Abstract
We have previously shown in a murine acute myelogenous leukemia (AML) model that leukemic mice can be cured with a B7 vaccine if immunized early in the disease and that CD8+ T cells are necessary for tumor rejection. However, when B7 vaccine is administered 2 weeks after leukemia inoculation, the effect is only prolonged survival, ending in death virtually of all the mice. To distinguish between tumor kinetics and tumor-induced immunosuppression as potential mechanisms eliminating the therapeutic potential of late B7 vaccines, we performed in vitro T-cell studies during leukemia progression and in vivo studies on the clinical outcome of late B7 vaccines in combination with prior cytoreductive chemotherapy. Our results show that CD8+ T cells from leukemic mice 1 and 2 weeks after leukemia inoculation proliferate more vigorously in response to in vitro activation than cells from normal mice and produce Th1-type cytokines interleukin-2 and interferon-γ. Cytotoxic T lymphocyte (CTL) assays demonstrate that cells from week-2 vaccinated mice (which succumb to their leukemia), surprisingly develop a stronger CTL activity than cells from week-1 vaccinated mice (which reject their leukemia). Finally, the combination of late chemotherapy and late B7 vaccine administration can cure only 20% of leukemic mice, whereas early chemotherapy and the same late B7 vaccine administration cure 100% of leukemic mice. These results demonstrate that in murine AML tumor growth does not induce T-cell anergy or a Th2 cytokine profile and suggest that tumor growth is most likely to be the limiting factor in the curative potential of late B7 vaccines.
FOR SEVERAL DECADES it was believed that the failure of the immune system to exert an effective surveillance in cancer patients and tumor-bearing animals arises from the lack of tumor-specific antigen expression on tumor cells. Accumulating evidence1-8 now suggests that this failure does not result from the absence of recognizable tumor antigens but, rather, from the inability of tumor cells to either initiate or complete an effective immune cytolytic response. Primary factors that have been implicated for this unfavorable host-tumor relationship are the inability of most of the tumor cells to provide T-cell costimulatory signals,9,10 and the absence of an appropriate cytokine microenvironment.11-13
A successful antitumor T-cell response involves the induction, recruitment, and effector functions of tumor-specific T cells and requires at least two distinct signals: a signal through the T-cell receptor (TcR) complex, and a costimulatory signal provided by the ligation of adhesion molecules on T cells with their counterreceptors on the surface of professional antigen-presenting cells (APCs).14,15 The CD28 signaling pathway is the most potent costimulatory pathway identified to date and is activated by binding of CD28 on T cells to either B7.1 (CD80) or B7.2 (CD86) expressed on the surface of professional APCs.16,17 Several studies in murine tumor models have shown that ectopic expression of the members of the B7 family of T-cell costimulatory molecules by tumor cells is effective at inducing protective immunity against a number of murine tumors.18-22 Recently, we have shown in a murine acute myelogenous leukemia (AML) model, that one intravenous (IV) injection of irradiated B7.1+ AML cells can provide mice with long-lasting systemic immunity against subsequent challenge with wild-type AML cells, and that CD8+ T cells are necessary for the tumor-specific immune response.23 Furthermore, one exposure to irradiated, B7.1+ AML cells can cure leukemic mice vaccinated early (1 week) after leukemia inoculation, whereas late (2 week) vaccinations only delay tumor growth. This discrepancy is consistent with other model systems, where an effective immune response could only be achieved against small pre-existing tumor burdens.18,21,22 These results have provoked controversy, as to whether tumor growth induces mechanisms of peripheral tolerance to cancer, thus eliminating the therapeutic potential of late vaccines. In support of a dysregulated immune system are reports of cancer patients with progressive disease and marked decreases in T-cell responses,24,25 and of T cells from both patients and tumor-bearing mice that produce Th2 (IL-4, IL-10) cytokines,26,27 which can inhibit a cell-mediated antitumor immune response.28
Although it is not yet known how T cells “interact” with tumor antigens in vivo, it has been speculated but not demonstrated that these interactions can induce tolerance in the host.29 Moreover, there is a scant information pertaining to the functional properties of T cells in response to hematopoietic tumor models. In murine AML tumor models, AML cells home to bone marrow and spleen after IV inoculation. This very close interaction between AML cells and T cells from the initial stages of the disease, in a presumably different cytokine and APC microenvironment than in any solid tumor model, could affect the function of the T cells in an unpredictable manner.
The current studies were undertaken to distinguish between rapid tumor growth and immunosuppression as potential mechanisms eliminating the therapeutic potential of late B7 vaccines and to investigate if the combination of cytoreductive chemotherapy and B7 vaccines can cure mice with advanced AML. We studied the in vitro functional properties of splenic CD8+ cells from mice with different tumor burdens (week 1 or 2 after AML inoculation) and the CTL activity of CD8+ T cells from mice vaccinated at week 1 or 2 of the AML course, previously shown to have different clinical outcomes. We show that CD8+ T cells from leukemic mice respond more vigorously than cells from normal mice to in vitro activation and produce Th1-type cytokines. The cytotoxic T lymphocyte (CTL) assays demonstrate that, despite the nontherapeutic clinical outcome of mice vaccinated at week 2, cells from these mice generate a stronger cytolytic response than cells from mice vaccinated at week 1, suggesting that progressive growth of tumor induces mechanisms that defeat a tumor-specific generated CTL activity. Finally, we show that the combination of chemotherapy, to reduce tumor burden, and B7 vaccines can lead to more effective clinical responses of mice with advanced disease.
MATERIALS AND METHODS
Mice.Female SJL/J mice, 6 to 8 weeks old, were purchased from Jackson Laboratories (Bar Harbor, ME) or Charles River Laboratories (NCI-Frederick Cancer Research & Development Center Frederick, MD). The animals were kept at the animal facility of Dana-Farber Cancer Institute (Boston, MA) according to the Institute's guidelines.
Murine AML model.The murine AML model used in this study has been previously described.23 Briefly, AML cells, originally obtained from radiation-induced AML in female SJL/J mice,30 are maintained by growing in syngeneic SJL/J mice. Mice injected IV or intraperitoneally (IP) with ≥104 AML develop leukemia and die in 4 to 5 weeks. In all experiments, freshly isolated or frozen spleen mononuclear cells from leukemic mice (4 to 5 weeks after the tumor inoculation) were used. Flow cytometry results demonstrated that these cells were essentially 100% myeloblasts.23
Purification of CD8+ T cells.Spleens from naive or leukemic SJL/J mice were removed aseptically and single cell suspensions were obtained on ice by gently pressing tissue pieces against the bottom of a petri dish with a syringe plunger. Cells were stained with Ly-2 monoclonal antibody (MoAb) (PharMingen, San Diego, CA), labeled with goat antirat IgG Microbeads (Milteny; Biotec, Sunnyvale, CA) and selected using magnetic MiniMacs separation columns (Milteny). Isolated cells were 90% to 94% pure as determined by immunofluorescent flow cytometry analysis (FACS) and appeared viable by exclusion of trypan blue and forward/side scatter analysis. The absolute numbers of purified CD8+ T cells from week 1 and week 2 leukemic mice were approximately the same.
Proliferation assays and reagents.Purified CD8+ T cells were cultured at 2 × 105 cells/well in U-bottomed 96-well plates in the presence of Concanavalin A (ConA; 2.5 μg/mL) or phorbol 12-myristate 13-acetate (PMA; 10 ng/mL) plus ionomycin (300 ng/mL). For TcR-crosslinking of CD8+ responder cells, wells were precoated with anti-CD3 MoAb 145-2C11 (2.5 μg/mL) for 90 minutes at 37°C. A feeder layer of 2 × 105 syngeneic, anti-Thy 1.2 and C′-treated and irradiated (2,000 rad) splenocytes was also added to each well. Supernatants were collected after 24 and 48 hours of culture and were assayed for cytokine levels as described below. Proliferation of responder cells was measured after 72 hours by the incorporation of 3H thymidine (1 μCi/well) for the last 20 hours of incubation.
ConA, PMA, and ionomycin were purchased from Sigma Chemical Co (St Louis, MO). The anti-Thy 1.2 MoAb used for T-cell depletion was isolated from tissue culture supernatants from hybridoma clone HO-13-4 (American Type Culture Collection, Rockville, MD; TIB # 99).31 Low-Tox-M rabbit complement (C′ ) was obtained from Accurate Corp (Westbury, NY). The anti-CD3 MoAb (145-2C11) was a generous gift from Dr S.J. Burakoff (Dana-Farber Cancer Institute).
Lymphokine ELISAs.Levels of interleukin-2 (IL-2), interferon-γ (IFN-γ), and IL-4 in tissue culture supernatants were determined by sandwich ELISA using specific antimurine MoAbs for capture and detection (PharMingen). A color reaction was developed using streptavidin-conjugated horseradish peroxidase (Genzyme, Cambridge, MA) followed by TMB peroxidase substrate (Kirkegaard & Perry Laboratories, Inc, Gaithersburg, MD). A standard curve was generated to determine the cytokine concentration in the sample. The lower detection limit of all cytokine assays was 1.0 U/mL. Each assay was performed in triplicate at least three times, and the results from a typical assay are reported.
The following MoAbs for capture and detection were used: IL-2, purified JES6-1A12 and biotin-conjugated JES6-5H432; IFN-γ, purified R4-6A233 and biotin-conjugated XMG1.234; IL-4, purified 11B1135 and biotin-conjugated BVD6-24G2.32 Recombinant murine IL-2 (rIL-2) was obtained from Boehringer Mannheim (Indianapolis, IN). Murine rIFN-γ was purchased from Amgen (Thousand Oaks, CA) and murine rIL-4 with a specific activity of 7 × 107 U/μg by FDCP-2 assay was obtained from Immunex Corp (Seattle, WA).
51Cr release assays.One or 2 weeks following injection of AML cells the mice were immunized with irradiated 105 B7-AML cells. One week after immunization their splenocytes were harvested and nylon-wool-enriched T cells were depleted of CD4+ and GR-1+ cells by magnetic cell separation. The resulting population of cells was used as effectors for the CTL assays. We could not detect any differences in the absolute numbers of purified CD8+ T cells from week 1 and week 2 vaccinated mice. FACS analysis showed that these cells were 90% to 94% CD8+ cells. Autologous AML cells or control EL-4 tumor cells were incubated with 51Cr (New England Nuclear, Boston, MA) for 2 hours and used as targets in the CTL assays.
The standard 4-hour CTL assays were set up with various effector to target (E:T) ratios in a total volume of 0.2 mL/well in a 96-well microtiter plate. All conditions were set up in quadruplicate. After a 4-hour incubation, 100 μL of supernatant was harvested from each well, and the quantity of 51Cr in the supernatants was determined using a gamma counter. Results are expressed as the percentage of specific lysis, calculated as 100 × (sample cpm − spontaneous cpm)/(maximum cpm − spontaneous cpm). The cpm of supernatant from wells containing target cells in normal media and from wells containing target cells in 1% Triton X-100 served as the spontaneous and the maximum release, respectively.
In vivo immunization studies.SJL/J mice were injected IV with live or irradiated (3,200 rad from a 137Cs source) B7.1 transduced AML cells. Expression of B7.1 on AML cells has been previously described.23 Briefly, E-86 cells were transduced with the LNCX-B7.1-sense or LNCX-B7.1-antisense (mock virus) retroviral constructs and two clones (B7.1 sense and B7.1 antisense), secreting high titers of virus, were used to infect the AML cells. AML cells (3 to 5 × 105 cells/mL) were exposed to viral supernatant for 24 to 48 hours. Infected, unselected AML cells were washed in phosphate buffered saline (PBS), counted, and the designated numbers of cells were used for the in vivo immunizations.
Cytoreductive treatment.Mice were injected IV in the tail vein with 105 wild-type AML cells in 0.3 ml saline. On days 7, 10, 14 (protocol I) or 3, 6, and 8 (protocol II) post AML inoculation, they received two consecutive IP injections of 200 mg/kg Ara-C (CHIRON Therapeutics, Emeryville, CA), 6 hours apart. Treated mice were vaccinated on day 16 after leukemia inoculation with B7-AML cells. Some mice remained without vaccination and 1 or 2 weeks after the end of chemotherapy (corresponding to 2 or 3 weeks post AML inoculation) they were killed and their spleen and bone marrow cells were stained with the GR-1 MoAb and analyzed by immunofluorescent flow cytometry.
Immunostaining and flow cytometry analysis.Cells were stained as previously described.23 The following antibodies were used in this study: CD80 (B7.1), Gr-1, CD4 (L3T4), CD8a (Ly-2), (PharMingen). After staining, the cells were fixed in 2% paraformaldehyde and analyzed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
Statistical analysis.Values are mean ± SEM. The statistical significance between any two groups was analyzed by Student's t-test.
RESULTS
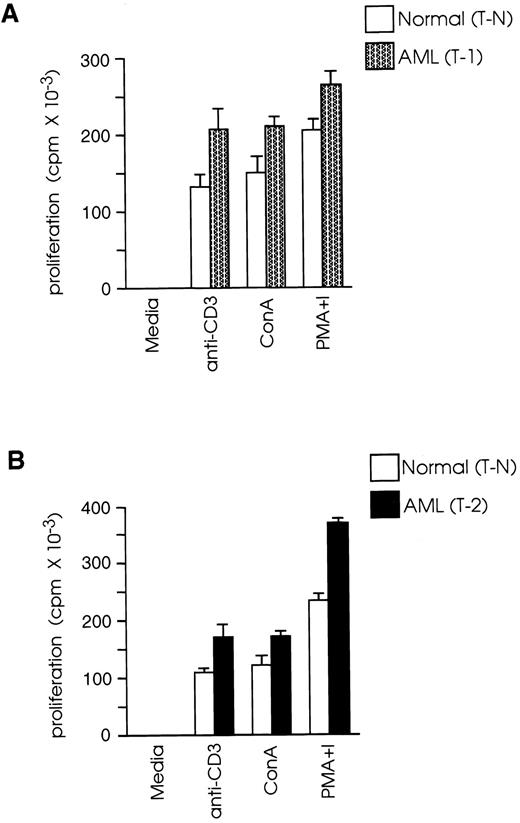
CD8+ T cells from leukemic SJL/J mice proliferate more vigorously than CD8+ T cells from normal SJL/J mice.In vivo depletion studies have shown that, in this AML model, rejection of the leukemic cells by vaccinated mice requires the presence of CD8+ but not of CD4+ T cells.23 Therefore, we studied the functional properties of CD8+ T cells from leukemic mice at 1 week (T-1) or 2 weeks (T-2) after leukemia inoculation and compared them with the functional properties of cells from normal mice (T-N). We examined the capacity of T-1, T-2, and T-N cells to proliferate in response to in vitro activation with: (1) a high density/high avidity TcR signal, provided by plate-bound anti-CD3; (2) ConA; and (3) PMA plus ionomycin. As shown in Fig 1A, T-1 cells showed 57% higher proliferative response than T-N in response to activation with anti-CD3 (P < .01), 39% higher response to ConA (P < .01), and 28% higher response to PMA plus ionomycin (P < .01). Similarly, T-2 cells had 55%, 41%, and 58% higher response than T-N cells in response to stimulation with anti-CD3, ConA, and PMA plus ionomycin, respectively (Fig 1B). These results revealed that T-1 and T-2 cells responded more vigorously than T-N cells not only to PMA plus ionomycin, a signal known to bypass the need for TcR stimulation by activating distal to PKC signaling pathways, but also to TcR stimulation (ConA or anti-CD3 MoAb), suggesting that the majority of T-1 and T-2 cells were not anergic.
CD8+ T cells from leukemic mice proliferate more vigorously than CD8+ T cells from normal mice. Splenic CD8+ T cells were isolated from normal and leukemic SJL/J mice, 1 week (T-1) or 2 weeks (T-2) after leukemia injection, by magnetic cell separation. T-1 (A) and T-2 (B) cells were cultured at 2 × 105 cells/well in the presence of ConA (2.5 μg/mL), PMA (10 ng/mL) plus ionomycin (300 ng/mL) or anti-CD3 MoAb (2.5 μg/mL) and a feeder layer of 2 × 105 syngeneic, anti-Thy 1.2 and C′-treated and irradiated (2,000 rad) splenocytes. 3H thymidine (1 μCi/well) was added during the last 20 hours of culture. Results are representative of three independent experiments (each experiment included two to three mice) and are shown as the mean and SD of triplicate culture.
CD8+ T cells from leukemic mice proliferate more vigorously than CD8+ T cells from normal mice. Splenic CD8+ T cells were isolated from normal and leukemic SJL/J mice, 1 week (T-1) or 2 weeks (T-2) after leukemia injection, by magnetic cell separation. T-1 (A) and T-2 (B) cells were cultured at 2 × 105 cells/well in the presence of ConA (2.5 μg/mL), PMA (10 ng/mL) plus ionomycin (300 ng/mL) or anti-CD3 MoAb (2.5 μg/mL) and a feeder layer of 2 × 105 syngeneic, anti-Thy 1.2 and C′-treated and irradiated (2,000 rad) splenocytes. 3H thymidine (1 μCi/well) was added during the last 20 hours of culture. Results are representative of three independent experiments (each experiment included two to three mice) and are shown as the mean and SD of triplicate culture.
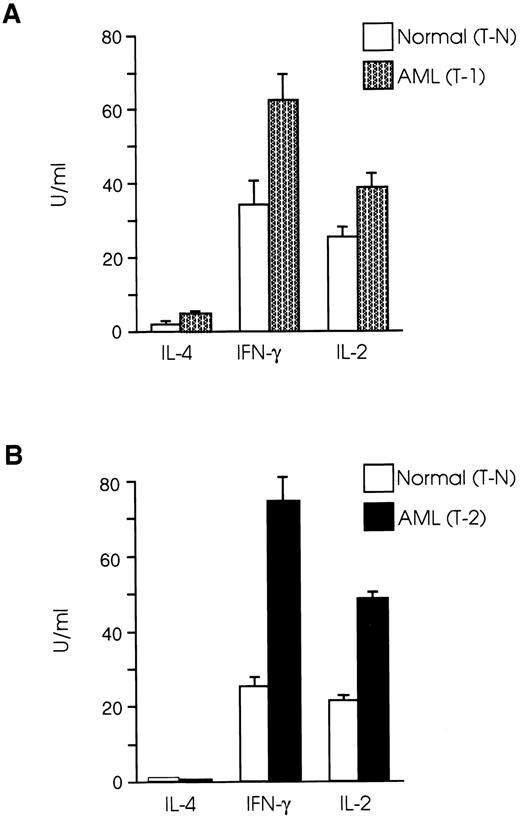
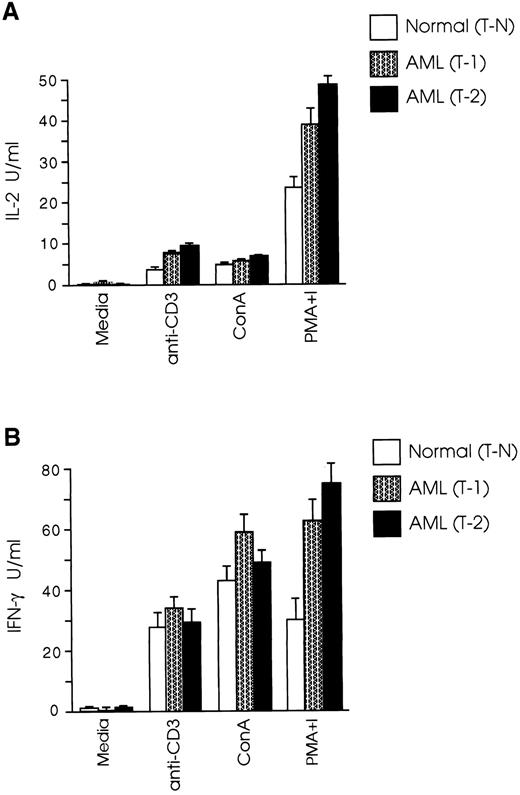
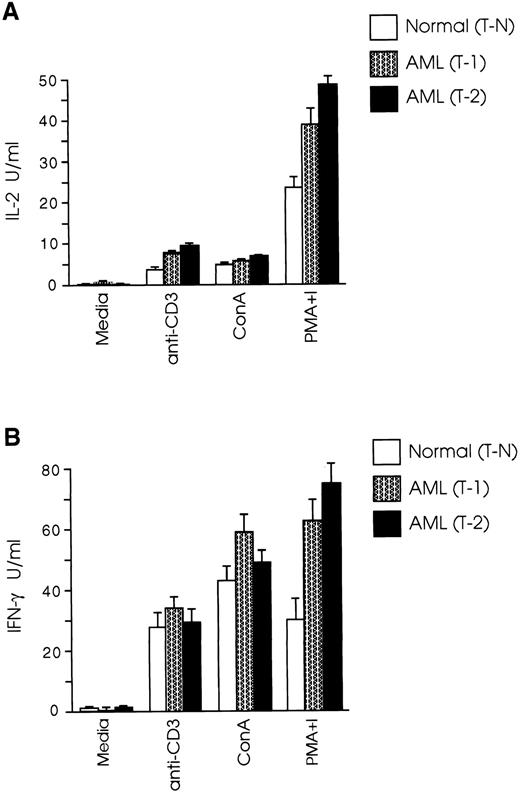
CD8+ T cells from leukemic SJL/J mice have a Th1 cytokine profile.Protective immune responses are coordinated to a large extent by cytokines produced by Th1 and Th2 T-cell subsets,36,37 whereas the role of the recently recognized Th0 subset38 has still to be defined. Th1 cells secreting IL-2 and IFN-γ induce cellular immune responses, whereas Th2 cells producing IL-4, IL-5, IL-6, and IL-10, promote humoral immune responses.39,40 It has been speculated that one possible mechanism leading to inability of an intact host to eliminate autologous tumor growth is the gradual loss of the Th1 cytokine profile and shift to Th2 type.26 27 We therefore examined the cytokine profile of splenic CD8+ T cells in our AML model and found that both T-1 and T-2 cells produced Th1 type cytokines, whereas the level of IL-4 production by CD8+ T cells from normal and leukemic mice was very low to undetectable (Fig 2A). Secretion of IFN-γ by T-1 cells was 82% higher than T-N cells (P < .005), and IL-2 levels produced by T-1 cells were 51% higher than those produced by T-N cells (P < .01). The differences in cytokine secretion were even more prominent when T-2 cells were used in the experiments. As shown in Fig 2B, IFN-γ levels produced by T-2 cells were nearly three times those produced by T-N cells (P < .001), and IL-2 production by T-2 cells was more than twice as great as that produced by T-N cells (P < .0001). We further examined the cytokine profile of T-1 and T-2 cells in response to anti-CD3 and ConA stimulation. As shown in Fig 3A, CD8+ T cells from both normal and leukemic mice produced low levels of IL-2 in response to anti-CD3 and ConA, as opposed to stimulation with PMA plus ionomycin. Figure 3B shows that T-1 and T-2 cells produced equivalent or higher levels of IFN-γ than normal cells in response to anti-CD3 and ConA, but the most striking differences were observed when the cells were stimulated with PMA plus ionomycin.
CD8+ T cells from leukemic SJL/J mice secrete Th1 type cytokines. (A) T-1 and (B) T-2 cells were cultured at 2 × 105 cells/well in the presence of PMA plus ionomycin and a feeder layer of irradiated 2 × 105 syngeneic, anti-Thy 1.2 and C′-treated splenocytes. Cytokine concentrations were determined in tissue culture supernatants taken after 24 hours (IL-2) or 48 hours (IFN-γ, IL-4) of the initiation of the culture. The graphs are representative of three independent experiments. Data are shown as the mean and SD of triplicate culture.
CD8+ T cells from leukemic SJL/J mice secrete Th1 type cytokines. (A) T-1 and (B) T-2 cells were cultured at 2 × 105 cells/well in the presence of PMA plus ionomycin and a feeder layer of irradiated 2 × 105 syngeneic, anti-Thy 1.2 and C′-treated splenocytes. Cytokine concentrations were determined in tissue culture supernatants taken after 24 hours (IL-2) or 48 hours (IFN-γ, IL-4) of the initiation of the culture. The graphs are representative of three independent experiments. Data are shown as the mean and SD of triplicate culture.
CD8+ T cells from leukemic mice secrete equal or higher levels of Th1 type cytokines than normal cells in response to TcR and mitogen stimulation. T-1 and T-2 cells were cultured at 2 × 105 cells/well in the presence of anti-CD3 or mitogens and a feeder layer of irradiated 2 × 105 syngeneic, anti-Thy 1.2 and C′-treated splenocytes. (A) IL-2 and (B) IFN-γ levels were determined as described in Materials and Methods. The graphs represent three separate experiments. Data are shown as the mean and SD of triplicate culture.
CD8+ T cells from leukemic mice secrete equal or higher levels of Th1 type cytokines than normal cells in response to TcR and mitogen stimulation. T-1 and T-2 cells were cultured at 2 × 105 cells/well in the presence of anti-CD3 or mitogens and a feeder layer of irradiated 2 × 105 syngeneic, anti-Thy 1.2 and C′-treated splenocytes. (A) IL-2 and (B) IFN-γ levels were determined as described in Materials and Methods. The graphs represent three separate experiments. Data are shown as the mean and SD of triplicate culture.
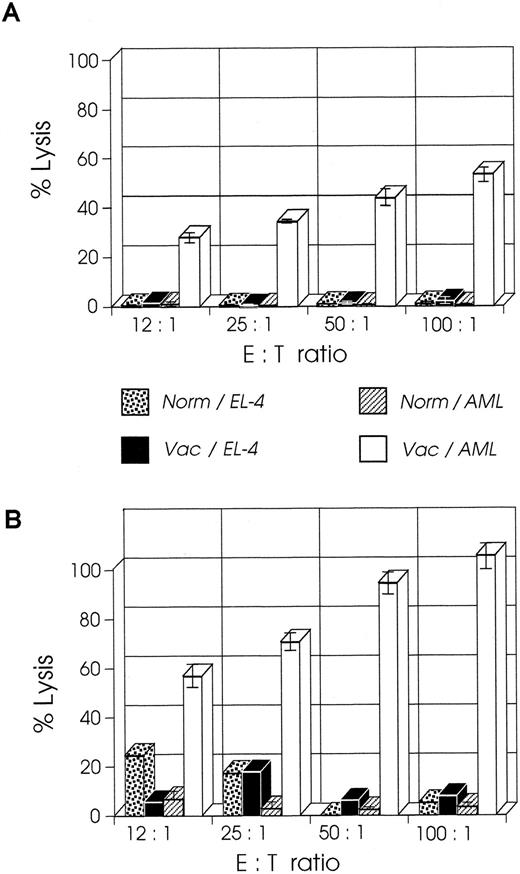
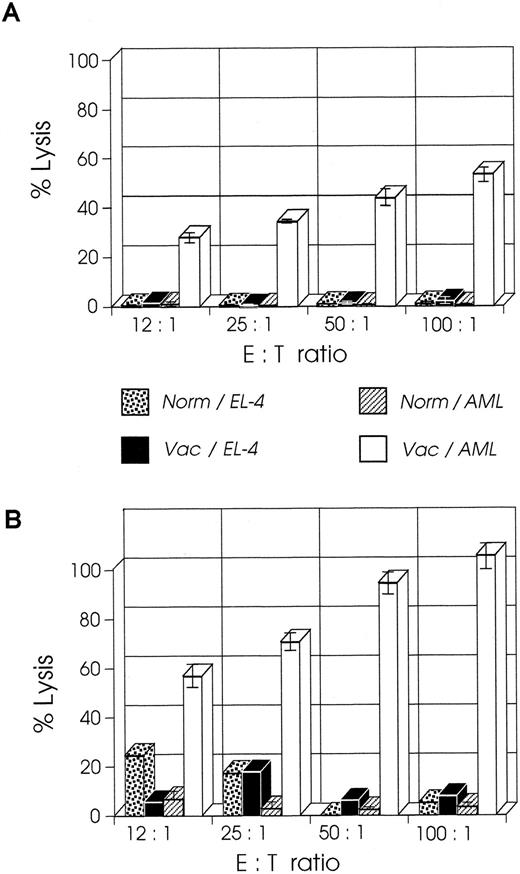
CD8+ T cells from mice vaccinated at week 2 have higher CTL activity than cells from mice vaccinated at week 1.We examined the CTL activity of CD8+ T cells from mice vaccinated at week 1 or 2 after leukemia injection; the former group of mice shows a curative response, whereas the latter group has only prolonged survival.23 As shown in Fig 4A, splenic CD8+ T cells isolated from mice a week after their vaccination with B7-AML cells generated a strong cytolytic response on stimulation with wild-type AML cells in the presence of 5 U/mL exogenous IL-2 and in the absence of APCs (costimulatory signals). When under the same experimental conditions splenic CD8+ T cells from mice vaccinated at week 2 were studied, surprisingly these cells generated a stronger in vitro cytolytic response, which at a 100:1 ratio of E:T cells was 100% (Fig 4B). The response was AML-specific because the same cells did not lyse alloantigen presenting EL-4 (H-2b) cells. These findings suggest that an anti-AML effector CTL population had been generated in vivo and that APC-derived costimulatory signals and restimulation were not required for the in vitro activation.
CTL activity of CD8+ T cells from week 2 vaccinated mice is higher than CTL activity from week 1 vaccinated mice. SJL/J mice were injected with 105 AML cells. One or 2 weeks later they were immunized with irradiated 105 B7-AML cells. One week after immunization CD8+ splenocytes were isolated as described in Materials and Methods and used as effector cells in various effector to target (E:T) ratios. Target cells (autologous AML or control EL-4 cells) were incubated with 51Cr for 2 hours. The standard 4-hour CTL assays were set up with various E:T ratios in a total volume of 0.2 mL/well in a 96-well microtiter plate. All conditions were set up in quadruplicate. After a 4-hour incubation, 100 μL of supernatant was harvested from each well, and the quantity of 51Cr in the supernatants was determined. (A) CTL activity of CD8+ cells from week 1 vaccinated mice on autologous AML cells (Vac/AML) and control EL-4 cells (Vac/EL-4). In the same experiment, CD8+ cells from normal SJL/J mice were tested for CTL activity on autologous AML cells (Norm/AML) and control EL-4 cells (Norm/EL-4). (B) CTL activity of CD8+ cells from week 2 vaccinated mice on autologous AML cells (Vac/AML) and control EL-4 cells (Vac/EL-4). Control cells from normal SJL/J mice were used as described in graph A.
CTL activity of CD8+ T cells from week 2 vaccinated mice is higher than CTL activity from week 1 vaccinated mice. SJL/J mice were injected with 105 AML cells. One or 2 weeks later they were immunized with irradiated 105 B7-AML cells. One week after immunization CD8+ splenocytes were isolated as described in Materials and Methods and used as effector cells in various effector to target (E:T) ratios. Target cells (autologous AML or control EL-4 cells) were incubated with 51Cr for 2 hours. The standard 4-hour CTL assays were set up with various E:T ratios in a total volume of 0.2 mL/well in a 96-well microtiter plate. All conditions were set up in quadruplicate. After a 4-hour incubation, 100 μL of supernatant was harvested from each well, and the quantity of 51Cr in the supernatants was determined. (A) CTL activity of CD8+ cells from week 1 vaccinated mice on autologous AML cells (Vac/AML) and control EL-4 cells (Vac/EL-4). In the same experiment, CD8+ cells from normal SJL/J mice were tested for CTL activity on autologous AML cells (Norm/AML) and control EL-4 cells (Norm/EL-4). (B) CTL activity of CD8+ cells from week 2 vaccinated mice on autologous AML cells (Vac/AML) and control EL-4 cells (Vac/EL-4). Control cells from normal SJL/J mice were used as described in graph A.
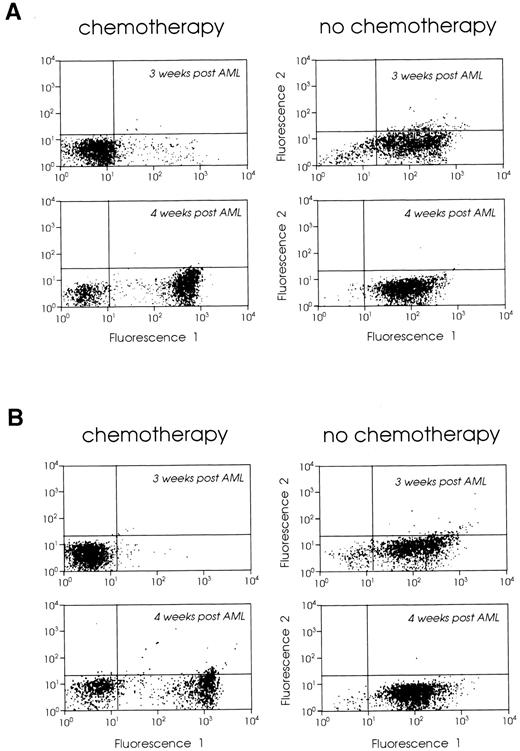
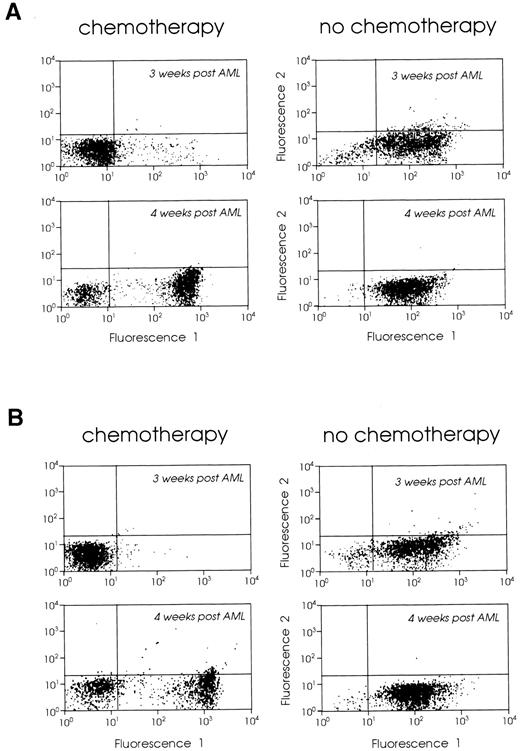
Cytoreductive treatment improves the vaccination potential of the leukemic cells.Tumor cell kinetics has been considered as a possible mechanism limiting the efficacy of tumor vaccines in animals with large tumor burdens.12 Based on our findings that vaccination of leukemic mice 2 weeks after leukemia inoculation had limited therapeutic effect but that the CTL response was still vigorous, we combined week 2 vaccines with prior cytoreductive treatment to determine if a better therapeutic effect could be generated. We treated mice with Ara-C, 2 × 200 mg/kg, administered 6 hours apart,41 on days 7, 10, and 14 (protocol I) or days 3, 6, and 8 (protocol II) after leukemia injection. These mice were then vaccinated with 105 irradiated B7-AML cells on day 16 after leukemia injection. As controls we used two groups of leukemic mice: (1) mice that received no treatment, and (2) mice that received chemotherapy but no vaccine. In order to confirm that our chemotherapy protocol was reducing the tumor burden, we treated groups of leukemic mice with either protocol I or II, and 1 or 2 weeks after the end of chemotherapy we analyzed their bone marrow and spleen cells using the GR-1 myeloid differentiation antigen as a phenotypic marker (not found on lymphoid or erythroid cells), as previously described.23 Mice treated with protocol II (days 3, 6, and 8) had almost no GR-1 positive bone marrow and spleen cells 1 week after the end of chemotherapy (0.8%) and had a low number of GR-1 positive cells (11%) 2 weeks later. The vast majority of bone marrow cells were CD3+ T cells (data not shown). However, mice treated with protocol I (days 7, 10, and 14) had a very small number of GR-1 positive cells in their bone marrow and spleen a week after the end of chemotherapy, but 2 weeks after chemotherapy both bone marrow and spleen were heavily infiltrated by leukemic cells (Fig 5A and B).
FACScan analysis of (A) bone marrow and (B) spleen cells from leukemic SJL/J mice with chemotherapy (left panels) or without chemotherapy (right panels). SJL/J mice were injected with 105 AML cells and treated with chemotherapy on days 7, 10, and 14 (protocol I). One or 2 weeks after chemotherapy (3 or 4 weeks after leukemia inoculation), bone marrow and spleen cells were stained with Gr-1 MoAb or control Ig. A total of 5,000 cells were analyzed by FACS for each sample.
FACScan analysis of (A) bone marrow and (B) spleen cells from leukemic SJL/J mice with chemotherapy (left panels) or without chemotherapy (right panels). SJL/J mice were injected with 105 AML cells and treated with chemotherapy on days 7, 10, and 14 (protocol I). One or 2 weeks after chemotherapy (3 or 4 weeks after leukemia inoculation), bone marrow and spleen cells were stained with Gr-1 MoAb or control Ig. A total of 5,000 cells were analyzed by FACS for each sample.
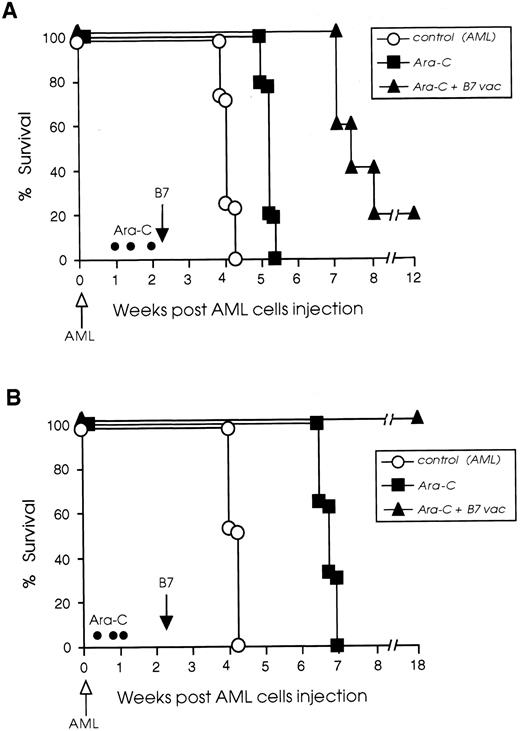
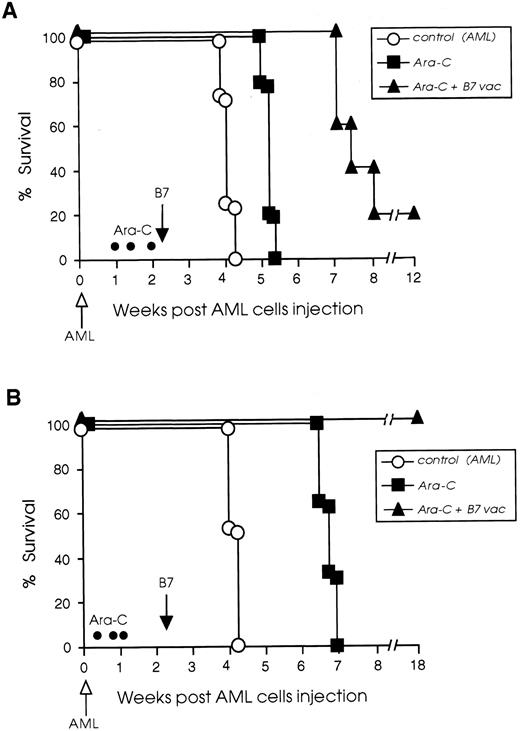
We have been able to improve the efficacy of late B7 vaccines by combining their administration with cytoreductive treatment. Two chemotherapy protocols were used and survival of chemotherapy-treated and vaccinated mice is illustrated in Fig 6. In the protocol I experiment, 20% of chemotherapy-treated and vaccinated mice rejected their tumor completely and 80% had 2 to 3 weeks prolonged survival compared with control leukemic mice, which received chemotherapy but no vaccine. The chemotherapy-treated but not vaccinated control mice had only 1 week prolonged survival compared with nontreated leukemic mice (Fig 6A). On the contrary, when protocol II was used for treatment, the combination of chemotherapy and B7 vaccine led to the cure of 100% of the leukemic mice. As shown in Fig 6B, mice treated only with chemotherapy had a 3-week prolonged survival compared with nontreated leukemic mice, whereas 100% of the mice that received both chemotherapy and B7 vaccine rejected their leukemia.
Cytoreductive treatment improves the vaccination potential of B7-AML cells. SJL/J mice were injected intravenously with 105 wild-type AML cells (open arrow) and then divided into three different groups. One group (○) received no further treatment. A second group (▪) was treated with Ara-C (•) on days 7, 10, and 14 (protocol I) or 3, 6, and 8 (protocol II), as described in Materials and Methods, but was not vaccinated. A third group of leukemic mice (▴) was treated with Ara-C, and on day 16 (black arrow) was vaccinated with 105 irradiated B7-AML cells. Graph A illustrates the survival of mice treated with protocol I and graph B illustrates the survival of mice treated with protocol II. Each experimental and control group included 8 to 10 mice.
Cytoreductive treatment improves the vaccination potential of B7-AML cells. SJL/J mice were injected intravenously with 105 wild-type AML cells (open arrow) and then divided into three different groups. One group (○) received no further treatment. A second group (▪) was treated with Ara-C (•) on days 7, 10, and 14 (protocol I) or 3, 6, and 8 (protocol II), as described in Materials and Methods, but was not vaccinated. A third group of leukemic mice (▴) was treated with Ara-C, and on day 16 (black arrow) was vaccinated with 105 irradiated B7-AML cells. Graph A illustrates the survival of mice treated with protocol I and graph B illustrates the survival of mice treated with protocol II. Each experimental and control group included 8 to 10 mice.
DISCUSSION
The experiments described in this report were motivated by recent studies on solid tumor models and our own study on a murine AML model demonstrating that B7 vaccines have curative potential only when administered to animals with small tumor burden. One speculation has been that tumor growth induces immunosuppressive mechanisms, with T-cell anergy or Th2 type cytokine profile of effector cells as potential candidates. Here we demonstrate that, in this AML model, tumor inoculation into naive mice induces activation rather than tolerance of CD8+ spleen cells during the first half of the leukemic course, and that the cells have a Th1 type cytokine profile. We also demonstrate that the limited clinical response of leukemic mice with large tumor burden to B7 vaccines cannot be attributed to the lack of tumor-specific CTLs. Additional evidence that tumor kinetics overwhelm an ongoing immune response in this AML model is provided from chemotherapy treatment experiments. These experiments clearly demonstrate that reduction of the tumor burden during the first week after leukemia injection can cure 100% of the mice vaccinated at week 2. However, when chemotherapy is provided during the second week of the leukemic course, 80% of the vaccinated mice have prolonged survival while 20% reject their tumor.
It has been well documented in experimental model systems, that T cells become anergized when they are antigenically stimulated in the absence of costimulatory signals.42,43 Anergized T cells become incapable of proliferating and producing IL-2 on restimulation through their TcR and costimulatory ligands due to a defect in the TcR ζ-chain phosphorylation and its sequential interaction with ZAP-70 (both essential for the initiation of TcR-mediated signal transduction).44,45 This defect can be bypassed by stimulation of T cells with PMA plus inonomycin which activates distal to PKC signaling pathways.46 In our experiments, splenic CD8+ T cells from leukemic mice proliferated more vigorously than normal CD8+ T cells not only in response to activation with PMA plus ionomycin, but more important, to antigenic stimulation through TcR. Although these findings cannot exclude the possibility that some T cells were anergic, they demonstrate that on a population basis, a considerable percentage of the cells had encountered an antigenic stimulation in vivo and had thus entered an activated state.
Wild-type AML cells in this model do not express B7.1 or B7.2 costimulatory molecules and therefore they cannot directly activate T cells as professional APCs. Our observation that CD8+ T cells from leukemic mice were in an activated state raises a multitude of questions regarding tumor antigen presentation during the AML course: (1) are tumor antigens exclusively presented by host bone marrow-derived APCs, which are able to provide antigen and costimulatory signals to T cells? (2) Do T cells “see” tumor antigens on the AML cells, even in the absence of costimulatory signals, thus becoming temporarily unresponsive due to TcR down-regulation? (3) Is the host immune response a continuous antagonism of the two aforementioned mechanisms, and could this explain why immunogenicity, route of injection, and number of injected cells play a specific role in the tumorigenicity of the tumor cells?
In support of the first hypothesis are two studies demonstrating that MHC class I-restricted tumor antigens are exclusively presented by host bone marrow-derived APCs47 and that, even in B7.1 tumor vaccines, the dominant mechanism of CTL priming is through the uptake and presentation of tumor antigens by bone marrow-derived APCs.48 If this is indeed the case, the number of surrounding APCs would have a very important impact on effectively taking up and presenting antigens provided by the growing tumor and would also explain why B7 vaccines can be therapeutic only under circumstances with relatively small tumor burden (especially in nonsolid tumors, in which the factor of a large, abnormally vascularized tumor mass, with limited traffic of effector cells, is excluded). We are currently investigating in our model if the simultaneous administration of tumorigenic AML cells and bone marrow-derived dendritic cells49 can prevent leukemia in naive hosts; and moreover, if the injection of autologous dendritic cells in leukemic mice can rescue them from lethal disease.
The second hypothesis, that T cells can recognize tumor-specific antigens, even in the absence of costimulatory molecules, is strengthened by recent observations that T-cell activation depends on the number of triggered TcRs, is independent of the nature of the triggering ligand, and that the activation threshold is decreased when costimulatory signals are provided.50 In the reported experimental system, T cells expressing very few TcRs (resulting from TcR down-regulation due to antigenic stimulation and lasting 5 to 8 days51 ) are triggered transiently but fail to be activated, because the number of TcRs is insufficient to allow these cells to sustain the signal and reach the activation threshold. Our findings do not support this as the only mechanism of tumor antigen–T-cell interaction in the AML model. If T cells, being exposed to an increasing number of AML cells in the splenic microenvironment, can be directly stimulated by them in the absence of costimulatory signals, we would expect the majority of T cells to be unresponsive in our in vitro activation experiments and, furthermore, this number to increase with disease progress. Additional evidence that T-cell unresponsiveness is not induced by tumor growth in this AML model, and that tumor antigen presentation mediated by APCs plays a definite role (thus their number being critical for an effective immune response), is provided by our observations that: (1) ≥104 is a tumorigenic dose when wild-type leukemic cells are injected IV or IP, but >105 is the tumorigenic dose when the subcutaneous (SC) route is used for injection; (2) when mice are immunized with a nontumorigenic dose (104) of live cells SC, they are capable of rejecting a subsequent SC tumor challenge with 106 cells but not an IV or IP challenge with the same number of cells (Dunussi-Joannopoulos et al, unpublished data). This is in agreement with previous reports on immunogenic tumor models, in which mice immunized with the minimal tumorigenic dose of irradiated, B7-devoid tumor cells, do not get tolerized and are capable of rejecting a subsequent tumor challenge with live cells.52 53
Another important issue is provided by the findings from the CTL assays. We have shown that the CTL activity of mice vaccinated at week 2 is surprisingly higher than the CTL activity of mice vaccinated at week 1 after leukemia injection and would expect the former group to reject leukemia as does the latter group. We can speculate that the effector CTL population in the vaccinated mice comprises a mixture of CTLs, part of which has been activated by APCs before vaccination and another part by the B7-AML cells provided by the vaccine. This can explain the presence of a higher number of effector T cells in the group of mice that have been vaccinated 2 weeks after leukemia injection and thus have had longer exposure to tumor antigen presenting APCs. Because mice vaccinated at week 2 finally fail to reject their leukemia despite the higher in vitro CTL activity, we propose two potential mechanisms as candidates for this failure: (1) mice vaccinated at week 2, when a large tumor burden has been established, can still recruit a high number of effector CTLs, but suppression of CTL activity by either the tumor itself or by tumor-induced suppressor cells leads to unfavorable clinical outcome; (2) mice vaccinated at week 2 may finally fail to eliminate leukemia, because the tumor-specific CTL clone develops increased susceptibility to “clonal exhaustion” (activation-induced cell death) as a result of heavy antigenic stimulation by the large tumor burden.54 It should be mentioned here that the reported55 slow Th2 effectors' death as opposed to the rapid Th1 effectors' death may account for the Th2 cytokine profile observed in cases with advanced disease.26 27
In conclusion, our data demonstrate that in this AML model, tumor growth does not induce tolerance or an altered Th2 type cytokine profile that might impede a cytotoxic T-cell–mediated immune response. Moreover, our results demonstrate that reduction of the tumor load with chemotherapy leads to more effective B7 vaccines, but it still needs to be determined which immune mechanisms regulate the final outcome of the vaccines and how tumor burden interferes with them. It is conceivable that in diseases like AML clinical trials based on genetically modified tumor cell vaccines will target residual disease after treatment either with chemotherapy or bone marrow transplantation. It is therefore clinically important that B7 vaccines not only initiate an antitumor effector T-cell response in these patients, but also sustain it and successfully lead to development of tumor-specific memory T cells able to “fight” tumor cell recurrence. To date, very little is known on the development from naive to tumor-specific memory T cells, and clearly more research in this direction is needed.
ACKNOWLEDGMENT
The authors thank John Delmonte Jr and Mahesh Karandikar for expert technical assistance.
Supported in part by the Andrew F. Gaffney Foundation, the Claudia Adams Barr Program of the Dana-Farber Cancer Institute. JLMF is a scholar of the Leukemia Society of America.
Address reprint requests to Kyriaki Dunussi-Joannopoulos, MD, PhD, Division of Pediatric Oncology, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA, 02115.