Abstract
CD6 and CD5 belong to a scavenger-receptor cysteine-rich (SRCR) super family of membrane glycoproteins that are expressed on chronic lymphocytic leukemia B (B-CLL) cells, normal T cells, and a small subset of normal B cells. CD6 configures in the membrane in relation to the cellular activation level and can act as a coreceptor for T-cell activation. We have examined a group of progressive and nonprogressive B-CLL cells. Most B-CLL cells were positive for CD6 and the expression of CD6 was increased after activation with Staphylococcus aureus Cowan I plus interleukin-2 or 12-O-tetradecanoylphorbol 13-acetate, although anti-CD6 antibodies did not increase proliferative responses to these stimuli. However, anti-CD6 stimulation was found to protect against anti-IgM–induced apoptosis in B-CLL. baxα upregulation and bcl-2 downregulation were found in anti-IgM– and glucocorticoid (GCC)-induced apoptotic cells, respectively. Furthermore, CD6 cross-linking downregulated baxα mRNA levels in anti-IgM–treated cells, resulting in an increased bcl-2/baxα ratio. CD6 activation also prevented bcl-2 mRNA downregulation and apoptosis induced by GCC in one of six GCC-sensitive patients. These data suggest that an interaction between CD6 and its ligand might contribute to B-CLL survival through the modulation of the Bcl-2/Bax ratio.
B-CELL CHRONIC lymphocytic leukemia (B-CLL) is characterized by the progressive accumulation of monoclonal B cells mature in appearance but biologically immature.1 The clinical course of B-CLL is widely variable. Indolent cases show no progression of the disease for an extended period of time, whereas others show rapid progression and have a poor prognosis.2 Progressive disease is associated with the increased growth of leukemic cells in peripheral blood that may result from both increased cell proliferation and cell survival that can be shown in vitro.3-6
Apoptosis is a distinct type of cell death having characteristic nuclear and cytoplasmic features.7 Susceptibility to apoptosis is regulated by gene products from the bcl-2 gene family, such as bcl-2 and bax.8 9 The bcl-2 gene was cloned from the t(14; 18) translocation breakpoint found in most patients with follicular lymphoma.10bax mRNA is differentially spliced, giving rise to baxα , baxβ , baxγ , and baxδ .11,12 Bcl-2 and Bax proteins form homodimers and heterodimers, which regulate susceptibility to apoptosis.8,11 Bcl-2 prevents apoptosis, whereas Bax contributes to cell death.13,14bcl-2 protects cells from apoptosis induced by a wide variety of agents, whereas overexpression of baxα results in accelerated cell death in transfected cell lines after an apoptotic stimulus.11,15 16
Bcl-2 expression is high in most B-CLL cells,17-19 and the bcl-2/baxα ratio is increased in cells from progressive patients.6 Susceptibility to apoptosis has been correlated to levels of bcl-2 in B-CLL cells.18,20,21 Apoptosis can be induced in both normal and neoplastic B cells by glucocorticoid (GCC) or anti-IgM treatment.22 We have studied the modulation of bcl-2 and baxα during anti-IgM– and GCC-induced apoptosis in B-CLL cells. baxα levels were increased both at mRNA and protein levels in B-CLL cells undergoing apoptosis induced by anti-IgM, whereas the bcl-2 levels were more affected by GCC.
The CD6 antigen is a membrane glycoprotein expressed in mature T cells, thymocytes, and a small subpopulation of normal B cells.23-25 Antibody-mediated CD6 activation can potentiate a proliferative T-cell response.26-28 CD6 is part of a large family of proteins, including CD5 and type I macrophage scavenger receptor.29 Human CD6 binds to the 100-kD activated leukocyte cell adhesion molecule (ALCAM) expressed on the surface of thymic epithelial cells, activated T cells, B cells, and monocytes.30
CD6 is also present on neoplastic B cells, but its function is not known.24 We investigated CD6 expression on leukemic cells from progressive and nonprogressive B-CLL patients and its association with survival and proliferation. CD6 is expressed on a large majority of B-CLL cells but is not correlated to disease progression. CD6 was not found to be involved in B-CLL proliferation in vitro, but rather in the protection of B-CLL cells against apoptosis induced by anti-IgM. We provide evidence suggesting that this protective effect may be mediated through the regulation of the Bcl-2/Baxα ratio.
MATERIALS AND METHODS
Reagents.Lymphoprep was bought from Nycomed Pharma Co (Oslo, Norway). Carbonyl iron, 2-aminoethylisothiouronium bromide, 12-O-tetradecanoylphorbol 13-acetate (TPA), aminoalkylsilane, dexametasone (Dex), and ethidium bromide were bought from Sigma Chemical Co (St Louis, MO). Phenol and chloroform were purchased from Fluka (Buchs, Switzerland). Primers for bcl-2 and baxα amplification and for bcl-2– and baxα -competitor construction were synthesised by Scandinavian Gene Synthesis AB (Köping, Sweden). G3PDH primers and G3PDH competitor were purchased from Clontech (Palo Alto, CA). RNAsin was bought from Promega Corp (Madison, WI). Dithiothreitol, Moloney murine leukemia virus reverse transcriptase, and first-strand buffer were bought from GIBCO BRL (Gaithersburg, MD). Random primers [pd(N)6 ], ultrapure dNTP, and agarose NA were obtained from Pharmacia AB (Uppsala, Sweden). Taq polymerase and the DNA molecular weight marker VI were from Boehringer Mannheim Scandinavia AB (Bromma, Sweden). Recombinant interleukin-2 (IL-2) was obtained from DuPont Scandinavia (Stockholm, Sweden). Staphylococcus aureus Cowan I (SAC) was bought from Calbiochem (San Diego, CA). Hoechst 33342 was from Molecular Probes, Inc (Eugene, OR). Rabbit antimouse Ig (RAM-Ig), fluorescein isothiocyanate (FITC)-conjugated swine antirabbit Ig and FITC-conjugated F (ab′)2 fragment of rabbit antimouse Ig were obtained from Dakopatts (Copenhagen, Denmark). Rabbit polyclonal Ig anti-Bax (P19) and control peptide Bax (P19P), corresponding to amino acids 43-61 mapping within an amino terminal domain of murine Bax, were from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Hamster IgG and FITC-conjugated goat antihamster Ig were from Organon Teknika (Turnhout, Belgium). Rabbit IgG was from Sigma Chemical Co. The anti-CD6 monoclonal antibody (MoAb), IOR-T1 (IgG2a, clone F5/43/27/F6), was produced as described.31 Anti-IgM MoAb (IgG1), purified mouse IgG1 MoAb, phycoerythrin (PE)-conjugated anti-CD19, and PE-conjugated anti-CD25 were from Dakopatts. PE-conjugated anti-CD5 and FITC-IgG1/PE-IgG2a–conjugated Simultest were from Becton Dickinson (Mountain View, CA). Purified mouse IgG2a MoAb was from Pharmingen (San Diego, CA). The hamster anti–Bcl-2 MoAb (6C8) was kindly provided by Dr S.J. Korsmeyer.32
Patients.Twenty-six B-CLL patients (14 women and 12 men) with a mean age of 73.5 ± 9.2 years (range, 54 to 89 years) were studied (Table 1). All patients had a monoclonal B-cell fraction (κ or λ light chains) and were staged according to Rai et al.33 No patient had received therapy for 30 days before the test. Parameters to consider disease progression have been described elsewhere.4-6
Cell isolation.Lymphocytes were obtained by sedimentation of heparinized blood, carbonyl iron treatment, and Lymphoprep centrifugation. T lymphocytes were removed by two consecutive cycles of rosetting with sheep erythrocytes treated with 2-aminoethyl isotiouronium bromide. Isolated cells were kept frozen in aliquots. Unfractionated B cells from tonsils were used as controls. Isolated nonrosetting, leukemic B cells and nonrosetting normal unfractionated tonsillar B cells contained less than 0.2% CD3+ cells as estimated by flow cytometry.
[3H] Thymidine incorporation.Isolated B cells were resuspended in RPMI 1640 medium supplemented with 2 mmol/L glutamine, antibiotics (100 IU penicillin/mL and 100 μg streptomycin/mL) and 0.5% bovine serum albumin. To cross-link anti-CD6 MoAb, flat-bottom 96-well culture plates were preincubated overnight at 4°C with RAM-Ig (10 μg/mL) in 0.05 mol/L bicarbonate buffer (pH 9.6). Unbound antibodies were removed by washing with phosphate-buffered saline (PBS). Cells were cultured at a concentration of 2 × 106/mL, at 37°C and 5% CO2 , and in the presence or absence of the various stimuli. SAC was used at 0.005%, TPA at 50 nmol/L, recombinant IL-2 at 100 U/mL, and anti-CD6 MoAb (IOR-T1) at 10 μg/mL. After culturing for 72 hours, including a 16-hour pulse with 1 μCi/well of 3H-thymidine, cells were harvested onto glass fiber filters (Skatron, Inc, Sterling, VA) and 3H-thymidine incorporation was measured in triplicate.
Flow cytometry.Isolated cells were phenotyped by immunofluorescence. For 30 minutes, 1 × 106 cells were incubated at 4°C with PE-anti-CD5, PE-anti-CD19, PE-anti-CD25, or PE-anti-CD3 or with unconjugated IOR-T1 MoAb or anti-IgM MoAb, plus FITC-F(ab′)2 antimouse IgG. FITC/PE-conjugated simultest was used as control. Isolated cells were also cultured in 24-well plates and were stimulated or not with SAC plus IL-2 or TPA for 72 hours to analyze CD6 expression by indirect immunofluorescence with IOR-T1.
Cells were fixed with 4% paraformaldehyde in PBS for 10 minutes for Bcl-2 and Bax staining. Cells were then permeabilized with 0.1% saponin in PBS with 0.1% bovine serum albumin and stained for 30 minutes at 4°C with either 6C8 MoAb (Bcl-2) and FITC-conjugated goat antihamster IgG or P19 (Bax) and FITC-conjugated swine antirabbit Ig. Cells incubated with hamster IgG or rabbit IgG were used as control for Bcl-2 or Bax staining, respectively. All the samples were analyzed in a Beckton Dickinson FACScan system equipped with an argon laser, using 10,000 cells for each determination and an acquisition rate lower than 400 cells/s.
Survival and apoptosis.Isolated B cells (2 × 106/mL) were cultured in RAM-Ig–coated 24-well plates in the presence or absence of anti-IgM (1.5 μg/mL), IOR-T1 MoAb (10 μg/mL), or Dex (1 μmol/L), singularly or in combination. Purified mouse IgG2a MoAb and purified mouse IgG1 MoAb were used as nonbinding isotype-matched controls for IOR-T1 and anti-IgM MoAb, respectively. Cells were cultured for 16 hours and viability was determined by trypan blue dye exclusion. To evaluate apoptosis, cells were washed, fixed with 4% paraformaldehyde in PBS, and put on aminoalkylsilane-treated slides. Chromatin was stained with 10 μg/mL of the DNA-specific fluorochrome Hoechst 3334234 for 10 minutes. Four hundred cells were independently examined by two persons using a UV microscope to register cells with condensed chromatin and nuclear fragmentation (apoptotic nuclei). Cells were also resuspended in a hypotonic solution (0.3% sodium citrate, 0.01% Triton X-100) containing 50 μg/mL of propidium iodide (PI) and analyzed by flow cytometry to identify the sub G0 peak corresponding to apoptosis.35
Competitive polymerase chain reaction (PCR).Total RNA was extracted from 10 × 106 isolated B cells after they were cultured at 2 × 106/mL in RAM-Ig–coated 12-well plates for 16 hours. RNA was extracted by phenol/chloroform, precipitated with ethanol, and quantitated by spectrophotometry. Three micrograms of total RNA was denatured and reverse transcribed using random hexanucleotides. Thereafter, 1 μL (75 ng) of cDNA was amplified in a 20-μL PCR reaction mixture. Two microliters of serial dilutions of competitor fragments with different lengths, but using the same primers as the target DNA, was added to the reaction. G3PDH competitor (MIMIC, 630 bp) was acquired from Clontech. Competitors for bcl-2 (230 bp) and baxα (602 bp) were built using composite primers and an exogenous DNA fragment (BamHI-EcoRI restriction fragment from v-erb). Cycling conditions were as follows: G3PDH and MIMIC for 30 cycles at 60°C; bcl-2 and bcl-2-competitor for 30 cycles at 72°C; baxα and baxα -competitor for 29 cycles at 66°C; 2 cycles at 67°C; 2 cycles at 68°C; and 2 cycles at 69°C as annealing temperature. An additional 13 minutes at 72°C and 13 minutes at 4°C were allowed at the end of the amplifications. The PCR products were then resolved by agarose gel electrophoresis and photographed. The negative of the photograph was analyzed by laser densitometry. Ratios of the absorbance of the relevant PCR product pairs were plotted against the concentration of the competitor DNA used. The point of intersection in the curve, where the amounts of target and competitor are equal, was extrapolated to the x-axis to determine the absolute values of the PCR products.6 The primers are as follows: sense bcl-2, 5′CGACGACTTCTCCCGCCGCTACCGC3′, antisense bcl-2, 5′CCGCATGCTGGGGCCGTACAGTTCC3′ (319 bp); sense baxα , 5′GCTCTGAGCAGATCATGAAGACAG3′, antisense baxα , 5′CACAAAGATGGTCACGGTCTGC3′ (488 bp); sense G3PDH, 5′TGAAGGTCGGAGTCAACGGATTTGGT3′, antisense G3PDH, 5′CATGTGGGCCATGAGGTCCACCAC3′ (983 bp).
Statistical analysis.Mean values were compared using the Student's t-test for paired samples. Pearson's coefficient of correlation was used to analyze correlations between independent observations, and two-tailed statistical significances were determined.
RESULTS
CD6 antigen expression on leukemic human B lymphocytes.CD6 expression on B-CLL cells has been reported previously.24 We examined CD6 expression on leukemic cells from 26 progressive and nonprogressive B-CLL patients. CD6 staining was higher on B-CLL cells than on normal B cells (P < .001, Table 2). There was no difference in CD6 expression between progressive and nonprogressive B-CLL patients. Although CD6 was expressed on the leukemic cells from most B-CLL patients, there was a great variation in the percentage of CD6+ cells between patients (Table 2). In 77% (20/26) of the patients, greater than 75% of the CD6+ B-CLL cells were found. Differences in the intensity of CD6 expression were also seen between B-CLL cases and mean fluorescence intensity (MFI) did not necessarily correlate with the percentage of CD6+ cells.
Anti-CD6 MoAb did not affect the in vitro proliferative response of B-CLL cells.CD6 cross-linking by IOR-T1 is comitogenic for T lymphocytes together with TPA or anti-CD3 MoAb.28 31 Leukemic cells were stimulated with SAC plus IL-2, TPA, or TPA plus IL-2, with or without CD6 cross-linking to investigate if CD6 activation could mediate similar activity on B-CLL cells. Data from 4 nonresponder (2 nonprogressive and 2 progressive) and 2 responder (progressive) patients are shown in Table 3. Cross-linking of CD6 with IOR-T1 did not affect the proliferative response of B-CLL cells.
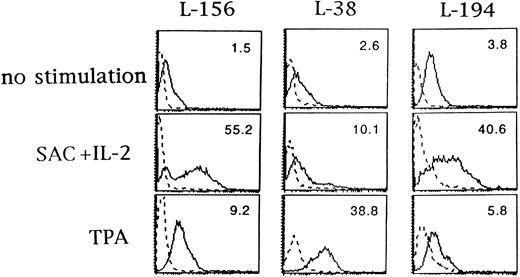
In vitro activation of B-CLL cells increased CD6 surface expression.Several surface molecules on B-CLL cells may be modulated by cytokines or mitogens, such as major histocompatability complex class II molecules, adhesion molecules, CD72, CD40, BB-1/B7, and CD23.36,37 The upregulation of the CD6 molecule in human T cells after activation is well known.38 Isolated B-CLL cells were cultured for 3 days in medium alone or in the presence of SAC plus IL-2 or TPA, and the expression of CD6 antigen was examined by immunofluorescence. CD6 expression was increased after SAC plus IL-2 or TPA stimulation in the three B-CLL cases studied (Fig 1). Unstimulated cells from patient L-194 expressed CD6 on greater than 90% of the cells, whereas the CD6-specific MFI was increased particularly by SAC plus IL-2. The percentage of CD6+ cells in isolated normal tonsillar B cells did not change after activation with SAC plus IL-2 or TPA (not shown), as reported.25
Upregulation of CD6 expression on B-CLL cells by activation with SAC plus IL-2 and TPA. B-CLL cells from L-156, L-38, and L-194 patients were cultured with or without SAC (0.005%) plus IL-2 (100 U/mL) or TPA (50 nmol/L) for 72 hours and analyzed for CD6 expression with IOR-T1 MoAb by flow cytometry. The dashed line represents background fluorescence and the solid line represents CD6 fluorescence. CD6-specific MFI is in the upper right corner of each panel. The x-axis corresponds to logarithmic fluorescence intensity and the y-axis to cell number.
Upregulation of CD6 expression on B-CLL cells by activation with SAC plus IL-2 and TPA. B-CLL cells from L-156, L-38, and L-194 patients were cultured with or without SAC (0.005%) plus IL-2 (100 U/mL) or TPA (50 nmol/L) for 72 hours and analyzed for CD6 expression with IOR-T1 MoAb by flow cytometry. The dashed line represents background fluorescence and the solid line represents CD6 fluorescence. CD6-specific MFI is in the upper right corner of each panel. The x-axis corresponds to logarithmic fluorescence intensity and the y-axis to cell number.
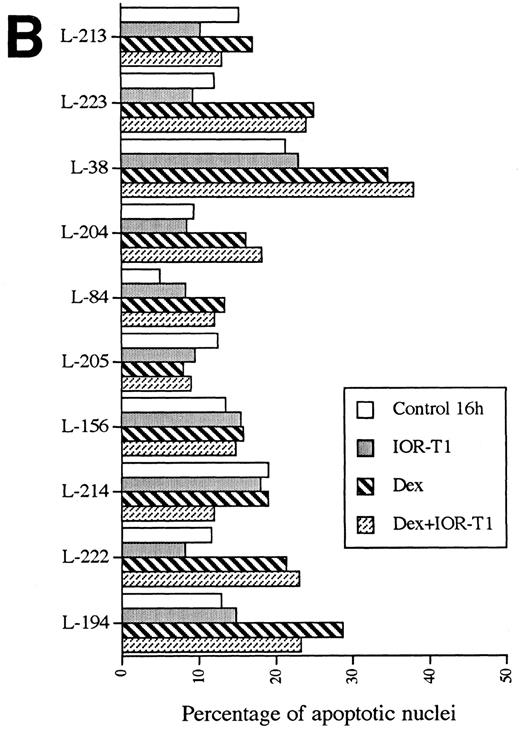
Anti-CD6 MoAb inhibited anti-IgM–induced apoptosis in B-CLL cells.To investigate if CD6 activation can influence B-CLL cell survival, the effect of IOR-T1 cross-linking on B-CLL cell apoptosis induced by anti-IgM MoAb or GCC was explored. Isolated leukemic B cells from 10 B-CLL patients were cultured in RAM-Ig–precoated plates, with anti-IgM MoAb or Dex in the presence or absence of IOR-T1. The percentage of apoptotic cells was determined after 16 hours by Hoechst staining (Fig 2A and B).
Anti-CD6 effect on anti-IgM– (A) or GCC-induced apoptosis (B). B-CLL cells 2 × 106/mL were incubated for 16 hours in RAM-Ig–coated 24-well plates with medium, anti-IgM, IOR-T1, or Dex, alone or in combination. The percentage of apoptotic nuclei was determined using Hoechst staining.
Anti-CD6 effect on anti-IgM– (A) or GCC-induced apoptosis (B). B-CLL cells 2 × 106/mL were incubated for 16 hours in RAM-Ig–coated 24-well plates with medium, anti-IgM, IOR-T1, or Dex, alone or in combination. The percentage of apoptotic nuclei was determined using Hoechst staining.
The percentage of apoptosis varied between 5% and 21.3% (mean, 13.2%; Fig 2A and B) when cells were cultured in medium alone. This was not significantly different from the percentage of apoptosis when cells were cultured in the presence of IOR-T1. However, a significant increase in the number of nuclear fragmented cells in the presence of anti-IgM MoAb was found (range, 15.2% to 44.4%; mean, 39.7%) in all 10 tested B-CLL cell populations as compared with the control (P < .001, Fig 2A). A significant reduction in cell viability was also induced by anti-IgM treatment (P < .01, not shown), which was correlated to IgM expression on the cell surface (r = .534, P < .05).
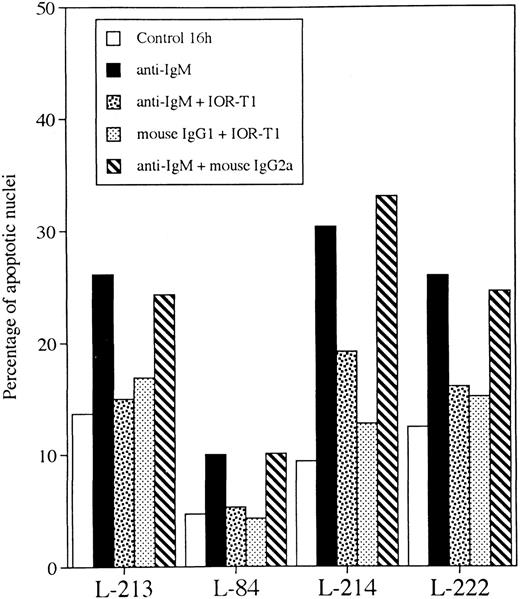
Anti-IgM–induced nuclear fragmentation was reduced in 8 of 10 B-CLL cell populations by IOR-T1 (P < .01, Fig 2A). Only cells from patients L-38 and L-156 were not protected by IOR-T1 against anti-IgM–induced apoptosis. The inhibitory effect of IOR-T1 on anti-IgM–induced apoptosis was also shown using PI staining (Fig 3). A nonbinding isotype-matched control was used to verify that the effect of IOR-T1 was specific and not mediated by the Fc receptor. Purified mouse IgG2a MoAb had no effect (Fig 4). The anti-IgM–induced apoptosis in B-CLL was not mediated by the Fc receptor either, because mouse IgG1 MoAb did not induce B-CLL cell apoptosis (Fig 4). Furthermore, IOR-T1 did not induce changes in the expression of surface IgM on B-CLL cells (not shown).
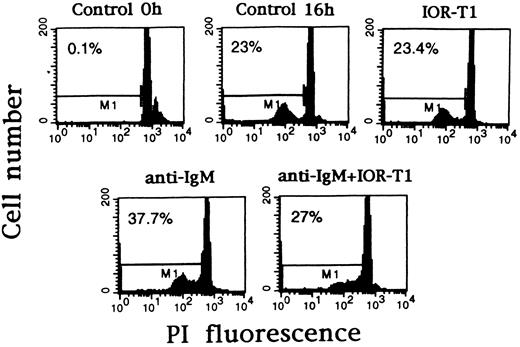
Anti-CD6 can prevent B-CLL cells from anti-IgM–induced DNA fragmentation. B-CLL cells from patient L-204 were incubated in RAM-Ig–coated 24-well plates with medium, anti-IgM, and/or IOR-T1. DNA fragmentation was determined after 16 hours using PI staining and FACS analysis. The percentage of apoptotic nuclei corresponding to M1 marker is in the left corner of each panel.
Anti-CD6 can prevent B-CLL cells from anti-IgM–induced DNA fragmentation. B-CLL cells from patient L-204 were incubated in RAM-Ig–coated 24-well plates with medium, anti-IgM, and/or IOR-T1. DNA fragmentation was determined after 16 hours using PI staining and FACS analysis. The percentage of apoptotic nuclei corresponding to M1 marker is in the left corner of each panel.
Anti-IgM– and IOR-T1-induced effects are not mediated by Fc receptor. B-CLL cells 2 × 106/mL were incubated for 16 hours in RAM-Ig–coated 24-well plates with medium, anti-IgM, IOR-T1, mouse IgG2a, or mouse IgG1 MoAbs in combination as indicated. The percentage of apoptotic nuclei was determined using Hoechst staining.
Anti-IgM– and IOR-T1-induced effects are not mediated by Fc receptor. B-CLL cells 2 × 106/mL were incubated for 16 hours in RAM-Ig–coated 24-well plates with medium, anti-IgM, IOR-T1, mouse IgG2a, or mouse IgG1 MoAbs in combination as indicated. The percentage of apoptotic nuclei was determined using Hoechst staining.
There was a significant increase in the percentage of apoptotic cells in GCC-treated B-CLL cells as compared with the control (P < .05, Fig 2B). However, cells from 4 patients (L-213, L-205, L-156, and L-214) were resistant to GCC and only 1 (L-194) of 6 GCC-sensitive cases was partially protected (35% reduction) by anti-CD6 MoAb from GCC-induced apoptosis (Fig 2B).
Anti-CD6 MoAb modulated bcl-2 and baxαmRNA levels in B-CLL cells undergoing anti-IgM– and GCC-induced apoptosis.The expression of bcl-2 and baxα mRNA was examined in B-CLL cells treated with anti-IgM or GCC. We analyzed bcl-2 and baxα mRNA by competitive PCR in L-194 and L-214 B-CLL cells in which IOR-T1 cross-linking counteracted anti-IgM– and/or GCC-induced apoptosis. IOR-T1 also inhibited the increase in cell mortality induced by anti-IgM in L-161 and L-206 B-CLL cases as determined by trypan blue staining (not shown). One case (L-156) in which IOR-T1 cross-linking did not modify anti-IgM– or GCC-induction of apoptosis was also included.
In cells treated with anti-IgM, the bcl-2/baxα ratio was reduced in every case as compared with cells cultured in medium alone (Table 4). There was a decrease in bcl-2 and an increase in baxα mRNA in cells from patients L-161, L-194, and L-206 treated with anti-IgM, with the most impressive effect being the upregulation of baxα mRNA. In patient L-214, the reduction of bcl-2/baxα ratio was a consequence of the baxα mRNA upregulation with anti-IgM. Anti-IgM also induced downregulation of bcl-2 mRNA, but had no effect on the baxα mRNA level in cells from patient L-156.
In patients L-161, L-194, L-214, and L-206, simultaneous cross-linking of anti-IgM and IOR-T1 induced a significant reduction in the baxα mRNA values but did not change the bcl-2 values as compared with anti-IgM alone (Table 4). There was a correlation between the increase in the bcl-2/baxα ratio (Table 4) and the IOR-T1–mediated protection against anti-IgM–induced cell death by apoptosis in these cases (Fig 2A). IOR-T1 did not significantly affect anti-IgM–induced changes in bcl-2 or baxα mRNA values in L-156 cells (Table 4), which was in agreement with the lack of IOR-T1–mediated protection against anti-IgM–induced apoptosis (Fig 2A). Although there was a small reduction in bcl-2 in the leukemic cells from patients L-161, L-194, and L-206, the bcl-2/baxα ratio was not significantly altered in the presence of IOR-T1 alone (Table 4).
GCC induced downregulation of bcl-2 mRNA in L-161 and L-194 cells. baxα values were also reduced by GCC treatment in cells from these two patients, but the bcl-2/baxα ratio remained lower than that of the controls (Table 4). GCC treatment did not either decrease the cell viability (not shown) or affect the bcl-2 and baxα mRNA expression in L-214 and L-206 B-CLL cells. There was also a small reduction of the bcl-2/baxα ratio in GCC-treated L-156 cells (Table 4), but no increased apoptosis (Fig 2B). IOR-T1 inhibited the bcl-2 downregulation induced by GCC treatment of L-194 cells, resulting in an increased bcl-2/baxα ratio (Table 4).
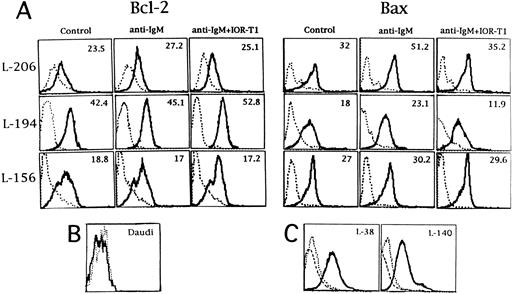
Modulation of Bax protein by IOR-T1 in anti-IgM–treated B-CLL cells.The effect of anti-CD6 cross-linking on the Bcl-2 and Bax protein levels in anti-IgM–treated L-206, L-194, and L-156 leukemic cells was analyzed. There was no change in the levels of the Bcl-2 protein with any of the treatments (Fig 5A). However, Bax protein levels were in accordance with the changes induced in the baxα mRNA expression (presented in Table 4). Bax protein was higher after anti-IgM treatment as compared with Bax protein levels in L-206 and L-194 cells cultured in medium (Fig 5A). Furthermore, Bax protein levels were comparable to control values when these anti-IgM–treated cells were stimulated in the presence of IOR-T1 (Fig 5A). No significant change in the Bax protein level was observed in L-156 cells with any of the treatments (Fig 5A). The specificity of the Bcl-2 antibody was shown by the negative staining of the Daudi cell line6 (Fig 5B). The specificity of the Bax antibody was shown by blocking the staining with a Bax peptide (Fig 5C).
(A) Expression of Bcl-2 and Bax protein in B-CLL cells from L-206, L-194, and L-156 patients. Cells were incubated in RAM-Ig–coated 24-well plates with medium, anti-IgM, or anti-IgM plus IOR-T1. Bcl-2 and Bax proteins were determined by flow cytometry after 16 hours. The solid line indicates Bcl-2 or Bax staining and the dotted line represents background control cells staining with nonspecific hamster IgG or rabbit IgG. Specific MFI (after the subtraction of background MFI) is in the right corner of each panel. (B) Negative control for Bcl-2 staining. The dotted and solid lines indicate background and Bcl-2 staining in Daudi cell line, respectively. (C) Specificity of the Bax antibody. Two different B-CLL cells were stained with anti-Bax antibodies that had been blocked with the specific peptide (AA 43-61; dashed line). The dotted and solid lines indicate background control and Bax staining, respectively. The x-axis corresponds to logarithmic fluorescence intensity and the y-axis corresponds to cell number.
(A) Expression of Bcl-2 and Bax protein in B-CLL cells from L-206, L-194, and L-156 patients. Cells were incubated in RAM-Ig–coated 24-well plates with medium, anti-IgM, or anti-IgM plus IOR-T1. Bcl-2 and Bax proteins were determined by flow cytometry after 16 hours. The solid line indicates Bcl-2 or Bax staining and the dotted line represents background control cells staining with nonspecific hamster IgG or rabbit IgG. Specific MFI (after the subtraction of background MFI) is in the right corner of each panel. (B) Negative control for Bcl-2 staining. The dotted and solid lines indicate background and Bcl-2 staining in Daudi cell line, respectively. (C) Specificity of the Bax antibody. Two different B-CLL cells were stained with anti-Bax antibodies that had been blocked with the specific peptide (AA 43-61; dashed line). The dotted and solid lines indicate background control and Bax staining, respectively. The x-axis corresponds to logarithmic fluorescence intensity and the y-axis corresponds to cell number.
DISCUSSION
CD6 is an important costimulatory molecule on T lymphocytes.26-28 However, CD6 function has not been properly determined in B cells, despite CD6 expression on leukemic and a subset of normal B cells.24 25 We studied CD6 expression in several B-CLL patients and looked for a correlation with clinical progression, in vitro survival, and proliferation. CD6 was expressed on cells from most B-CLL patients. Differences in CD6 expression between patients were unrelated to clinical progression. CD6 activation did not promote proliferation. However, CD6 was upregulated on activated B-CLL cells, in contrast with the absence of CD6 modulation in activated normal B cells.
To our knowledge, this is the first report on bcl-2 and baxα mRNA modulation during anti-IgM–induced apoptosis in B-CLL cells. Strong induction of the baxα mRNA and protein levels was the most relevant alteration induced by IgM ligation. In addition, CD6 activation reduced anti-IgM–induced apoptosis and decreased baxα mRNA and protein levels. Although more data are required, it seems that the inability of IOR-T1 to protect some B-CLL cells (L-38 and L-156) from anti-IgM–induced apoptosis is associated with a low expression of the CD6 molecule in such cells.
Downregulation of bcl-2 could be an important event for GCC-induced apoptosis in B-CLL cells. In one patient, IOR-T1 protected against GCC-induced apoptosis and inhibited the bcl-2 downregulation induced by GCC. On the other hand, lack of CD6-mediated protection for GCC-induced apoptosis was not related to the CD6 surface expression, because high expression of IOR-T1 was not accompanied by a protective effect.
It has been suggested that the Bcl-2/Bax ratio determines cell susceptibility to a given apoptotic stimulus.11 Enhanced bcl-2 expression has been associated with the protective effects of IL-4, interferon α, and interferon γ against apoptosis induced in B-CLL cells.20,21,39bcl-2 and bax expression has already been associated with spontaneous and GCC-induced apoptosis in B-CLL cells.6,40 McConkey et al40 found an association between resistant B-CLL cells to GCC with constitutively high Bcl-2 and Bax protein levels and Bax downregulation after treatment. Induction of anti–APO-1 — mediated apoptosis in B-CLL cells appeared to correlate with bcl-2 mRNA downregulation.18baxα upregulation was observed in IgM-mediated apoptosis in a human B-lymphoma cell line.41 The present data reinforce the importance of bcl-2 and bax gene regulation in anti-IgM– and GCC-induced apoptosis in B-CLL cells, together with a modulatory effect of CD6 cross-linking by IOR-T1 MoAb that results in reduction of apoptosis.
IOR-T1 cross-linking prevented anti-IgM–induced apoptosis in most of the cases. However, the activation of CD6 failed to protect against spontaneous apoptosis and could only protect against GCC-induced apoptosis in one case. This is not surprising, because culture under suboptimal conditions, anti-IgM and GCC treatment represent stimuli that may generate different intracellular apoptotic pathways. Nothing is currently known about the transduction signals elicited via CD6 that are linked to the modulation of the Bcl-2/Bax ratio. Anti-CD6 MoAb can activate protein tyrosine kinase in T lymphocytes in the presence of TPA.31 It will be interesting to explore the possibility of whether protein kinases can be triggered by CD6 in B-CLL cells and what their relationship is with the ability of CD6 to rescue cells from apoptosis.
Clinical progression was found to have no correlation to CD6 expression, cytolytic effects of GCC and anti-IgM treatment, or the protective effects of CD6 engagement. However, the gene modulation that occurs as a consequence of these stimuli could be important in the molecular mechanisms that contribute to disease progression. CD6 and CD6 ligand (CD6L) can play a significant role in T-B cells and stromal tissue interactions. Because both these molecules have been detected in activated normal T cells, it will be important to investigate the expression of CD6L and CD6-CD6L interactions in B-CLL cells. There is some evidence to support a regulatory role for T cells in B-CLL.4 42-44 Whether CD6 interactions can actually constitute a bridge for communication between autologous cells and leukemic B cells could be essential to understanding better and eventually restricting the accumulation of monoclonal leukemic B cells.
In summary, we found that bcl-2 and baxα mRNA are differently regulated during anti-IgM– and GCC-induced apoptosis in B-CLL cells. baxα upregulation was the most frequent alteration in anti-IgM–treated cells, whereas modulation of bcl-2 and other bcl-2 family members could be more important for GCC-induced apoptosis in B-CLL cells. CD6 cross-linking counteracted anti-IgM–induced baxα upregulation and decreased cell death by apoptosis. However, CD6 cross-linking did not reduce GCC-induced apoptosis in most cases. Our results provide evidence that CD6 activation is a mechanism promoting B-CLL cell survival through the regulation of the Bcl-2/Bax ratio. CD6 activation might also provide other survival signals apart from those connected to Bcl-2/Bax modulation.
ACKNOWLEDGMENT
The authors are indebted to Dr S.J. Korsmeyer for providing the antibody 6C8.
Supported by funds from the Swedish Cancer Association, the Cancer Society in Stockholm, The Swedish Medical Association, and The Swedish Institute.
Address reprint requests to Lyda M. Osorio, MD, Microbiology and Tumor Biology Center (MTC), Box 280, S-171 77, Karolinska Institute, Stockholm, Sweden.