Abstract
The site and mechanism of platelet production by bone marrow megakaryocytes (MKs) has been the subject of extensive studies, but is still a matter of controversy. However, the recent discovery of the Mpl ligand (Mpl-l), also called megakaryocyte growth and development factor (MGDF ) or thrombopoietin, has resulted in considerable progress in the understanding of the maturation of the MK lineage. To better understand the mechanism of platelet production, we examined the late stage of MK maturation by electron microscopy in cells cultured in the presence of Mpl-l. Human bone marrow CD34+CD38+ cells, which contain late MK progenitors, were purified by flow cytometry and cultured in a serum-free liquid medium containing recombinant human Mpl-l (MGDF 10 ng/mL) for 7 days. In this system, the majority of cultured cells were large MKs with lobulated polyploid nuclei. The MKs displayed a smooth surface with harmonious cytoplasmic maturation and abundant, regularly distributed demarcation membranes and α-granules, and even some dense granules. Interestingly, approximately 30% of the MKs observed displayed morphologic evidence of platelet production: at optical microscopy, MKs formed long filamentous cytoplasmic extensions (proplatelets) that fragmented into platelet-sized particles. Moreover, flow cytometric analysis of this cultured cell population showed GPIIb-positive particles of the size of platelets. Electron microscopic observation showed that MKs producing platelets displayed thin pseudopods on the surface, and that the channels of the demarcation membrane system were dilated, allowing long strands of cytoplasm to extend from the cell periphery. These cytoplasmic strands displayed beading with constrictions separating platelet-sized segments; the more distal to the cell core, the smaller the fragments were. They eventually detached from the cell core into the culture medium either occasionally still elongated or, more often, separated into individual platelets. Parallel longitudinal and perpendicular microtubules were visualized in the constricted regions of these cytoplasmic strips; immunogold study of tubulin localization confirmed this subcellular distribution. On both sides of the constricted areas, vacuoles were noted, the fusion of which might have led to the detachment of individual platelets. Finally, in close proximity to the platelet-forming MKs, numerous microparticles were shed. Although some of these particles might correspond to transverse sections of pseudopods, this did not seem to be the case, since they were rarely seen around thrombin-stimulated MKs with surfaces bristled by numerous pseudopods. Flow cytometry showed that apart from shed cytoplasmic fragments of platelet size, numerous smaller particles strongly labeled for CD41 were also released by mature MKs. In conclusion, this study describes the ultrastructure of human platelet production in cultured MKs, involving the formation of proplatelets and the shedding of microparticles.
PLATELETS are formed by bone marrow megakaryocytes (MKs) and arise from fragmentation of the mature cytoplasm.1 Although their mode of formation has always been of considerable interest, the site and mechanism of platelet shedding is still a matter of controversy.2 Indeed, documenting the morphologic and ultrastructural aspects of platelet release by MKs has been hampered by two major difficulties. First, although it is known that MKs are located adjacent to the marrow sinusoids,3 it is rare to observe authentic platelet shedding within the marrow. It is known that intact MKs are able to cross the sinusoidal barrier and to enter the blood circulation1-6; from there, they enter the pulmonary circulation, and it is well established that at least some degree of platelet production appears to take place in the pulmonary microvasculature.5,7-11 Second, before the discovery of the Mpl ligand (Mpl-l), also called megakaryocyte growth and development factor (MGDF ) or thrombopoietin, culture conditions for human MKs in either semisolid or liquid medium did not permit sufficiently adequate maturation to achieve the terminal stage of platelet production.12 13
Various theories have been proposed to explain how platelets are released from MKs, involving requirements for a specific microenvironment of stromal cells (and associated cytokines) and extracellular matrix, as well as the physical forces of blood circulation.6,13,14 Moreover, several issues concerning the subcellular organization of MK cytoplasmic fragmentation remain unclear. For example, is the MK plasma membrane the precursor of the platelet membrane,15-17 and does the MK shed its cytoplasm all at once or progressively? The increase in platelet size observed soon after induction of thrombocytopenia is of unknown etiology.18
Finally, it is a striking feature that in clinical states such as immunologic thrombocytopenic purpura (ITP) in which platelet destruction is accompanied by increased production, the hemorrhagic syndrome is often less pronounced than might be predicted from the platelet count. We thus questioned whether platelet microparticles, which carry hemostatic properties and are released during platelet activation,19 might also be shed during platelet production.
Since the recent discovery of Mpl-l,20-23 the quality of maturation of MK cultures has improved substantially,20 and platelet production has been observed in such cultures.24 25 CD34+38+ human bone marrow cells purified by flow cytometry (which contain late MK progenitors) were grown in a liquid serum-free medium containing recombinant human Mpl-l. Fully mature MKs were observed that produced ultrastructurally normal platelets. We thus have been able to document the morphologic changes of MKs undergoing platelet production, and found that the phenomenon of platelet release was preceded by the extension of proplatelet-like expansions from the cytoplasm and accompanied by the shedding of microparticles carrying platelet membrane receptors.
MATERIALS AND METHODS
Cell cultures.Human bone marrow was harvested from normal adult donors during hip surgery, after obtaining informed consent and in accordance with the institutional guidelines of the Committee on Human Investigation. Bone marrow precursor cells (CD34+) were isolated by the immunomagnetic bead or the Miltenyi technique.24 Among this population, CD34+38+ cells were separated by cell sorting. Briefly, cells were labeled with a R-PE anti-CD34 monoclonal antibody (HPC-A2; Becton Dickinson, Mountain View, CA) and a fluorescein isothiocyanate (FITC) anti-CD38 monoclonal antibody (IOB6; Immunotech, Luminy, France). After one wash, cells were sorted on a FACSvantage flow cytometer (Becton Dickinson) equipped with an argon ion laser tuned to 488 nm and operating at 500 mW, and then cultured for 7 days in serum-free liquid medium containing recombinant human Mpl-l (rHu PEG-MGDF; generously provided by Amgen, Thousand Oaks, CA) at a final concentration of 10 ng/mL. Cell culture was performed under conditions previously described.26
Electron microscopy.Cultured cells were studied by electron microscopy. They were washed twice in Hanks' medium at 4°C, fixed in 1.25% glutaraldehyde in Gey's buffer for 1 hour, and washed twice. Cells were then postfixed with osmium tetroxide, dehydrated, and embedded in Epon. Thin sections were examined with a Philips CM 10 electron microscope (Philips, Eindoven, The Netherlands) after uranyl acetate and lead citrate staining. Around 100 proplatelet-forming MKs were examined.
Immunoelectron microscopy.Alternatively, cells were fixed in 1% glutaraldehyde in phosphate buffer (0.1 mol/L, pH 7.4) for 1 hour and embedded in glycol-methacrylate. Immunolabeling for glycoprotein (GP)Ib, GPIIb-IIIa, and P-selectin was performed as described previously.27 Tubulin immunolocalization was performed with a mixture of two monoclonal antibodies, anti–α- and anti–β-tubulin (Sigma, St Louis, MO), followed by goat anti–mouse Igs conjugated to 10 nm colloidal gold (Amersham, Les Ullys, France).
RESULTS
The addition of Mpl-l to cultures of CD34+CD38+ cells had a beneficial effect on the quality of cytoplasmic maturation. This effect has been documented by both optical and electron microscopy, which led to the following observations:
At light microscopy, round MKs began to deform at day 5 of culture and showed a pseudopod that progressively elongated. Ultimately, this process became extremely long and thin, with regular swelling (Fig 1). The central part of the MK, containing the nucleus surrounded by a rim of cytoplasm, was directed eccentrically. Finally, this long process was noted to break irregularly at several constriction sites, giving rise to detached (pro)platelets, some of them remaining adherent to the plastic. In the presence of Mpl-l and depending on the culture, up to 70% of MKs (mean, 30%) exhibited a filamentous form. In contrast, in the absence of Mpl-l, this phenomenon was rare (<10%).
Mature MK cultured with Mpl-l, developing proplatelets (arrowheads) as seen by phase-contrast microscopy: (a) a pseudopod extends, becoming extremely long and thin; (b) this process develops regular swellings. These proplatelets are in the continuity of the MK core (arrows).
Mature MK cultured with Mpl-l, developing proplatelets (arrowheads) as seen by phase-contrast microscopy: (a) a pseudopod extends, becoming extremely long and thin; (b) this process develops regular swellings. These proplatelets are in the continuity of the MK core (arrows).
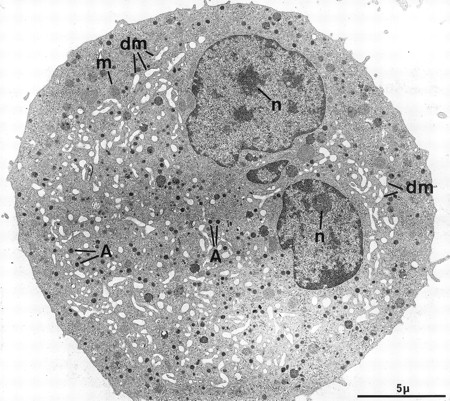
At electron microscopy, apart from the high proportion of megakaryocytic cells in the cultured population (contaminated by a few macrophages and basophils), mature MKs could be divided into two main categories. The first population consists of fully mature, resting MKs and is composed of large cells with a smooth surface (Fig 2). The nucleus displays several rounded lobes, with abundant euchromatin and prominent nucleoli. These MKs have abundant cytoplasm, numerous α-granules randomly scattered throughout the surface, only a few multivesicular bodies, and a well-developed demarcation membrane system with open cisternae also randomly distributed throughout the cytoplasm. Some dense granules, attesting to a good terminal maturation stage, are also present.26 These cells closely mimick MKs maturing in the bone marrow, and accounted for more than half of the cells found in our experimental culture conditions. However, a minority of MKs had a less even organelle development, displaying an inadequate demarcation membrane system with flat cisternae or an insufficient number of storage granules.
Example of a harmoniously developed MK, grown for 7 days from CD34+CD38+ progenitors in the presence of Mpl-l. The cell nucleus displays several lobes with abundant euchromatin, and nucleoli (n). The cytoplasm has harmoniously matured with numerous α-granules (A) and a well-developed demarcation membrane system (dm). m, mitochondria.
Example of a harmoniously developed MK, grown for 7 days from CD34+CD38+ progenitors in the presence of Mpl-l. The cell nucleus displays several lobes with abundant euchromatin, and nucleoli (n). The cytoplasm has harmoniously matured with numerous α-granules (A) and a well-developed demarcation membrane system (dm). m, mitochondria.
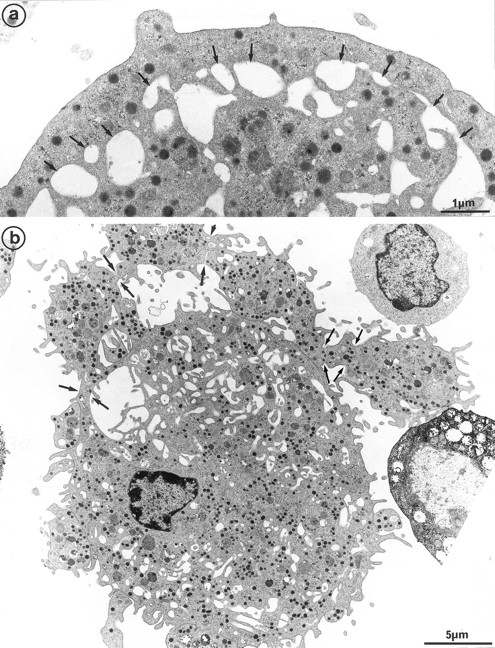
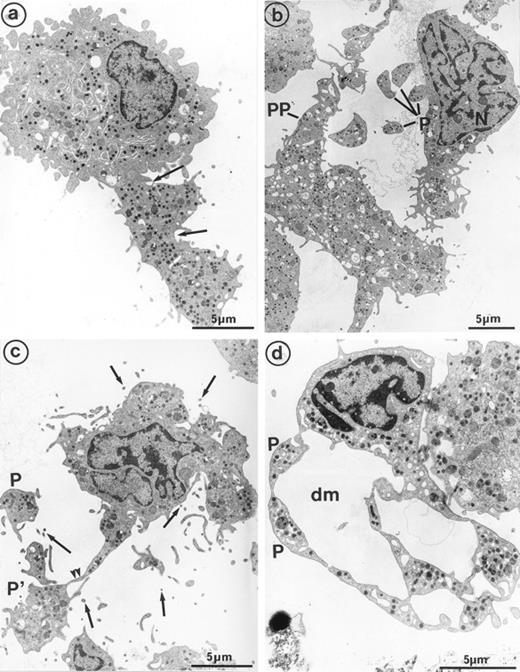
The second population had not been observed by us in any other culture conditions before the use of Mpl-l. It is noteworthy that the described morphologic changes were also present in single cell experiments, ruling out the necessity of other cytokines produced by accessory cells (not shown). A mean of 15% of the MKs had a tighter nucleus with closely apposed nuclear lobes and an indented shape, the chromatin being mostly condensed. The cell surface was uneven, often bristled with thin pseudopods. The appearance of these cells was consistent with their undergoing active platelet shedding. But because electron microscopy takes point-in-time looks at continually changing processes, dynamic relationships could only be inferred from electron microscopic data. Nevertheless, the following morphologic events were observed, leading to the eventual platelet shedding: (1) Alignment and dilatation of the peripheral demarcation membranes (Fig 3a). (2) Unfolding of cytoplasmic sheets and extension of cytoplasmic expansions still attached to the cell body, equivalent to “proplatelets” (Fig 3b). (3) These extensions could be several within a cell, and appeared to be most often still attached to the cell body. (Some large proplatelet-like territories appeared to be separated from the cell core, but this might be an artifact related to the section plan.) The cytoplasmic extensions displayed a beaded appearance, with constriction points separating discrete platelet-like territories. Along a proplatelet, future platelet delimited areas were decreasing from 10 μm to 2 μm in size, the smaller being the furthest from the cell core and the earliest to detach (Fig 4). (4) Careful observation of the constriction zones showed that MK cytoplasmic extensions elongated with the appearance of a central longitudinal bundle of microtubules (Fig 5). Immunogold labeling for tubulin was able to evidence this structure. (5) Some microtubules, transverse to the long axis of the cytoplasmic extension, appeared on both sides of the constriction zone (again confirmed by tubulin immunolabeling); and a central vacuole formed and widened in the constriction zone center, leading to the further detachment of a newly formed platelet (Fig 5). (6) Platelets of various size were found in the supernatant of the culture medium. We have studied this shed platelet population by electron microscopy and immunoelectron microscopy (Fig 6).
Cultured MK grown for 7 days from CD34+CD38+ progenitors in the presence of Mpl-l, and presenting signs of platelet formation. (a) This mature MK displays alignment and dilatation of some peripheral demarcation membranes, individualizing an outer ring of cytoplasm (arrows). (b) The peripheral sheet of cytoplasm has unfolded from the cell core. Constriction zones are regularly disposed along this cytoplasmic extension (arrows).
Cultured MK grown for 7 days from CD34+CD38+ progenitors in the presence of Mpl-l, and presenting signs of platelet formation. (a) This mature MK displays alignment and dilatation of some peripheral demarcation membranes, individualizing an outer ring of cytoplasm (arrows). (b) The peripheral sheet of cytoplasm has unfolded from the cell core. Constriction zones are regularly disposed along this cytoplasmic extension (arrows).
Sample of cultured MK grown for 7 days from CD34+CD38+ progenitors in the presence of Mpl-l, showing some evidence of platelet formation and shedding. (a) A cytoplasmic process extends straight from the cell core. Constriction zones seem to individualize already distinct platelet fields of large size, close to the MK core (arrows). (b) The appearance of this mature MK is consistent with the process of shedding platelets: The nucleus (N) displays condensed heterochromatin, and its shape is indented. Several platelet-sized cytoplasmic fragments have clearly detached from the MK (P). A large cytoplasmic fragment deprived of nuclear material and possibly corresponding to a detached proplatelet (PP) is also shown. (c) Before releasing platelets, a constriction zone (arrowheads) is formed between the mother cell and the forming platelet (P′). A few detached platelets (P) seem to have already detached, as well as numerous microparticles (arrows). (d) Considerable dilatation of the demarcation membrane system (dm) leads to the demarcation of future platelet fields (P).
Sample of cultured MK grown for 7 days from CD34+CD38+ progenitors in the presence of Mpl-l, showing some evidence of platelet formation and shedding. (a) A cytoplasmic process extends straight from the cell core. Constriction zones seem to individualize already distinct platelet fields of large size, close to the MK core (arrows). (b) The appearance of this mature MK is consistent with the process of shedding platelets: The nucleus (N) displays condensed heterochromatin, and its shape is indented. Several platelet-sized cytoplasmic fragments have clearly detached from the MK (P). A large cytoplasmic fragment deprived of nuclear material and possibly corresponding to a detached proplatelet (PP) is also shown. (c) Before releasing platelets, a constriction zone (arrowheads) is formed between the mother cell and the forming platelet (P′). A few detached platelets (P) seem to have already detached, as well as numerous microparticles (arrows). (d) Considerable dilatation of the demarcation membrane system (dm) leads to the demarcation of future platelet fields (P).
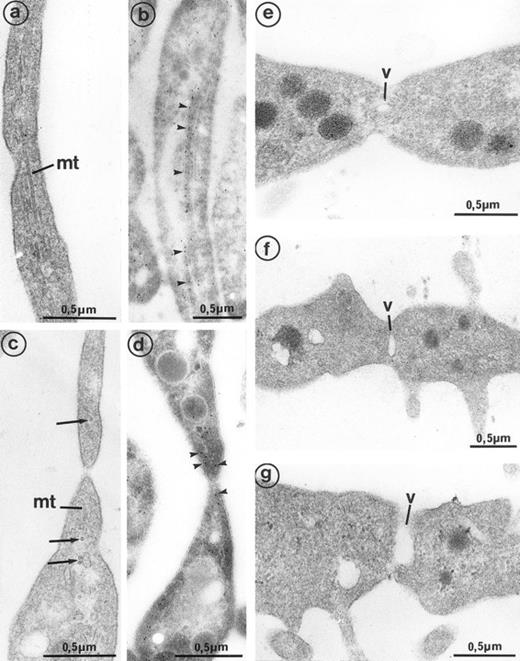
High magnification of the constriction zones observed on mature MKs (grown for 7 days from CD34+CD38+ progenitors in the presence of Mpl-l). (a) Some elongated parallel microtubules (mt) are present along the length of the cytoplasmic extension separating the platelet buds. (b) Immunogold labeling for tubulin confirms its presence in the length of the elongated arm separating platelet territories (arrowheads). (c) On each side of the constriction area, transverse microtubule fragments (mt) are observed near the longitudinal microtubules (arrows). (d) Immunogold labeling for tubulin confirms its concentration on each side of the constriction zone (arrowheads). (e, f, and g) A vacuole of increasing size (v) is formed at the constriction zone, which may lead to detachment of the platelet fragment.
High magnification of the constriction zones observed on mature MKs (grown for 7 days from CD34+CD38+ progenitors in the presence of Mpl-l). (a) Some elongated parallel microtubules (mt) are present along the length of the cytoplasmic extension separating the platelet buds. (b) Immunogold labeling for tubulin confirms its presence in the length of the elongated arm separating platelet territories (arrowheads). (c) On each side of the constriction area, transverse microtubule fragments (mt) are observed near the longitudinal microtubules (arrows). (d) Immunogold labeling for tubulin confirms its concentration on each side of the constriction zone (arrowheads). (e, f, and g) A vacuole of increasing size (v) is formed at the constriction zone, which may lead to detachment of the platelet fragment.
(a) Platelet-sized cytoplasmic fragments, shed by 7-day cultured MKs, exhibit the usual platelet cytoplasmic organelles: α-granules (A), open canalicular system (ocs), and microtubule transverse sections (mt) at each extremity of the cell. Note that microtubules also extend along the length of the cell (arrows), which is unusual in circulating platelets. Immunogold labeling for tubulin occasionally shows the presence of the protein at (b) the 2 platelet poles (arrowheads) and (c) more frequently along the platelet length (arrowheads). (d) GPIIb-IIIa has the classic platelet-like distribution on the platelet fragments shed by MK: plasma membrane (arrowheads), open canalicular system (ocs), and α-granules (A).
(a) Platelet-sized cytoplasmic fragments, shed by 7-day cultured MKs, exhibit the usual platelet cytoplasmic organelles: α-granules (A), open canalicular system (ocs), and microtubule transverse sections (mt) at each extremity of the cell. Note that microtubules also extend along the length of the cell (arrows), which is unusual in circulating platelets. Immunogold labeling for tubulin occasionally shows the presence of the protein at (b) the 2 platelet poles (arrowheads) and (c) more frequently along the platelet length (arrowheads). (d) GPIIb-IIIa has the classic platelet-like distribution on the platelet fragments shed by MK: plasma membrane (arrowheads), open canalicular system (ocs), and α-granules (A).
The shape and size of shed platelets were mostly irregular. They contain the usual organelles encountered in peripheral blood platelets (eg, α-granules, open canalicular system, and microtubules). The microtubules were often elongated along one side of the platelets rather than circumferential, but some microtubular transverse sections were frequently observed at the platelet extremities, corresponding to those observed on each side of the constriction areas of proplatelets. Platelets of various size were found in the supernatant of the culture medium.
The platelet glycoproteins GpIb (not shown), GpIIb-IIIa (Fig 6b), and P-selectin (not shown) were distributed according to the classic blood platelet distribution (ie, plasma membrane, open canalicular system, and α-granule membrane, respectively).
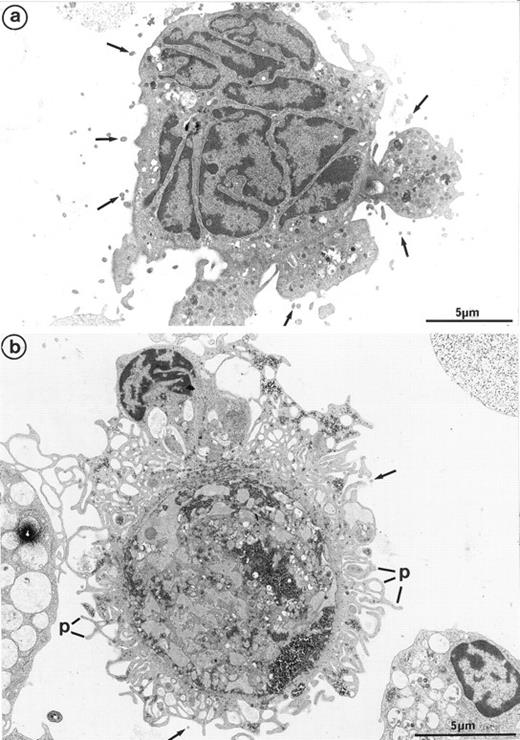
Interestingly, platelet-producing MKs were surrounded by numerous microparticles from 0.1 to 0.3 μm in diameter (Fig 7a). It is conceivable that the particles might be transverse sections of cell surface–connected pseudopods. However, they were also scattered in the vicinity of smooth areas of the plasma membrane and present along dilated channels of demarcation membrane with a smooth surface. These areas were devoid of pseudopods that could have been transversely sectioned. Moreover, these particles were not observed in the vicinity of thrombin-activated MKs, the surface of which was bristled with numerous long and thin pseudopods29 (Fig 7b). Some of these particles seemed to arise from fragments of the MK elongated arms, located between constriction zones, which were not included in the newborn platelet and were thus released into the surroundings. By immunoelectron microscopy, GpIIb-IIIa was also found to be strongly expressed on the shed microparticles.
(a) MK grown for 7 days from CD34+CD38+ progenitors in the presence of Mpl-l: numerous microparticles (arrows) surround this platelet-shedding mature MK. (b) Cultured MK having undergone thrombin activation (1 U/mL for 5 min): the plasma membrane is bristled with numerous pseudopods (p). However, unlike platelet-shedding MKs, only a few microparticle profiles are seen around the cell surface (arrows), probably corresponding to some pseudopod transverse sections.
(a) MK grown for 7 days from CD34+CD38+ progenitors in the presence of Mpl-l: numerous microparticles (arrows) surround this platelet-shedding mature MK. (b) Cultured MK having undergone thrombin activation (1 U/mL for 5 min): the plasma membrane is bristled with numerous pseudopods (p). However, unlike platelet-shedding MKs, only a few microparticle profiles are seen around the cell surface (arrows), probably corresponding to some pseudopod transverse sections.
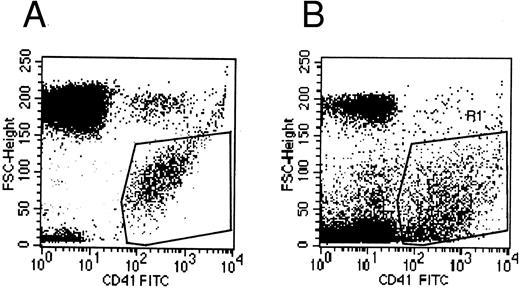
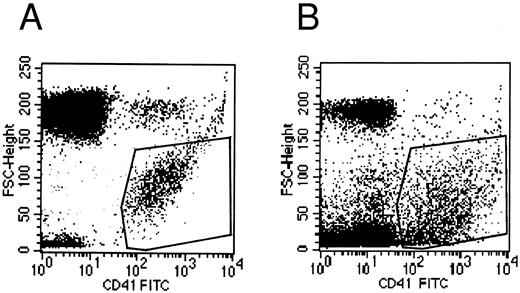
Flow cytometry.Flow cytometric experiments were performed on the supernatant of MK cultures. Numerous platelet-size particles were found, corresponding to the shed platelets observed by electron microscopy, as well as an abundant population of particles of smaller size. This latter population expressed the membrane receptor GpIIb-IIIa, and corresponded to the microparticles observed by ultrastructure (Fig 8).
Flow cytometric histograms of CD41+ cell populations. (A) Flow cytometric analysis of normal blood platelets showing the range of normal platelet size. (B) Flow cytometric analysis of the culture supernatant: an abundant population of small CD41+ particles is detected in addition to the platelet-sized elements, which probably represents the released microparticles.
Flow cytometric histograms of CD41+ cell populations. (A) Flow cytometric analysis of normal blood platelets showing the range of normal platelet size. (B) Flow cytometric analysis of the culture supernatant: an abundant population of small CD41+ particles is detected in addition to the platelet-sized elements, which probably represents the released microparticles.
DISCUSSION
In this study, we documented the ultrastructural subcellular events of platelet production observed in vitro from human MKs cultured in the presence of Mpl-l. The phenomenon of platelet release by either bone marrow or cultured mature MKs and the subcellular changes leading to it have been rarely observed. One or several of the experimental conditions used here may have been critical to achieve good quality cell maturation, including the use of Mpl-l, the high cell concentration in the culture, and the use of the late CD34+38+ MK progenitors. Indeed, it was recently reported that Mpl-l is not a direct inducer of proplatelet formation in human MKs.30 Nevertheless, we and others had previously been unable to achieve experimental MK maturation of such good quality as to allow convincing and consistent in vitro platelet formation. The fact that functional platelets can now be produced from MKs grown in vitro has been described by another group,24 but the morphologic events that accompany platelet production have not been shown precisely. Again, it is important to emphasize that the dynamic sequence of events is necessarily an extrapolation from the observation of fixed, immobilized cells; however, this was made possible thanks to our culture conditions, which provided a large yield of MKs reaching the terminal maturation stage of platelet production. Observations of this event presented herein permit an approach to answer several unresolved questions.
Firstly, the observation of platelet production in culture without an adherent layer rules out the absolute necessity for the stromal microenvironment (and associated cytokines)3,13,14 and the dynamic forces of blood circulation.5,6 Secondly, the ultrastructural changes that lead to platelet shedding reveal that the cell periphery is involved in the future platelet membrane, since it seems to be the initial site of unfolding of the cytoplasm.16 The dilated demarcation membrane system delimits the fracture boundaries,31 which leads to the detachment of a shred of cytoplasm forming a proplatelet-like extension identical to the one initially observed by Thiéry and Bessis32 and further described by others.17,33-36 Although proplatelet-like cytoplasmic extensions had been observed on MKs grown in culture by several groups,17,34-37 the fact that this phenomenon was reversible and could be neutralized by low temperature and microtubule depolymerizing agents38,39 raised the question of whether it was related to the event of platelet production or to cell damage and in vitro activation. Choi et al in recent studies on in vitro human platelet formation,24 25 also observed the step of proplatelet formation.
As observed by phase-contrast microscopy, several such extensions may be present, and display a beaded conformation. Platelet-like fragments detached from the tip of these extensions. Interestingly, in a single proplatelet, the closer to the cell core, the larger the segments were budding, with a mean diameter of 10 μm; whereas the more distal to the cell core, the segments were smaller, and approximated the normal platelet size. This might explain the rapid increase in platelet volume as an early response to thrombocytopenia, observed within 8 hours following the decrease in platelet count.18 Instead of detachment from the tip of the proplatelet, in case of increased need, platelets might detach earlier and closer to the core of the cell where the delimited platelet fragment is larger. However, large proplatelet fragments appeared to detach in the culture medium that can be compared with the large MK cytoplasmic fragments observed in the pulmonary circulation,7 and it remains unclear by which mechanism they reach average platelet size.
The segments appeared to be delimited by constricted areas in which bundles of microtubules were running parallel to the axis of the proplatelet as observed previously in rat40 and guinea pig17 MKs. On both sides of the constriction zones, transversal microtubules appeared to form a net that, by increasing local rigidity, possibly results in breakage and platelet liberation. Thus, the newly formed platelets were bearing longitudinally aligned microtubules and were often lacking the circumferential arrangement of microtubules. The formation of the microtubule coil circumferentially distributed around circulating platelets has also been the subject of controversy. Here, we show that platelets detach from the tip of a cytoplasmic process along which a bundle of microtubules stretches.41 Even in these newly formed platelets, microtubules are often found longitudinally disposed along the long axis, which is consistent with the elegant study by Behnke42 showing that platelets display longitudinal microtubules and that the newly formed blood platelets are elongated and able to curve and become round, thus forming the circumferential microtubule belt. Our findings are consistent with this and other studies,33,43 and with the observation that “stress” platelets, a possible equivalent of young platelets, and ITP platelets have an elongated shape with longitudinal microtubules.44 However, our study disagrees with those that have suggested that the microtubule coil is formed as early as in the MK.17 Nonetheless, the authors illustrate their findings with a micrograph of a newly formed platelet with a longitudinal alignment of microtubules (Fig 8 of Tablin et al17 ), consistent with our observation.
Finally, platelet-shedding MKs were bristled with cytoplasmic pseudopods, and numerous microparticles were present in close proximity. Some of these fragments might have been generated from breakage of the narrow elongated arm of the MK, in the vicinity of which small detached cytoplasmic fragments were released. Platelet dust was first described by Wolf,45 who found that this material contained most of the platelet-related coagulant activity of whole plasma and serum. Crawford46 and George et al47 extended these observations and concluded that these microparticles were formed in vivo by the breakage of activated pseudopods. Many human pathologic states have been found to be associated with microparticle shedding, namely prethrombotic and thrombotic states such as cardiac surgery with cardiopulmonary bypass, ITP, and heparin-induced thrombocytopenia. Microparticles have been associated with platelet activation, but their physiologic role, prothrombotic or anticoagulant, is still unclear.47-49 Our ultrastructural observations of microparticle shedding in platelet production by cultured MKs was confirmed by flow cytometry, which showed that platelet-size elements were found in the supernatant of MK cultures and were accompanied by a dense cloud of very small fragments strongly expressing the CD41 marker. Although it cannot be excluded that some of these particles had been shed by the newly formed platelets activated in vitro, they were absent from the surrounding quiescent non–platelet-shedding MKs and were more numerous in the vicinity of MKs in the process of releasing platelets than near groups of detached platelets. Some of these particles seemed to have detached from elongated cytoplasm territories not included in the formed platelets. Nevertheless, this study provides evidence that apart from platelet shedding, smaller membrane-bound fragments are simultaneously released that might contribute to ensure normal hemostasis. The clinical evolution of patients with ITP whose hemorrhagic syndrome and bleeding time are not as significant as might be predicted from their platelet count might also be partly explained by the compensating effect provided by circulating microparticles formed during increased platelet production. Such a possibility needs to be confirmed by in vivo studies and might be an important phenomenon that should be taken into account during recovery from thrombocytopenia.
ACKNOWLEDGMENT
The authors gratefully acknowledge Dr Pam Hunt and J.L. Nichol (Amgen, Thousand Oaks, CA) for the generous gift of recombinant human MGDF; M. Jean-Marc Massé for excellent photography; Dr Paul-Henri Roméo for constant support; Dr Jean-Philippe Rosa for helpful discussions; and Dr Samuel Burstein for carefully editing the manuscript and for the generous gift of Tab.
Supported by l'Association pour la Recherche sur le Cancer (ARC) and la Fondation pour la Recherche Médicale.
Address reprint requests to Elisabeth M. Cramer, MD, INSERM U.91–Hôpital Henri Mondor Créteil, 94010 Créteil, France.