phorbol 12-myristate 13-acetate (PMA), the function-activating anti-CD44 monoclonal antibody H90, and cytokines such as granulocyte-monocyte colony-stimulating factor, interleukin-3 (IL-3), and stem cell factor. Enhancement through PMA required several hours, was protein-synthesis–dependent, and was associated with an increase of CD44 cell surface expression, whereas stimulation of adhesion by H90 monoclonal antibody and cytokines was very rapid and without alteration of CD44 expression. H90-induced activation occurred at 4°C and lasted for at least 2 hours, whereas activation by cytokines required incubation at 37°C and was transient. These data, which show for the first time that CD34+ HPC can directly adhere to HA via CD44, point out that this adhesive interaction to HA is a process that may also be physiologically regulated by cytokines.
IN NORMAL ADULTS, hematopoietic progenitor cells (HPC) reside in the bone marrow, where they nest within a specific stroma that controls their proliferation and differentiation along different hematopoietic lineages.1 Various cellular elements of the stroma together with their biosynthetic products, which include extracellular matrix components and cytokines, constitute the hematopoietic microenvironment of the bone marrow. Although the function of cytokines in the regulation of hematopoiesis has been extensively investigated, the role of adhesive interactions is still poorly understood. One important function of adhesive receptors is the retention of HPC in the bone marrow. In addition, adhesive receptors may also be involved in the regulation of HPC proliferation and development because, in murine and human long-term bone marrow cultures, the addition of function-blocking monoclonal antibodies (MoAbs) to adhesive receptors such as CD44 and VLA-4 leads to a profound inhibition of hematopoiesis.2-4
A number of adhesive receptors are expressed on the surface of CD34+ HPC, such as integrins α2β1 (VLA-2), α4β1 (VLA-4), α5β1 (VLA-5), α6β1 (VLA-6),5,6 and αLβ2 (LFA-1)3,7; the proteoglycan CD443,7,8; CD36; L-selectin; and galactosyl- or mannosyl-containing homing receptors.1 However, on freshly isolated CD34+ HPC, most of these receptors and particularly integrins are expressed in an inactive, ligand nonbinding form. Integrins can be activated into a ligand-binding form by the phorbol ester phorbol 12-myristate 13-acetate (PMA),9,10 function-activating MoAbs,11-14 cross-linking of antigens belonging to an Ig family such as CD31,15 and mitogenic cytokines.6 16
To date, few studies have dealt with the involvement of CD44 in the interaction between CD34+ HPC and the bone marrow stroma and in the regulation of normal hematopoiesis. CD44 has been reported to be the principal receptor for hyaluronan (HA),17,18 a glycosaminoglycan abundantly present in the bone marrow extracellular matrix.19,20 However, many cells expressing the CD44 molecule do not bind HA.21,22 As an example, HA-binding by normal T lymphocytes and several T-lymphoma cells required activation by certain MoAbs to CD44 or by phorbol ester.22-25 It was also reported that IL-5 could induce B lymphocytes to bind HA via CD44.26 CD44 is constitutively active in many lymphoid cell lines,23,24 whereas it is inactive and refractory to phorbol ester and activating MoAb treatments in myeloid cell lines, with the exception of KG1 and KG1a.20,22,23 27
Concerning normal CD34+ HPC, it has been shown that CD44 might interact with integrin VLA-4 in the mediation of CD34+ HPC adhesion to fibronectin,28 but it is still unknown whether CD44 could directly mediate adhesion of CD34+ HPC to HA. We report here that CD44, although strongly expressed on all CD34+ HPC, could only mediate adhesion to HA of a small subset of CD34+ HPC, which comprise granulo-monocytic and erythroid committed progenitors, as well as more primitive progenitors (pre–colony-forming units [pre-CFU]). In addition, we showed that the CD44-mediated adhesion of CD34+ cells to HA could be increased by phorbol ester, a function-activating MoAb to CD44, and cytokines.
MATERIALS AND METHODS
MoAbs
The J173 anti-CD44 MoAb (IgG1) was obtained from Immunotech (Marseille-Luminy, France). For flow cytometry, the J173 and anti–HLA-DRII MoAbs (IgG2a) were fluorescein isothiocyanate (FITC)-conjugated, and anti-CD38 (Leu-17, IgG1) and anti-CD34 (HPCA-2, IgG1) MoAbs were phycoerythrin (PE)-conjugated; all were provided by Becton Dickinson (San Jose, CA). Murine IgG1 and IgG2a conjugated with either FITC or PE were made available by Immunotech. The function-activating anti-CD44 MoAb H90 was kindly donated by Dr A. Bernard (Nice, France).29
Chemicals and Cytokines
Human umbilical cord HA (ref. 53730) was obtained from Fluka (Mulhouse, France). PMA, cycloheximide, phosphate-buffered saline without calcium or magnesium (PBS), bovine serum albumin (BSA), 2-mercaptoethanol (2-ME), and Streptomyces hyaluronidase came from Sigma (St Louis, MO). Na512CrO4 was purchased from NEN (Wilmington, DE). Recombinant human stem cell factor (SCF ), granulocyte-monocyte colony-stimulating factor (GM-CSF ), and IL-3 originated from Amgen Biologicals (Thousand Oaks, CA).
Normal Bone Marrow CD34+ Cells
Hematopoietic cells were extracted from normal bone marrow by grounding the trabeculae with a potter and vortexing the samples several times in PBS. Low-density mononuclear cells were separated by density gradient centrifugation on Ficoll-hypaque density 1.077 g/mL (Pharmacia LKB, Uppsala, Sweden) and resuspended in RPMI 1640 plus 10% fetal calf serum (FCS). Mature monocytes were removed by adhesion on plastic for 2 hours, and after two washes in PBS, the most differentiated precursors and stromal cells were discarded by adhesion on soy bean agglutinin CELLector flasks (Applied Immune Sciences Inc, Menlo Park, CA). CD34+ cells were finally recovered by adhesion on ICH3 anti-CD34 MoAb-coated CELLector flasks (Applied Immune Sciences Inc), according to the manufacturer's instructions. The purity of CD34+ cells was checked by flow cytometric analysis. All samples contained more than 95% CD34+ cells. To assess that the adhesive functions of CD44 were not altered by the CD34+ cell purification procedure, we verified that cell labeling with an anti-CD34 MoAb did not change the adhesive function of CD44 to immobilized HA. We also checked that the purification procedure did not alter the expression level of CD44.
Immunostaining and Flow Cytometry
CD34+ cells were suspended in RPMI 1640 containing 0.2% BSA, referred to as label medium, at 106 cells/mL and then incubated at 4°C for 30 minutes with 2 μg/mL conjugated MoAbs FITC-J173 and PE-Leu17 or FITC-anti-DRII and PE-HPCA-2. After two washes in label medium, MoAb binding was measured by flow cytometry relative to isotype-matched control antibodies by using a Profile II flow cytometer (Coulter Immunology, Hialeah, FL) equipped with a 15-mW argon laser and calibrated using a panel of fluorescent beads (Immunobrite; Coulter). Adjustment of the crossover fluorescence was obtained by compensation of the two single-stained samples to limit superposition of the fluorochrome emission spectra. Forward light scattering and the two fluorescence signals were stored in listmode files. Each measurement was performed on 5,000 cells.
51Cr Adhesion Assay to Immobilized HA
Ninety-six–well tissue culture-treated plates (Costar Corp, Cambridge, MA) were incubated overnight at room temperature with 100 μL of distillated water containing 5 mg/mL HA. Unbound HA was removed by aspiration and replaced with 100 μL of RPMI 1640 plus 3% BSA. After 2 hours at 37°C, plates were washed three times with RPMI 1640 supplemented with 10 mmol/L HEPES, 1 mmol/L sodium pyruvate, and 0.2% BSA, referred to as cell adhesion medium.
CD34+ cells were starved overnight at 37°C, in the absence of cytokines, at 2 × 105 cells/mL in serum-free medium made of Iscove's modified Dulbecco's medium (IMDM) supplemented with 10 mg/mL BSA, 200 μg/mL iron-saturated human transferrin (Sigma), 10 μg/mL insulin, 10 μg/mL low-density lipoproteins, and 10−5 mol/L 2-ME. As previously reported,6 16 we verified that overnight starvation did not significantly alter either cell viability as determined by trypan blue exclusion, by clonogenicity, or the expression level of CD44. Starved CD34+ cells were then washed twice and resuspended in 500 μL cell adhesion medium. They were labeled with 51Cr by incubation with 50 to 100 μCi of Na512CrO4 (10 mCi/mL) for 1 hour at 37°C. Cells were washed three times in cell adhesion medium, resuspended to 2 × 105 cells/mL, and chilled on ice for 10 minutes before the assay. One hundred microliters of the labeled cell suspension was placed in triplicate into HA-coated wells. Cytokines SCF, IL-3, and GM-CSF were added at specified concentrations. The entire procedure was performed on ice. Plates were centrifuged at 1,000 rpm for 5 minutes at 4°C to sediment cells into direct, uniform contact with treated surfaces. Plates were quickly warmed for 2 minutes to 37°C in a water bath before transfer to a humidified incubator at 37°C for the indicated periods of time. Assay medium was removed by aspiration and wells were washed three times by the addition of 150 μL of the cell adhesion medium and vigorous flicking off. After the last wash, cells were lysed in 150 μL 1% sodium dodecyl sulfate (SDS) and 0.1 mol/L NaOH solution. After 10 minutes, lysates were added to 2 mL Optiphase hisafe (Wallac, Milton Keynes, UK) and counted after 10 minutes using a beta counter. Nonspecific cell adhesion was determined in wells treated with BSA. The percentage of adherent cells was determined by dividing the radioactivity in the adherent fraction by the radioactivity contained in 100 μL of the initial labeled cell suspension.
To analyze the effect of anti-CD44 MoAbs on the adhesion of CD34+ cells to immobilized HA, 105 cells (either 51Cr-labeled or unlabeled according to the experimental purpose) were incubated for 15 minutes at 4°C in 500 μL cell adhesion medium containing 5 μg/mL of anti-CD44 MoAbs J173 or H90. Control cells were incubated with 5 μg/mL IgG1. Cells were then processed for adhesion assay as described.
Effect of PMA and of Cycloheximide on the Adhesion of CD34+ Cells to HA
CD34+ (5 × 105) cells were suspended in 1 mL RPM1 1640 medium/10% FCS and incubated for 1, 3, and 16 hours at 37°C with 10−7 mol/L PMA. In specified experiments, cells were treated overnight with 10 μg/mL cycloheximide. After viability determination by trypan blue exclusion, PMA-treated and untreated control cells were washed, labeled with 51Cr, and processed for adhesion assay as described above.
Clonogenic Features of HA-Adherent CD34+ Cells
Adhesion assays were also performed for studying clonogenic properties of cells adhering to HA either spontaneously or after treatment with the function-activating anti-CD44 MoAb H90 or with cytokines. After starvation in serum-free medium deprived of cytokines as described above, CD34+ cells were resuspended in sterile cell adhesion medium at 2 × 104 cells/mL and 100-μL aliquots were seeded in triplicate into HA-coated wells. After incubation at 37°C for 15 minutes, nonadherent cells were removed as described above. Adherent cells were detached by gentle scraping in serum-free medium and processed for short-term methylcellulose and pre-CFU assays.
Short-term methylcellulose assay.Input and HA-adherent CD34+ cells were cultured in 35-mm nontissue culture grade Petri dishes (Falcon; reference 1008) containing 1 mL of complete methylcellulose medium purchased from StemCell Technologies Inc (Vancouver, British Columbia, Canada; 0.8% methylcellulose in IMDM, 30% FCS, 1% deionized BSA, 10−4 mol/L 2-mercaptoethanol). Colony-stimulating factors were provided as 5% of phytohemagglutinin-human leukocyte-conditioned medium (Hemostim H2400; StemCell Technologies Inc). Erythropoietin purified from human urine was added at 3 U/mL (StemCell Technologies Inc). Plates were incubated at 37°C in a fully humidified atmosphere containing 5% CO2 in air. Burst-forming unit-erythroid (BFU-E) and colony-forming unit-granulocytes-monocytes (CFU-GM) were scored at day 16 following the standard criteria, and their number was expressed as the percentage of colonies obtained in the input, ie, cells without adhesion step, as determined in a parallel experiment.
Pre-CFU assay.This assay, which is also referred to in the literature as delta assay, measures after 7 days in liquid culture the generation of nascent CFU-GM from precursors that were initially more primitive than those giving rise to colonies in short-term colony assays in methylcellulose medium.30-32 Briefly, CD34+ cells from the input and from the HA-adherent fractions were recovered as described above and resuspended at 2 × 103 cells/100 μL in IMDM supplemented with 30% FCS, 1% deionized BSA, 10−4 mol/L 2-ME, 3 mmol/L L-glutamine, and IL-1, IL-3, IL-6, GM-CSF, granulocyte colony-stimulating factor (G-CSF ), and SCF, all at 10 ng/mL. Triplicate 100 μL cell suspension cultures were established in 96-well plates. After 7 days at 37°C in the presence of 5% CO2 in air, the content of each well was resuspended and washed in IMDM. Twenty percent of the cells was plated in duplicate in a short-term methylcellulose assay as described above to determine the number of nascent CFU-GM present after 7 days in liquid cultures.16 30-32
Statistical Analysis
Data were expressed as means ± 1 standard error (SE). Means were compared using the Student's t-test. They were considered significantly different when P < .05.
RESULTS
CD44 Mediates Adhesion of CD34+ Cells to HA. The Anti-CD44 MoAb H90 Increases This Adhesion
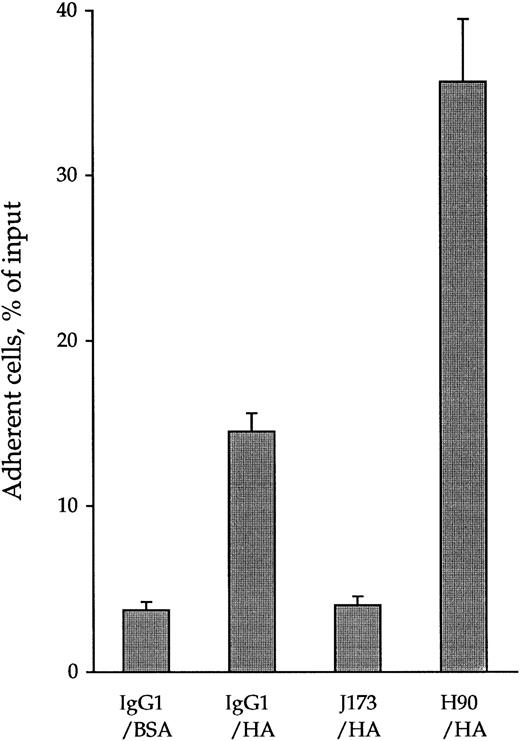
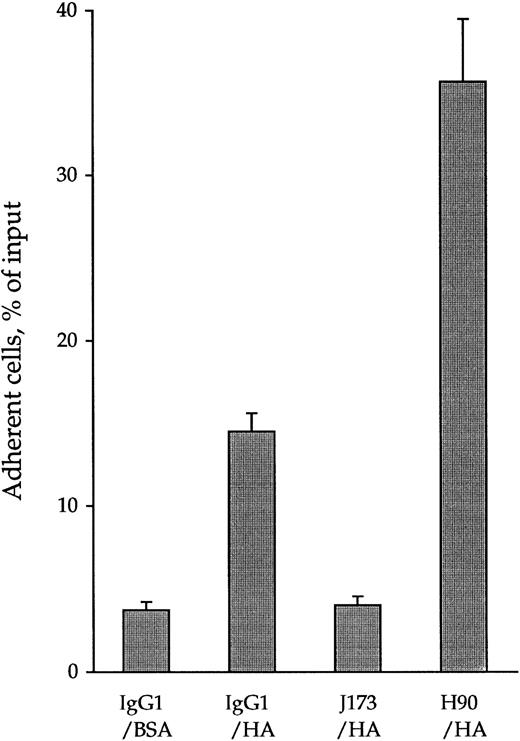
By using 51Cr-labeled cells in quantitative cell adhesion assays, 13.3% ± 1.1% of CD34+ cells were found to spontaneously adhere to immobilized HA (Fig 1). This adhesion reached a plateau after 15 minutes at 37°C and lasted for at least 120 minutes. It was specifically mediated by CD44-HA interaction because J173, an MoAb inhibiting CD44-HA interaction,27 or pretreatment of HA-coated wells with Streptomyces hyaluronidase lowered cell adhesion to levels obtained in control BSA-coated wells (3.7% ± 0.5%). In contrast, treatment of CD34+ cells with H90, an anti-CD44 MoAb recognizing another epitope than J173,27 increased their adhesion up to 35.7% ± 5.7% of the input. This increase reached a plateau after 15 minutes and lasted for at least 120 minutes. This H90-dependent activation of CD44 was not temperature-dependent, because we observed the same increase of CD34+ HPC adhesion to HA when cells were pretreated with H90 at 4°C (data not shown).
Opposite effects of anti-CD44 MoAbs J173 and H90 on spontaneous adhesion of CD34+ cells to immobilized HA. 51Cr-labeled CD34+ cells (2 × 105 cells/mL) were incubated at 4°C for 15 minutes with 5 μg/mL of IgG1, J173, or H90 MoAbs in the cell adhesion medium and put in contact for 15 minutes at 37°C with immobilized HA in 96-well culture plate (100 μL per well, 3 wells per point). After discarding the nonadherent cells by two washes, the adherent cell-associated radioactivity was measured using a β scintillator counter as described in the Materials and Methods. Data, expressed as the percentage of the radioactivity of the input, are the means ± 1 SE calculated from five independent experiments.
Opposite effects of anti-CD44 MoAbs J173 and H90 on spontaneous adhesion of CD34+ cells to immobilized HA. 51Cr-labeled CD34+ cells (2 × 105 cells/mL) were incubated at 4°C for 15 minutes with 5 μg/mL of IgG1, J173, or H90 MoAbs in the cell adhesion medium and put in contact for 15 minutes at 37°C with immobilized HA in 96-well culture plate (100 μL per well, 3 wells per point). After discarding the nonadherent cells by two washes, the adherent cell-associated radioactivity was measured using a β scintillator counter as described in the Materials and Methods. Data, expressed as the percentage of the radioactivity of the input, are the means ± 1 SE calculated from five independent experiments.
Clonogenic Features of CD34+ HPC Adherent to HA, Spontaneously and After H90 MoAb Activation
Short-term methylcellulose assay.We investigated whether CD34+ cells that adhered to immobilized HA contained committed progenitors cloning in a short-term methylcellulose assay. As shown in Table 1, the CD34+ cells spontaneously adherent to HA comprised 19.6% ± 2.5% of CFU-GM and only 7.3% ± 1.0% of BFU-E contained in the input. Pretreatment of CD34+ cell with the MoAb H90, which enhanced adhesion to HA (Fig 1), raised the percentage of HA-adherent BFU-E up to 34.0% ± 2.5% of the input. On the contrary, the proportion of adherent CFU-GM was not significantly altered (Table 1). To verify whether preincubation with the H90 MoAb could have altered the proliferation of clonogenic progenitors, an aliquot of H90-treated CD34+ cells was immediatly seeded in the short-term methylcellulose assay, without prior adhesion to HA. They generated the same number of BFU-E and CFU-GM as the untreated cells (data not shown).
Pre-CFU assay.It has been shown that normal CD34+ cells comprise primitive progenitors designated pre-CFU, which are not clonogenic in short-term methylcellulose assay, but can still generate nascent CFU-GM after 7 days in liquid culture, in the presence of growth factors.16 30-32 We enumerated 760 ± 82 pre-CFU or nascent CFU-GM generated from 2 × 103 HA-adherent CD34+ cells cultured for 7 days in these conditions and 1,033 ± 185 nascent CFU-GM from the same number of nonadherent cells.
Antigenic Profiles of HA-Adherent CD34+ Cells
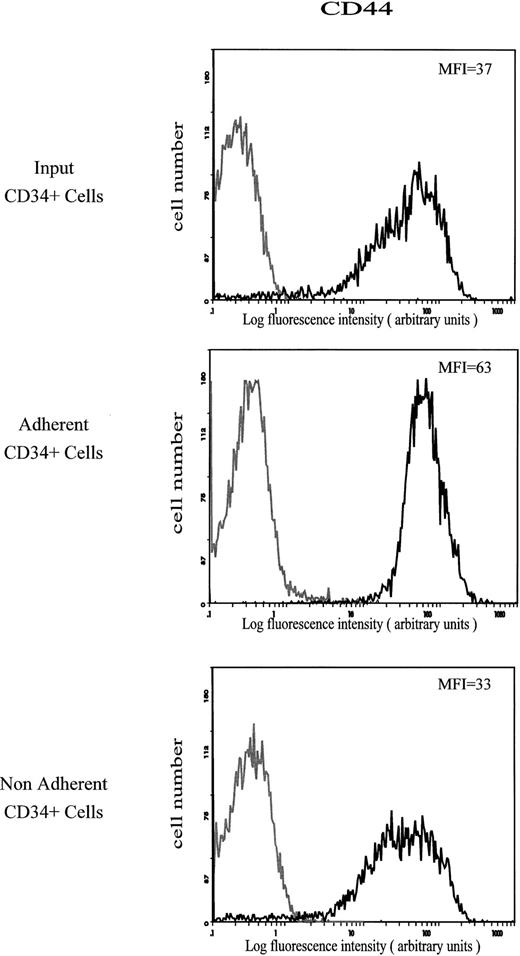
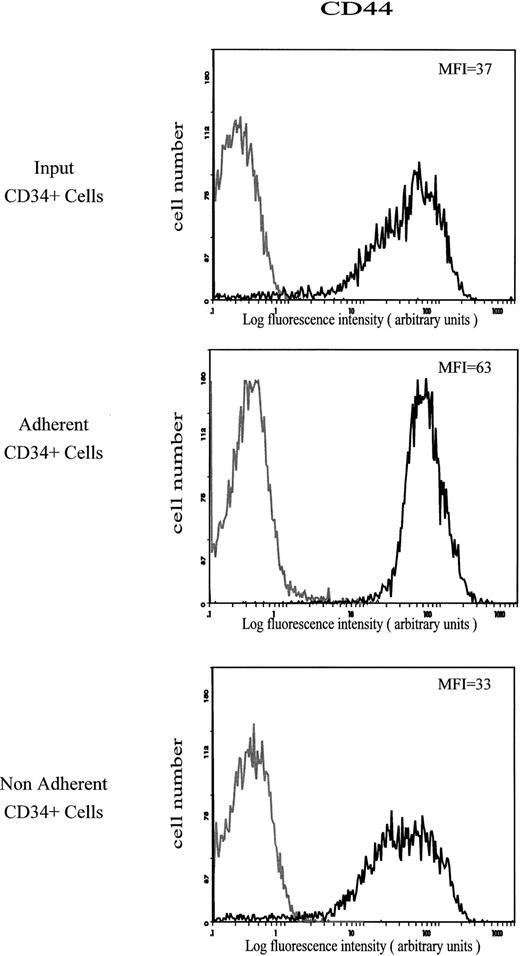
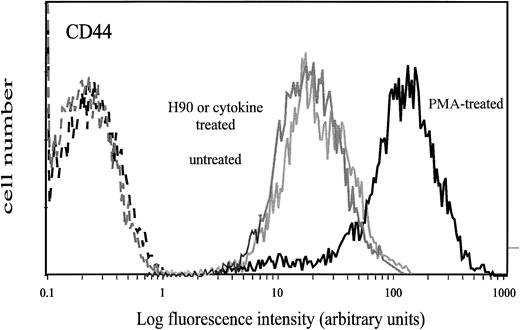
Expression of CD44 antigen.The amount of CD44 on CD34+ cells that adhered spontaneously to HA was characterized by a mean fluorescence intensity (MFI) value of 63. This value was much higher than on the input and on the nonadherent CD34+ cells (MFI of 37 and 33, respectively; Fig 2). The level of CD44 was not modified on CD34+ cells treated with the MoAb H90, which stimulates cell adhesion to HA (Fig 3).
Cell surface expression of CD44 on input, HA-adherent and HA-nonadherent CD34+ cells. CD34+ cells were stained with FITC-conjugated anti-CD44 MoAb J173. Negative controls (grey lines) are cells labeled with FITC-conjugated IgG1. Flow cytometric analysis was performed as described in the Materials and Methods. Data are from a representative experiment. Four independent experiments gave similar results.
Cell surface expression of CD44 on input, HA-adherent and HA-nonadherent CD34+ cells. CD34+ cells were stained with FITC-conjugated anti-CD44 MoAb J173. Negative controls (grey lines) are cells labeled with FITC-conjugated IgG1. Flow cytometric analysis was performed as described in the Materials and Methods. Data are from a representative experiment. Four independent experiments gave similar results.
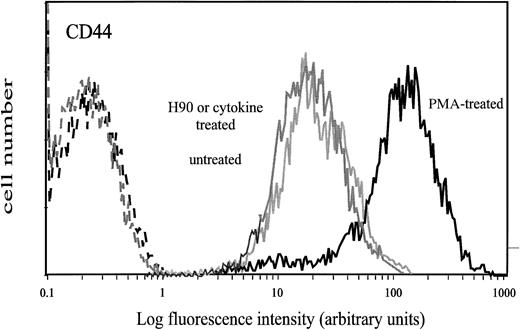
Surface expression of CD44 on CD34+ cells treated with PMA, H90 MoAb, or cytokines. Cells were treated with either 10−7 mol/L PMA for 16 hours, H90 MoAb (5 μg/mL), or cytokines SCF (10 ng/mL), GM-CSF (0.1 ng/mL), or IL-3 (10 ng/mL) for 15 minutes at 37°C as described in the Materials and Methods. Control cells were either untreated cells or cells treated with IgG1. Thereafter, cells were washed and labeled with FITC-conjugated J173 MoAb. Negative controls (dotted lines) were treated cells labeled with FITC-conjugated IgG1. Flow cytometry analysis was performed as described in the Materials and Methods.
Surface expression of CD44 on CD34+ cells treated with PMA, H90 MoAb, or cytokines. Cells were treated with either 10−7 mol/L PMA for 16 hours, H90 MoAb (5 μg/mL), or cytokines SCF (10 ng/mL), GM-CSF (0.1 ng/mL), or IL-3 (10 ng/mL) for 15 minutes at 37°C as described in the Materials and Methods. Control cells were either untreated cells or cells treated with IgG1. Thereafter, cells were washed and labeled with FITC-conjugated J173 MoAb. Negative controls (dotted lines) were treated cells labeled with FITC-conjugated IgG1. Flow cytometry analysis was performed as described in the Materials and Methods.
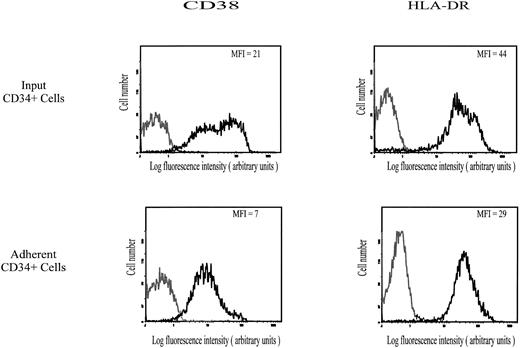
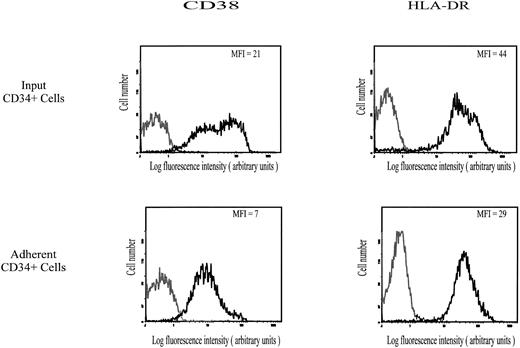
Expression of CD38, HLA-DRII, and CD34 antigens.As shown in Fig 4, expression of CD38 and HLA-DRII antigens on CD34+ cells that spontaneously adhered to HA was characterized by MFI values of 7 and 29, respectively, ie, lower than on the input population (MFI of CD38 and HLA-DRII equal to 21 and 44, respectively). On the contrary, the CD34 level was similar in both populations (data not shown). It is very unlikely that expression of CD38, which may have some HA-binding activity,33 was partly masked by bound HA, because treatment of adherent cells with Streptomyces hyaluronidase did not increase the binding of PE-conjugated anti-CD38 MoAb. Taken together, these data suggest that the subset of CD34+ hematopoietic cells spontaneously adhering to HA may be enriched with CD38lowHLA-DRIIlow cells.
Cell surface expression of CD38 and HLA-DR II on input and HA-adherent CD34+ HPC. CD34+ HPC were stained with MoAbs to CD38 (PE-conjugated) and to HLA-DR (FITC-conjugated). Negative controls (grey lines) are cells labeled with PE-conjugated IgG1 or FITC-conjugated IgG2a. Flow cytometric analysis was performed as described in the Materials and Methods. Data are from a representative experiment. Four independent experiments gave similar results.
Cell surface expression of CD38 and HLA-DR II on input and HA-adherent CD34+ HPC. CD34+ HPC were stained with MoAbs to CD38 (PE-conjugated) and to HLA-DR (FITC-conjugated). Negative controls (grey lines) are cells labeled with PE-conjugated IgG1 or FITC-conjugated IgG2a. Flow cytometric analysis was performed as described in the Materials and Methods. Data are from a representative experiment. Four independent experiments gave similar results.
PMA-Enhanced Adhesion of CD34+ Cells to HA
Cell treatment by PMA has been reported to activate the HA-binding ability of CD44.22,23,25 27 Thus, we incubated CD34+ cells with 10−7 mol/L PMA for 1, 3, and 16 hours before measuring their adhesion to immobilized HA. CD34+ cells treated for 1 hour and 3 hours adhered to HA in the same proportion as untreated cells (about 13%). However, after 16 hours of incubation with PMA, up to 48% ± 7% of CD34+ cells adhered to HA. No significant adhesion was observed on BSA-coated wells. Trypan blue exclusion test showed that more than 95% of the treated cells were viable during the entire experiment. This PMA-induced adhesion to HA required protein synthesis, because it was completely inhibited by cycloheximide, an inhibitor of protein synthesis. It was associated with an increase of CD44 expression (MFI = 110 v 37; Fig 3). As previously observed on untreated CD34+ cells, we noticed that expression of CD44 was stronger on adherent than on nonadherent PMA-treated cells (MFI of 150 and 66, respectively). In contrast, the expression of CD38 and HLA-DRII antigens was not modified by PMA treatment, whereas the expression of CD34 was only slightly increased. Adhesion of PMA-treated CD34+ cells to HA was decreased to 29% ± 3% by the anti-CD44 MoAb J173, which was fully active in inhibiting the spontaneous adhesion of untreated cells (Fig 1). This partial inhibition was not due to an incomplete binding of J173, because all CD34+ cells were brightly labeled by this MoAb (Fig 3) and neither the inhibition of cell adhesion to HA nor the labeling of CD34+ cells was increased by using fourfold more J173 MoAb (20 μg/mL).
Cytokines SCF, GM-CSF, and IL-3 Enhance CD34+ HPC Adhesiveness to HA
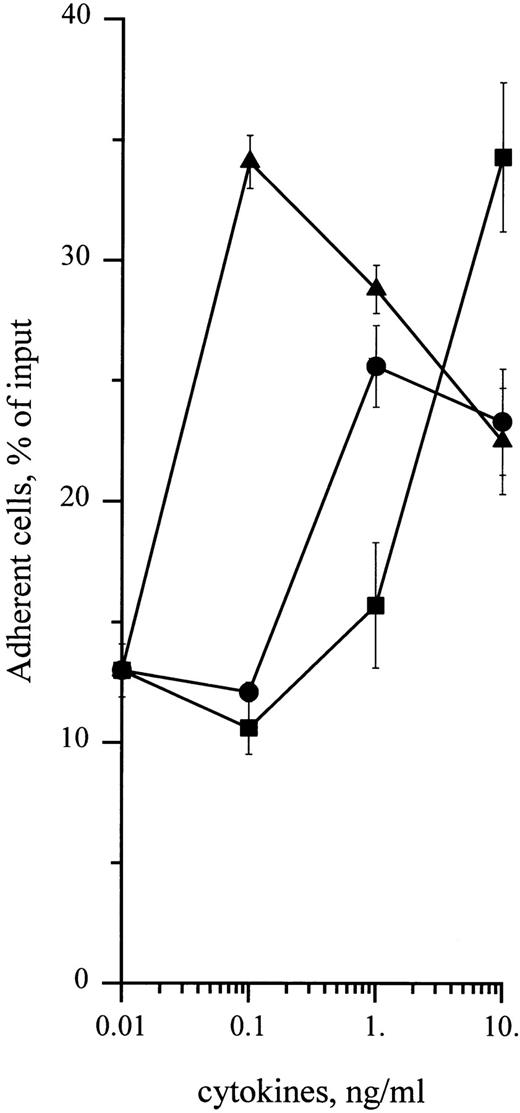
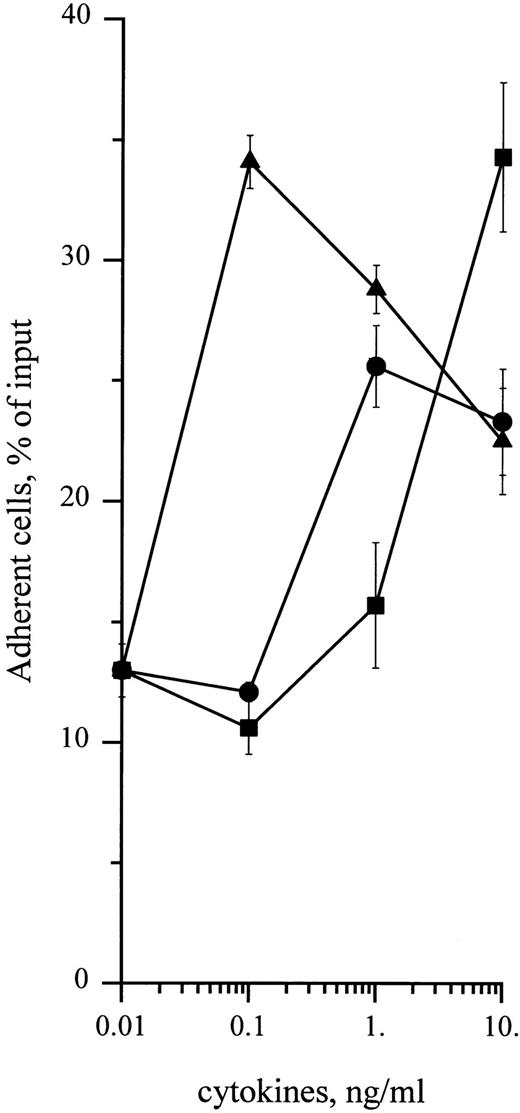
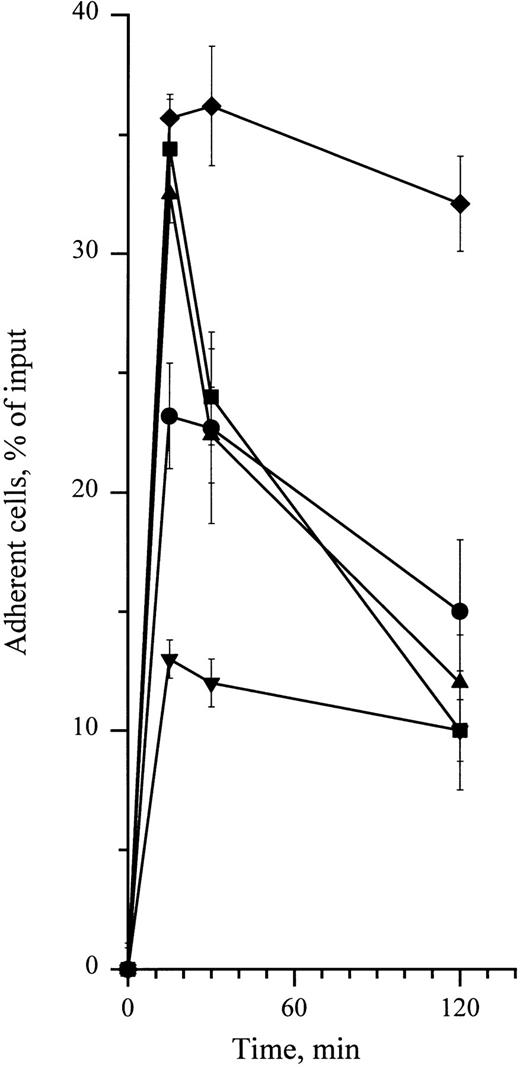
To determine whether cytokines could also enhance adhesion of CD34+ cells to HA, cells were treated with 0.1 to 10 ng/mL of SCF, GM-CSF, or IL-3. As shown in Fig 5, these cytokines enhanced adhesion of CD34+ cells in a dose-dependent manner, with a twofold increase with 10 ng/mL SCF and a threefold increase with 10 ng/mL IL-3 and 0.1 ng/mL GM-CSF. These levels of adhesion in response to cytokines were significantly higher than spontaneous levels (P < .05, Student's t-test). Stimulation of cell adhesion to HA by cytokines was transient and greatly decreased at 30 minutes in the case of IL-3 and GM-CSF and at 120 minutes in the case of SCF (Fig 6). Adhesion stimulated by these three cytokines was fully inhibited in the presence of J173 MoAb, indicating that this process was fully mediated by CD44. However, it was not due to an increase of cell surface CD44 level, which remained unchanged, as shown by flow cytometric analysis (Fig 3). Finally, in the presence of a combination of SCF, IL-3, and GM-CSF at 10 ng/mL each, adhesion of CD34+ cells to HA did not show a higher increase than the one observed with individual cytokines, indicating that these growth factors did not synergize to enhance normal CD34+ cell adhesion. Short-term methylcellulose assay shows that treatment with either SCF, IL-3, or GM-CSF increased the proportion of HA-adherent CFU-GM by about 2.5 times, whereas the percentage of HA-adherent BFU-E was not significantly changed (Table 1).
Dose-dependency of cytokine-stimulating effect on CD34+ HPC adhesion to HA. Adhesion assays were performed in the presence of 0.1 to 10 ng/mL of (•) SCF, (▪) IL-3, or (▴) GM-CSF. Cell adhesion was measured at 15 minutes as described in the Materials and Methods. Data are means ± 1 SE calculated from three independent experiments.
Dose-dependency of cytokine-stimulating effect on CD34+ HPC adhesion to HA. Adhesion assays were performed in the presence of 0.1 to 10 ng/mL of (•) SCF, (▪) IL-3, or (▴) GM-CSF. Cell adhesion was measured at 15 minutes as described in the Materials and Methods. Data are means ± 1 SE calculated from three independent experiments.
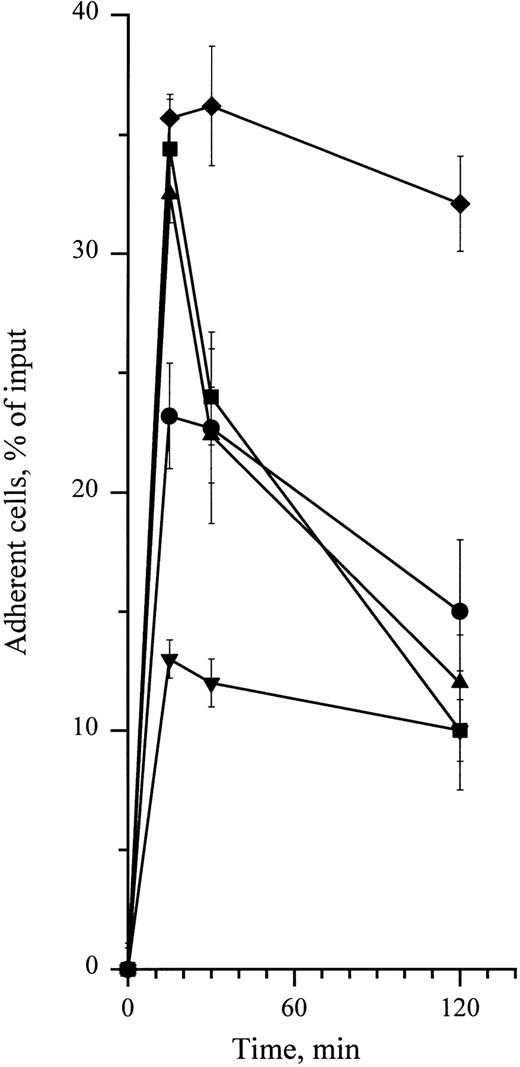
Time-dependency of cytokine-stimulating effect on CD34+ cell adhesion to HA. Adhesion assays were performed in the presence of 10 ng/mL (•) SCF and (▪) IL-3 and 0.1 ng/mL of (▴) GM-CSF. (♦) Cells preincubated at 4°C for 15 minutes with 5 μg/mL of the anti-CD44 MoAb H90. (▾) Cells treated with the same dose of IgG1 in the same conditions. Cell adhesion was measured at 15 minutes, 30 minutes, and 2 hours. Measures were performed in triplicate as indicated in the legend to Fig 1. Data are means ± 1 SE calculated from three independent experiments.
Time-dependency of cytokine-stimulating effect on CD34+ cell adhesion to HA. Adhesion assays were performed in the presence of 10 ng/mL (•) SCF and (▪) IL-3 and 0.1 ng/mL of (▴) GM-CSF. (♦) Cells preincubated at 4°C for 15 minutes with 5 μg/mL of the anti-CD44 MoAb H90. (▾) Cells treated with the same dose of IgG1 in the same conditions. Cell adhesion was measured at 15 minutes, 30 minutes, and 2 hours. Measures were performed in triplicate as indicated in the legend to Fig 1. Data are means ± 1 SE calculated from three independent experiments.
DISCUSSION
CD44 probably plays an important role in hematopoiesis, because the addition of function-blocking anti-CD44 MoAb completely inhibits both myelopoiesis and lymphopoiesis in murine and human long-term bone marrow cultures.2,3 In favor of this hypothesis is the increasing evidence that CD44 is a signalling molecule. For instance, CD44-hyaluronan interaction activates intracellular signalling in lymphoma cell lines26,34,35 and macrophages36 and enhances cell proliferation during eosinopoiesis.37 Despite these lines of evidence, little is known of the functions of this molecule in human hematopoiesis. It has been reported that CD44 is the principal cell surface receptor for HA.17,18 However, most hematopoietic cells, despite expressing high levels of CD44, bind HA poorly or do not bind it at all.20-24,26 27 Therefore, it appears clear that high levels of CD44 expression does not imply adhesion to HA.
Although normal CD34+ HPC strongly express CD44,3,7,8 their adhesiveness to HA has never been investigated. In this report, we show that only a small subpopulation of normal human bone marrow CD34+ HPC spontaneously adhere to immobilized HA, and that this adhesive interaction is specifically mediated by CD44. In addition, we report that adhesion of CD34+ cells can be increased by the phorbol ester PMA, by a function-activating anti-CD44 MoAb H90, and by the cytokines SCF, GM-CSF, and IL-3. The avidity of CD44 for HA has been measured by means of quantifying adhesion of 51Cr-labeled CD34+ hematopoietic cells to immobilized HA. This technique has previously been used to show HA-binding by hybridoma18 and immature myeloid cell lines27 and to study adhesion of CD34+ hematopoietic cells to various immobilized extracellular matrix components.6,16,38 Because of its high sensitivity and reproducibility, it is relevant to affirm that 13% of normal human CD34+ hematopoietic cells spontaneously and reproducibly adhere to immobilized hyaluronan. This spontaneous adhesion was specifically mediated by CD44-HA interaction because both pretreatment of CD34+ HPC with the function-blocking anti-CD44 MoAb J17327 and pretreatment of HA-coated wells with Streptomyces hyaluronidase prevented adhesion to HA. Because CD34+ cells displayed a similar level of CD44 before and after their isolation from bone marrow, the limited rate of their spontaneous adhesion to HA could not be due to the internalization or degradation of cell surface CD44 molecules during the CD34+ cell purification procedure. In addition, preliminary experiments showed that starvation did not affect CD34+ cells adhesion to HA.
Spontaneously adherent CD34+ cells comprised granulo-monocytic and erythroid committed progenitors, as well as more primitive progenitors, pre-CFU. Because in these experiments CD34+ HPC were put in contact with HA for a very short time (only 15 minutes), the possibility that this interaction might have perturbed subsequent HPC growth, in short-term methylcellulose and pre-CFU assays, is very unlikely. Indeed, it has been reported that at least 2 weeks were necessary for HA to alter the proliferation of CD34+ HPC.37 Furthermore, we did not detect any alteration of CD34+ HPC growth when cells were preincubated for 15 minutes with the function-activating anti-CD44 MoAb H90 or cytokines.
A high level of CD44 seems to be associated with the adhesiveness of CD34+ HPC to HA, because CD34+ hematopoietic cells spontaneously adherent to HA express higher levels of CD44 (MFI = 63) than the nonadherent ones (MFI = 37, Fig 2). In good accord with this observation, very high levels of CD44 have been detected on CFU-GM,39 which have a high rate of adhesion to HA (20%). Conversely, the low proportion (7%) of erythroid progenitors adhering to HA may be related to the lower amount of CD44 expressed on these cells.39 In addition, spontaneously adherent cells expressed less CD38 and HLA-DRII antigens, which suggests that another subset of very immature progenitors may also spontaneously adhere to HA. We are currently testing this hypothesis by determining whether the very primitive progenitors, designated long-term culture-initiating cells (LTCIC), which have been shown to strongly express CD44,3 spontaneously adhere to HA.
However, it appears clear that adhesion of CD34+ HPC to HA does not exclusively depend on strong CD44 expression. As reported with myeloid cell lines KG1 and KG1a,27 many CD44bright CD34+ HPC do not adhere to HA (Fig 2). In addition, the augmentation of CD44 expression did not fully account for the PMA-stimulated CD34+ cell adhesion to HA. Moreover, the function-activating anti-CD44 MoAb, H90, as well as the cytokines SCF, IL-3, and GM-CSF increased this adhesion to HA without alteration of CD44 expression. Interestingly, Lesley et al suggested that murine lymphocytes express CD44 molecules with different affinity states to HA and distinct ability to rapid activation by function-stimulating anti-CD44 MoAbs.21-24 40 Our data strongly suggest that, similar to murine lymphocytes, human CD34+ HPC display both constitutively active and inactive CD44 molecules in regard to HA-binding ability. Therefore, the fact that CFU-GM have a much higher level of spontaneous adhesion to HA than BFU-E may result not only from a higher amount of CD44, but also from the presence of more active CD44 molecules.
The PMA-induced adhesion was detected after several hours at 37°C and was inhibited by the protein synthesis inhibitor cycloheximide, thus implying protein synthesis as an important part of this process. PMA may induce the neosynthesis of CD44 molecules, because cytofluorometric analysis with FITC-J173 MoAb showed a much higher amount of CD44 on PMA-treated than on untreated CD34+ cells (MFI = 110 v 37, Fig 3). However, this higher CD44 expression did not fully account for PMA-increased adhesion to HA. Indeed, the anti-CD44 J173, which fully inhibited spontaneous and cytokine-enhanced adhesion of CD34+ HPC to HA, inhibited only 40% of PMA-induced adhesion to HA. Because all CD34+ cells were maximally labeled by J173, this suggests that PMA may induce neosynthesis of structurally distinct CD44 molecules, such as alternative spliced isoforms with very high affinity for HA41-43 or of HA-binding proteins distinct from CD44, such as hyaluronectin.44
In sharp contrast with PMA-enhanced adhesion which requires 24 hours, both H90 MoAb and cytokines increased adhesion of CD34+ HPC to HA in 15 minutes (Fig 6). This enhanced adhesion was exclusively mediated by CD44, because it was fully abrogated by the function-blocking anti-CD44 MoAb J173. In addition, it was not accompanied by a detectable augmentation of CD44 cell surface expression (Fig 3). The whole of these features suggest that both H90 MoAb and cytokines activate a preexistent pool of inactive CD44 molecules and that this activation may involve conformational changes. The stimulating effect of MoAb H90 was not uniform within the CD34+ HPC population, because it strongly stimulated adhesion of BFU-E, whereas it had no effect on CFU-GM. As discussed above, conformational differences in CD44 molecules may result in a different ability to bind the H90 MoAb27 or in a different sensitivity to its activating effect. According to this hypothesis, BFU-E may display more CD44 molecules with higher affinity for the H90 MoAb or that are rapidly activable by this MoAb than CFU-GM.
Despite some similarities between CD44 activation by MoAb H90 and cytokines, distinct molecular mecanisms are involved in these two processes. H90 was able to stimulate adhesion even at 4°C, and this stimulation lasted for at least 2 hours, whereas cytokine-induced adhesion required incubation at 37°C and was transient, peaking at 15 minutes and returning to basal levels within 2 hours (Fig 6). Preliminary experiments performed on the cytokine-dependent CD34+ myeloid cell line TF1, a suitable model to study regulation of adhesive receptors expressed by normal human CD34+ HPC,6,16 argue in favor of this hypothesis. Indeed, we have found that the protein-kinase inhibitor staurosporine at 500 nmol/L abolished cytokine-stimulated adhesion but not H90-stimulated adhesion of TF1 cells to HA (unpublished data). As hypothesized by Lesley et al to account for MoAb-induced activation of CD44 in murine lymphocytes,22-24,40 the rapid and stable stimulation of CD34+ HPC adhesion to HA initiated by H90 MoAb may result from a stable change of CD44 molecule conformation or distribution, which, in turn, would result in the increase of CD44 affinity for HA. This is in good accord with the fact that activation by H90 MoAb is neither temperature dependent nor protein-kinase dependent. Conversely, as with the example of β1 integrins,6,45-47 cytokine receptors, once bound to their ligand, activate several cascades of protein kinases that could directly phosphorylate CD44 molecules and modulate their affinity for HA21,48 or alter the cytoskeleton proteins in contact with the cytoplasmic domain of CD44 molecule,21,48,49 resulting into the conformational change required for enhanced affinity for HA. The evidence that the cytoplasmic domain of CD44 plays an important role in HA-binding fit this hypothesis.49 As in the case of β1 integrins,6,45-47 the involvement of the protein-kinase cascade in the activation of CD44 by cytokines is reflected by the transient nature of this process50 and its blockade by both low temperatures and, as shown in TF1 cells, by protein-kinase inhibitors such as staurosporine. Contrarily to H90 MoAb, SCF, IL-3, and GM-CSF activate the adhesion to HA of CFU-GM, but have no effect on the adhesion of BFU-E. However, because these cytokines have been shown to stimulate the proliferation and differentiation of both CFU-GM and BFU-E they are likely to trigger intracellular signalling in both populations of progenitors.51-54 Because this intracellular signalling is thought to activate CD44 at the level of the intracytoplasmic domain of CD44 (or of intracytoplasmic-associated molecules), it is suggested that the intracytoplasmic domain of CD44 molecules from CFU-GM and BFU-E may differ, as reported in the case of CD44 molecules from various cell lines.55 56
It is noteworthy that no more than 40% to 50% of CD34+ cells could adhere to HA, despite high levels of CD44 and stimulation by various molecules. This feature may be at least partly accounted for by the above hypothesis that CD44 molecules endowed with HA-binding capacity (either constitutive or inducible) may be displayed only by certain progenitor subsets. Another answer to this apparent paradox may be that an important proportion of CD44 molecules expressed by CD34+ HPC have ligands different from HA. Thus, it has been shown that CD44 expressed on lymphocytes and fibroblasts could also bind fibronectin, chondroitin sulfate, collagens,57 and serglycin.58 Conversely, it has been reported that CD44 did not directly mediate the adhesion of CD34+ HPC to fibronectin,28 and we did not observe any adhesion of CD34+ cells to chondroitin sulfate (data not shown). However, it is not yet known whether CD44 could mediate adhesion of CD34+ cells to collagens or to serglycin.
In conclusion, the present data show for the first time that the CD44-mediated adhesion of CD34+ cells to HA can be modulated by various activating molecules, including PMA, an anti-CD44 MoAb, and the cytokines SCF, IL-3, and GM-CSF. Strikingly, these activating molecules and particularly cytokines can also enhance the integrin-mediated adhesion of CD34+ HPC to fibronectin.6,9-14 16 Taken together, these data strongly suggest that cytokines may not only control the proliferation and the differentiation of hematopoietic progenitors, but also the adhesive interactions between HPC and various proteic and glucidic components of the bone marrow stroma. This last function of cytokines may be of considerable importance to regulate the homing of HPC in the bone marrow, particularly in relation to cell grafting. Moreover, the augmentation of HPC adhesion to specific substrata such as fibronectin and HA could be an important modulator of HPC proliferation and differentiation in response to cytokines.
ACKNOWLEDGMENT
The authors are indebted to J. Chaker for technical assistance, to Prof P. Berczeller for critical reading of the manuscript, and to N. Vriz for editorial assistance.
Supported by grants from Institut National de la Santé et la Recherche Médicale, Assistance Publique, and Association pour de Nouvelles Recherches Biomédicales.
Address reprint requests to Florence Smadja-Joffe, PhD, Laboratoire de Différenciation Hématopoı̈étique Normale et Leucémique, INSERM U268, Hôpital Paul Brousse, 14, avenue P.V. Couturier, 94800 Villejuif, France.