Abstract
Bone marrow transfusion is a well-established method for induction of mixed hematopoietic chimerism and donor-specific tolerance in animal models. This procedure, however, is inapplicable in clinical transplantation using cadaveric donors due to the interval (1 week to 7 months) between tolerance induction and organ transplantation. For clinical use, it is essential that allografts be placed at the time of bone marrow transfusion. In the present study, we performed skin transplantation within 1 hour after a nonlethal conditioning regimen. Recipient mice were treated with anti-CD3, anti-CD4, low-dose total body irradiation (3 to 6 Gy TBI) and fully mismatched or haploidentical donor bone marrow cells. Stable multilineage chimerism and specific T-cell nonresponsiveness developed. Donor skin grafts were permanently accepted. These results suggest that this single day protocol has clear potential for application in both cadaveric and living-related organ transplantation.
IMPROVEMENTS in immunosuppressive protocols largely account for the current level of success observed in organ transplantation. However, this success has a reverse side. It means life-long treatment of patients with immunosuppressive therapy, which is associated with many, sometimes serious, side-effects. In addition, the continuous risk of chronic rejection, which is difficult to treat, remains. For these reasons, one of the major goals in transplantation immunology is the search for methods of inducing donor-specific tolerance to transplantation antigens.
It has been demonstrated in several animal models that the establishment of mixed hematopoietic chimerism through bone marrow transfusion provides an effective means for the development of specific transplantation tolerance.1-8 Induction of stable mixed chimerism across major histocompatibility complex (MHC) barriers involves a preparative regimen of allogeneic bone marrow cells, short-term immunosuppression, and myeloablation. Conditioning of the host is needed for the permanent engraftment of allogeneic stem cells.
Previously, we have developed a murine model in which recipient mice were treated with anti-CD3, anti-CD4, low-dose total body irradiation (TBI) (3 to 6 Gy) and haploidentical or fully mismatched allogeneic donor bone marrow cells. This well-tolerated, nontoxic protocol results in the development of stable mixed chimerism and permanent transplantation tolerance without any clinical evidence of graft-versus-host-disease (GVHD).9 10
So far, the clinical application of our and other experimental models has been hampered by the relatively long interval between tolerance induction and the actual transplantation, which varies in different models from 1 week to 7 months.1-10 Therefore, in the present study, we have shortened this time interval and performed skin transplantation within 1 hour following conditioning. We report here that stable multilineage mixed chimerism and permanent donor-specific tolerance for skin can be successfully achieved across multiple histocompatibility barriers without using a lethal conditioning approach. Furthermore, this regimen does not require prolonged treatment of the host before transplantation, making it even more attractive for clinical application.
MATERIALS AND METHODS
Animals.Eight- to 14-week-old male mice with different H-2 histocompatibility haplotypes, C57BL/10 (B10, H-2b), B10.D2 (H-2d), and B10.BR (H-2k), were bred at the Central Laboratory of the Netherlands Red Cross Blood Transfusion Service under specific pathogen-free conditions. H-2b/d F1 hybrids were obtained by crossing C57BL/10, H-2b females with B10.D2, H-2d males.
Monoclonal antibodies (MoAbs).For immunosuppression of the host, anti-CD3 (145-2C11; generously provided by Dr J.A. Bluestone, University of Chicago, Chicago, IL) and anti-CD4 (GK1.5; American Type Culture Collection, Rockville, MD) monoclonal antibodies were used as previously reported.9
Bone marrow transfusion.Using sterile procedures, bone marrow cells were flushed from donor femoral and tibial bones and resuspended in Iscove's modified Dulbecco's medium supplemented with 10% heat-inactivated fetal calf serum (FCS). Erythrocytes were lysed with an ammonium chloride buffer. Bone marrow cells were depleted of T cells by treatment with anti-Thy–1.2 monoclonal antibody (F7D5; Serotec, Wiesbaden, Germany) and complement (low tox M rabbit complement; Cedarlane, Hornby, Ontario, Canada), and contained less than 0.2% T cells as measured by flow cytometry analysis. Recipient animals were reconstituted with 15.106 allogeneic bone marrow cells in 0.25 mL of saline containing 5% heat-inactivated autologous serum via caudal vein injection.
Conditioning regimen.B10 mice were treated once with 3 or 6 Gy TBI and a single dose of 400 μg anti-CD3 intraperitoneal (IP). For reconstitution, 15.106 (6.108/kg) haploidentical (B10 × B10.D2)F1 or fully mismatched B10.D2 bone marrow cells or a mixture of 15.106 B10.D2 and 5.106 (2.108/kg) syngeneic bone marrow cells were injected intravenously (IV). Mice were irradiated 3 to 4 hours before MoAb and bone marrow injection. Just before conditioning, recipient mice were given 500 μg of GK1.5, a depleting anti-CD4 MoAb, to reduce the contribution of CD4 positive T cells to the cytokine release syndrome, associated with anti-CD3 treatment.11 Treatment with anti-CD4 alone or with lower doses of anti-CD3 or TBI did not induce stable chimerism and permanent tolerance, as previously shown.9 10
Skin transplantation.Within 1 hour after completing the conditioning regimen, tail skin transplantation was performed. Each recipient mouse was given a control B10 (syngeneic), a B10.D2 or (B10 × B10.D2)F1 (donor), and a B10.BR (third party) skin graft. The grafts were inspected three times a week and considered to be rejected when no viable donor skin was detectable. Second donor-type skin grafts were transplanted 4 months after the first skin grafts.
Flow cytometry analysis.Engraftment of donor cells was determined by two-color flow cytometry using a FACScan (Becton Dickinson, Mountain View, CA). All procedures were performed in phosphate-buffered saline (PBS) containing 0.2% bovine serum albumin and 0.05% sodium azide. Fluorescence data were collected by using logarithmic amplification on 104 viable cells as determined by forward light scatter intensity. To quantify chimerism, cells were stained with H-2Dd-fluorescein isothiocyanate (FITC) and H-2Kb-phycoerythrin (PE) (Pharmingen, San Diego, CA). For lineage analysis of chimerism, PE-conjugated MoAbs against T cells (Thy-1.2, CD4, and CD8), B cells (B220), granulocytes (GR-1), and macrophages (MAC-1) (all from Pharmingen) were used in double staining with antidonor antibody (H-2Dd-FITC).
Cell-mediated lympholysis (CML).CML assays were performed as described previously.9 Radioactivity was measured in a gamma counter (Packard Instrument Co, Meriden, USA). The percentage of specific 51Cr release was calculated by the following formula: % Specific Lysis = [(cpm Experimental − cpm Background 51Cr Release)/(cpm 10% Triton X-100 Release − cpm Background 51Cr Release)] × 100. Data are presented as percent of control. All samples were performed in triplicate.
RESULTS
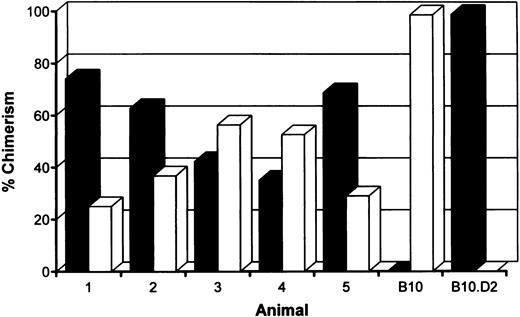
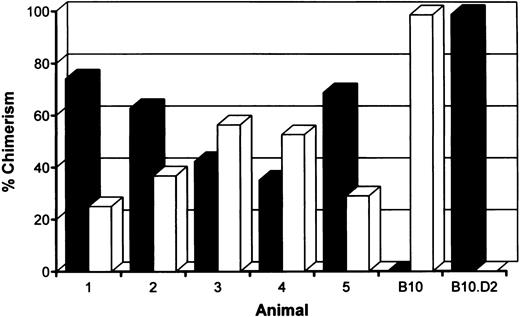
Induction of stable mixed chimerism.To develop a model for clinical use, we have performed our tolerance inducing protocol and the actual allotransplantation on the same day. B10 (H-2b) recipient mice were treated with anti-CD3 and anti-CD4 MoAbs, 6 Gy TBI, and 15.106 fully allogeneic B10.D2 (H-2d) bone marrow cells. To maintain the host own immune repertoire as much as possible, we also reduced the level of chimerism by transfusion with a combination of 5.106 syngeneic and 15.106 allogeneic T-cell depleted bone marrow cells. Within 1 hour following reconstitution, treated animals received skin grafts of recipient (B10), donor (B10.D2) and third party (B10.BR, H-2k) origin. The presence of donor-type cells was measured by fluorescence-activated cell sorting (FACS) analysis on day 49 and day 119, just before the second skin transplantation. Stable mixed chimerism was induced in five of six mice conditioned with a mixture of syngeneic and allogeneic bone marrow cells (Fig 1). As shown before, mice transfused with allogeneic bone marrow cells were repopulated almost completely with cells of donor origin.9,10 No persistent donor bone marrow engraftment was seen after omission of TBI, anti-CD3, or allogeneic bone marrow cells from our conditioning regimen (data not shown).9
Percentages of donor (▪) (H-2d)- and host (□) (H-2b)-derived cells in peripheral blood of mixed chimeras measured 4 months after conditioning by two-color FACS analysis. B10 hosts were treated with anti-T–cell MoAbs, 6 Gy TBI and a mixture of 5.106 syngeneic and 15.106 fully mismatched allogeneic (B10.D2) donor bone marrow cells.
Percentages of donor (▪) (H-2d)- and host (□) (H-2b)-derived cells in peripheral blood of mixed chimeras measured 4 months after conditioning by two-color FACS analysis. B10 hosts were treated with anti-T–cell MoAbs, 6 Gy TBI and a mixture of 5.106 syngeneic and 15.106 fully mismatched allogeneic (B10.D2) donor bone marrow cells.
Because of the prospective HLA matching policies widespread in kidney transplant centers, kidney transplantation across a full HLA disparity does not occur frequently. Therefore, we also tested a haploidentical donor-recipient combination. In this more clinical relevant situation, especially to living-related transplantations, even lower doses of TBI (3 Gy) can be used. All B10 (H-2b) mice receiving MoAbs (B10xB10.D2)F1 (H-2b/d), T-cell depleted bone marrow cells, and 3 or 6 Gy TBI showed stable mixed chimerism (Table 1).
Multilineage chimerism.To determine engraftment of donor-type hematopoietic cells in conditioned mice, one representative animal of each group was killed 9 to 12 months following reconstitution. Cells from the spleen, lymph nodes, peripheral blood, bone marrow, and thymus were collected and screened by flow cytometry. The percentage of chimerism was determined, as well as the contribution of donor-derived lymphoid (T, both CD4 and CD8 subsets, and B cells) and myeloid (granulocytes and macrophages) cells. Results of typing spleen, lymph nodes, and peripheral blood of haploidentical conditioned mice are shown in Table 2 and Fig 2. Donor-type cells were also clearly present in bone marrow and in the thymus (data not shown). Data obtained from fully MHC disparate conditioned mice were comparable. In conclusion, all analyzed chimeric mice showed multilineage donor-chimerism in the lymphoid tissues and the periphery. The level of chimerism induced was dependent on the conditioning regimen (see also Fig 1 and Table 1).
(A) The percentages of host-type (H-2b pos/d neg) and donor-type (H-2b pos/d pos) cells were determined by FACS analysis 9 months after conditioning in lymph node cells of control B10 and (B10 × B10.D2)F1 animals and representative F1 → B10 chimeras (no. 12 and no. 17, Table 1). (B) Analysis of donor-derived (H-2dpos) lymphoid (T and B cells) and myeloid (granulocytes and macrophages) lineages in lymph node cells of a representative chimera (no. 12).
(A) The percentages of host-type (H-2b pos/d neg) and donor-type (H-2b pos/d pos) cells were determined by FACS analysis 9 months after conditioning in lymph node cells of control B10 and (B10 × B10.D2)F1 animals and representative F1 → B10 chimeras (no. 12 and no. 17, Table 1). (B) Analysis of donor-derived (H-2dpos) lymphoid (T and B cells) and myeloid (granulocytes and macrophages) lineages in lymph node cells of a representative chimera (no. 12).
Absolute correlation between substantial levels of mixed chimerism and permanent transplantation tolerance.To examine donor-specific unresponsiveness in vivo, all mice received host, donor, and third party skin grafts directly following bone marrow transfusion. Second skin transplantations were performed 4 months later.
Most importantly, in all recipients, there was an absolute correlation between a substantial level of chimerism and tolerance. All chimeric mice accepted host-type and first and second donor-type skin grafts permanently, without evidence of rejection (Fig 3 and Table 1). In contrast, donor-type skin grafts were rapidly rejected by nonchimeric control animals. Tolerance induction was specific, as third party (B10.BR) skin grafts were rejected by all mice. Furthermore, chimeras appeared healthy without stigmata of GVHD.
Graft survival of donor-type (B10.D2) skin on normal B10 mice (n = 4) and mixed chimeras (transfused with fully allogeneic bone marrow cells [n = 5] or a combination of syngeneic and allogeneic bone marrow cells [n = 5]). First skin transplantation was performed within 1 hour after the conditioning regimen, second skin transplantation was performed 4 months later.
Graft survival of donor-type (B10.D2) skin on normal B10 mice (n = 4) and mixed chimeras (transfused with fully allogeneic bone marrow cells [n = 5] or a combination of syngeneic and allogeneic bone marrow cells [n = 5]). First skin transplantation was performed within 1 hour after the conditioning regimen, second skin transplantation was performed 4 months later.
Thus, we clearly show that a combination of a sublethal dose of TBI, anti-T–cell MoAbs, and MHC incompatible bone marrow cells can ensure permanent tolerance to simultaneously transplanted allogeneic skin of the bone marrow donor strain.
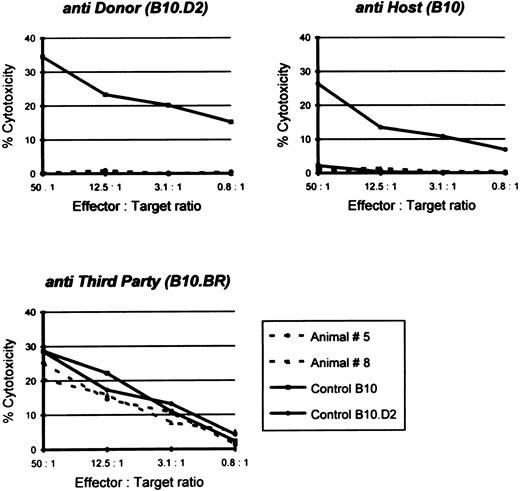
Donor-specific unresponsiveness in vitro.Another important criterion for permanent or classical tolerance induction is immunologic tolerance to host and donor antigens and reactivity to third party antigens in vitro. Therefore, splenocytes from mixed chimeras were examined using a cellular cytotoxicity assay. As shown in Fig 4, spleen cells from mixed chimeras were unresponsive to either donor (B10.D2) or host-type (B10) target cells, whereas the cytotoxic T lymphocyte (CTL) response against third party (B10.BR) cells remained obviously present. Similarly, splenocytes isolated from semiallogeneic-conditioned chimeric mice failed to lyse either host or donor (F1) targets, but showed CTL reactivity to third-party targets (data not shown). In contrast, lymphocytes from untreated control animals exhibited a vigorous reactivity against donor, as well as third party targets (Fig 4).
Specific CTL lysis of 51Cr-labeled host, donor, and third party targets by splenocytes from B10, B10.D2, and two representative chimeras: animal no. 5 transfused with a combination of syngeneic and allogeneic bone marrow cells (68.7% donor-type splenocytes; see also Fig 1) and animal no. 8 transfused with only allogeneic bone marrow cells (95.1% donor-type splenocytes). CTL response was examined 8 months after conditioning. Spontaneous release was less than 25%.
Specific CTL lysis of 51Cr-labeled host, donor, and third party targets by splenocytes from B10, B10.D2, and two representative chimeras: animal no. 5 transfused with a combination of syngeneic and allogeneic bone marrow cells (68.7% donor-type splenocytes; see also Fig 1) and animal no. 8 transfused with only allogeneic bone marrow cells (95.1% donor-type splenocytes). CTL response was examined 8 months after conditioning. Spontaneous release was less than 25%.
DISCUSSION
The ultimate goal in transplantation medicine is to achieve successful organ and tissue transplantation without the need for continuous immune suppression. We and others have demonstrated previously that development of stable mixed chimerism through bone marrow transfusion provides an effective means for the induction of tolerance across MHC barriers.1-10 However, for clinical application in cadaveric organ transplantation, the delay between conditioning and the actual allotransplantation has to be eliminated because organs can only be preserved for a limited amount of time. In addition, in living-related transplantation, simultaneous conditioning and organ transplantation would shorten the donation and transplantation protocols. In the present murine study, we have performed the conditioning regimen and transplantation across major MHC barriers on the same day.
Previously, we showed that sublethally irradiated mice transfused across a fully allogeneic barrier were specifically tolerant to both donor and recipient antigens, and yet fully reactive to third party antigens.9,10 However, in lethally irradiated and fully allogeneic reconstituted mice, significant deficiency in primary humoral responses12,13 and deficient cellular immune responses to intracellular pathogens14 were demonstrated. Furthermore, in clinical allogeneic bone marrow transplantation, complete reconstitution with donor-type cells creates a great risk for the development of GVHD. To minimize these risks, we tried to reduce the level of chimerism in our murine model.10 In this way, we would be able to save as many host-derived antigen presenting cells as possible, necessary for an optimal functioning host immune system. Moreover, it has been reported that mixed chimerism is associated with protection against acute GVHD.15 We manipulated the level of chimerism by varying the dose of TBI, anti-T–cell MoAbs and bone marrow cells or by adding syngeneic bone marrow cells.10 As we discussed before,10 a substantial level of stable mixed chimerism is needed to induce permanent donor-specific tolerance.
The results presented here clearly demonstrate that skin transplantation can be performed successfully directly following donor-specific bone marrow transfusion. We have shown before9 10 that conditioned mice permanently accepted fully mismatched or, with more clinical relevance, haploidentical skin allografts transplanted 2 months (first graft) and 6 months (second graft) after conditioning. The establishment of stable mixed chimerism was confirmed by the presence of both donor- and host-derived cells in lymphoid organs and the periphery, 2, 4, and 9 months after conditioning. This lasting chimerism, as well as the presence of donor-type cells in both lymphoid (T and B cells) and myeloid (macrophages and granulocytes) lineages, suggest that engraftment of donor hematopoietic stem cells has occurred.
A very important observation is that our single day protocol results in ‘real’ or classical transplantation tolerance. All chimeric mice showed specific T-cell nonresponsiveness in vitro and permanently accepted host-and donor-type skin grafts. Second donor-type skin grafts, transplanted 4 months later, were also indefinitely accepted. Third party transplants were rejected.
In the clinical setting, donor bone marrow (stem cells) can be obtained from a living-related donor or from a cadaveric donor at the time of organ procurement. The Pittsburgh group of Starzl recently reported their preliminary results combining high-dose donor bone marrow (3.108/kg) with solid organ (kidney, liver, heart) and cell (pancreatic islet) transplantation without pretransplant radiation or other cytoreduction therapy.16-19 They suggest that simultaneous infusion of donor bone marrow at the time of whole organ transplantation leads to augmentation of microchimerism without the risk for GVHD.18-20 However, patients are still receiving immunosuppressive drugs. Burke et al21 also initiated donor bone marrow infusion as an adjuvant to quadruple immunosuppression for the solid organ kidney/pancreas transplant protocol. Preliminary results demonstrated a higher degree of chimerism in the group receiving bone marrow. Long-term acceptance of renal allografts and multilineage chimerism were demonstrated after administration of donor bone marrow to irradiated, antithymocyte globulin-treated cynomolgus monkeys, without further immunosuppression.6
As stated before, the level of mixed chimerism can be reduced by administration of both donor and autologous bone marrow cells. In the clinical situation, the problem of collecting autologous bone marrow cells can be overcome by using autologous granulocyte colony-stimulating factor (G-CSF )–mobilized peripheral blood stem cells. These autologous cells can be collected and frozen anytime when the patient is on the waiting list for organ transplantation. At the time of transplantation, these cells can be added to the T-cell depleted donor bone marrow inoculum.
Engraftment of bone marrow cells across MHC barriers requires a brief course of immunosuppressive agents and myeloablation. Bone marrow transfusion after conditioning with lethal TBI is associated with considerable toxicity and mortality. For use in patients needing solid organ transplants, it would be desirable to develop a nontoxic method for inducing tolerance across HLA mismatches. In the present study, we used a low, nonlethal dose of TBI (3 to 6 Gy) for myeloablation. Currently, we are investigating if other ‘space’-creating myeloablative agents can be used for tolerance induction.
Thus, in a murine model, the actual allotransplantation can be performed directly following the tolerance inducing protocol. Stable multilineage chimerism and donor-specific skin graft tolerance were achieved in a haploidentical and a fully mismatched donor-recipient combination. Because skin is more immunogenic than any other graft type,22 we suggest that our well-tolerated method can be used for donor-specific tolerance induction in organ transplantation.
ACKNOWLEDGMENT
We thank T. Jansen Hendriks for performing the murine experiments, Drs M.A. van der Pol for technical assistance, and S.S. Weinreich and C.E. van der Schoot for critically reading the manuscript.
Supported by Grants No. C90.953 and C94.1398 from the Dutch Kidney Foundation (Bussum, The Netherlands).
Address reprint requests to Claire J.P. Boog, PhD, Department of Transplantation Immunology, Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands.



![Fig. 3. Graft survival of donor-type (B10.D2) skin on normal B10 mice (n = 4) and mixed chimeras (transfused with fully allogeneic bone marrow cells [n = 5] or a combination of syngeneic and allogeneic bone marrow cells [n = 5]). First skin transplantation was performed within 1 hour after the conditioning regimen, second skin transplantation was performed 4 months later.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/7/10.1182_blood.v89.7.2596/6/m_bl_0047f3.jpeg?Expires=1767778323&Signature=gp7ghySo2y~3Lj3mEUDNys4yqbIiXdYewp9kFBkWH~DbPdnov63J~SAG5OIYuJUFgeey6aZKH8Gx9cDeNOQfObiVl5PqeWm88JokiWeUJgUbo9osYVpBX~dYzIZP-TSpIHNC5JSet19BgTKGUMNE5WljDi86KdwPHULQfBWYxDmSWzZ6tWloC1UqHG~u-q0FfQmLxypdZgBCkRa51WmT6xNQioNoyaDtsdZ0P9xoOhRkSBrMnNlwCjARkKJhP2jke0dVJV7upmZDEV0WLTUSzBNeZNTgZMh8E20TWbjvTi97IjPkSMdJdpZDOKy2Y4RhdWzzIBoyplR6BtoK-b65iQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 3. Graft survival of donor-type (B10.D2) skin on normal B10 mice (n = 4) and mixed chimeras (transfused with fully allogeneic bone marrow cells [n = 5] or a combination of syngeneic and allogeneic bone marrow cells [n = 5]). First skin transplantation was performed within 1 hour after the conditioning regimen, second skin transplantation was performed 4 months later.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/7/10.1182_blood.v89.7.2596/6/m_bl_0047f3.jpeg?Expires=1767778324&Signature=1lSBMINJIE4Sgcv~iRGugITvADUAVzYpafbX-9IiAg1GuJ9R1zTzq~EQcp~qkCz0lfYdp9lehR3weyfO73mTXdo~wTgkbsdV0lfSS~vSjZU-yeR8pj3HXkuUxUtnuL~3R7bXyC5hyiKlIK5pU5hRxYEr0Sz8poS-pWW~ai3I5lRkS2~zEXW1Q0N3KazLFCPE4XVeDNks2z7DQC-2I90DXePbERCgoaWb9s5CaVKJuifli9vo7Y7yTXNgj22~s2tSttgF69c-BAFusw2FAxNeUkYDbU4WTfcOfY3eBZrXoWDfN7d-ExHC1i5o8sR3F7H8VF-2LMMlRj1ih0Ol9dNoYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
