Abstract
We assessed the chimerism of CD34+ bone marrow cells before donor leukocyte infusion (DLI) on nine occasions in seven patients with leukemic relapse after allogeneic marrow transplantation. The patients suffered from acute lymphoblastic leukemia (n = 1), acute myeloid leukemia (n = 3), and chronic myeloid leukemia (CML; n = 3). Two patients received a second DLI because of disease progression after the first one. The origin of the CD34+ cells was determined by analyzing variable number of tandem repeats with polymerase chain reaction and, in sex-mismatched cases, by fluorescence in situ hybridization. Before DLI CD34+ cells were exclusively of donor origin in four patients. In another patient 41% of CD34+ cells were derived from the donor. No aplasia occurred in these patients after DLI, whereas in the two patients with exclusively recipient hematopoiesis severe aplasia lasting for 5 and 13 weeks necessitated hematopoietic stem cell support. One patient who had only 5% CD34+ donor cells before DLI recovered without stem cell support after 10 days. Two patients in relapse of CML showed a high percentage of BCR-ABL− CD34+ cells of recipient origin before DLI. These BCR-ABL− cells of recipient type did not prevent severe aplasia which indicates that the assessment of BCR-ABL+ hematopoiesis alone is insufficient for predicting aplasia. Our data indicate that in case of sufficient donor hematopoiesis before DLI no persistent aplasia will occur. Thus, evaluation of donor hematopoiesis allows prediction of aplasia after DLI and makes early therapeutic interventions possible.
THE PROGNOSIS of patients who relapse after allogeneic bone marrow transplantation (BMT) is dismal. Although sustained remissions can be achieved after a second marrow graft, survival rates remain poor because of the high incidence of transplant-related complications.1 Other therapeutic strategies, such as conventional chemotherapy and cytokine application, offer little prospect for a prolonged survival.2,3 Kolb et al4 and Porter et al5 have achieved sustained remissions in patients with chronic myeloid leukemia (CML) and, to a lesser extent, in patients with acute leukemia by transfusion of leukocytes from the original marrow donor. Graft-versus-host disease (GVHD) and marrow aplasia in up to 80% and 50% of patients given donor leukocyte infusion (DLI) are responsible for up to 20% mortality in patients achieving remission.4-6 The incidence and severity of myelosuppression after DLI vary and cannot be predicted before treatment so far. However, there is evidence that patients with acute leukemia who received donor leukocytes in chemotherapy-induced remission or with CML in cytogenetic relapse are less prone to aplasia than patients in advanced stage of CML or in relapse of acute leukemia.4 Here we show that assessment of the hematopoietic chimerism in patients who relapse after BMT and quantification of residual donor hematopoiesis can predict the risk of developing DLI-induced aplasia. This information is of important clinical value because it allows the application of supportive therapies such as infusion of donor stem cells early after DLI in patients with a high risk of marrow aplasia.
MATERIALS AND METHODS
Patients.Seven patients with relapsed leukemia after BMT from HLA-identical siblings were giving donor leukocyte infusions from their original BM donors. Their characteristics are shown in Table 1. All patients were conditioned with fractionated total body irradiation (TBI) combined with cytosine arabinoside and etoposide (patient 1) or cyclophosphamide (patients 2 to 7). GVHD prophylaxis consisted of cyclosporine A (CSA) either alone (patients 1 and 4) or in combination with methotrexate (patients 2, 3, 5, 6, and 7) as described.7 Marrow grafts were not T-cell depleted. According to the Seattle criteria,8 acute GVHD grade 1 occurred in patients 2 and 3. Chronic GVHD was not seen. Except patient 1, all patients achieved hematologic, cytogenetic, and molecular remissions. Despite hematologic remission, patient 1 remained BCR-ABL+ in the reverse transcriptase-polymerase chain reaction (RT-PCR). Patients 1 and 5 received two cycles of DLI because of disease progression. They are described as 1a and 5a before first DLI and 1b and 5b before second DLI in Table 1. Before DLI, patients 1b, 2, 3, and 5a were in hematologic relapse and patients 1a, 5b, 6, and 7 were in hematologic remission. Therapy of relapse consisted of interferon-α in patients with CML and chemotherapy in patients with acute myeloid leukemia (AML). No immunosuppressive therapy was administered. Aplasia was defined as granulocyte counts <0.5 × 109/L and platelet counts <20 × 109/L and confirmed by marrow aspiration.
Infusion of donor leukocytes.DLI were performed as reported by Porter et al.5 Unstimulated peripheral blood mononuclear cells (PBMNC) were freshly obtained from the original BM donor by a continuous flow cell separator (Cobe Spectra S 3000; Cobe International, Denver, CO). Between 190 and 300 mL were collected per session, which was well tolerated by all donors. The number of CD3+ cells was evaluated with a fluorescence-activated cell sorter (FACS). A total of 4.05 to 4.48/108 CD3+ cells/kg recipient body weight (bw) were infused in four weekly infusions in patients 1, 2, and 3, respectively (Table 1). In patients 4, 5, 6, and 7 the number of infused CD3+ cells was reduced to 0.5 to 1.2 × 108/kg bw in patients 4, 5, 6, and 7.
RT-PCR for detection of BCR-ABL transcripts.Analyses were performed in patients with CML and Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (ALL) after BMT and DLI as described.9 Integrity of DNA was controlled by RT-PCR of ABL-specific sequences. Reverse transcription was performed with oligo dT random primers followed by two successive PCR amplifications with ABL- and BCR-specific primers. With 1 μL first-round product the nested PCR using a set of primers located within the first round amplicon was done. The one-step and two-step PCR products were analyzed on polyacrylamide gradient gels (4% to 20%) and visualized by ethidium bromide staining. Each sample was tested at least twice in duplicates. Sensitivity of the one-step assay was one BCR-ABL+ cell in 104 normal cells and that of the two-step assay one BCR-ABL+ cell in 106 normal cells, respectively. The specificity of the PCR products was proven by Southern blot hybridization as described.9
Analysis of variable number of tandem repeats with PCR (VNTR-PCR).We used a VNTR region within the intron 40 of the von Willebrand factor gene for the distinction between donor and recipient cells. PCR and evaluation were performed as described previously.10 Amplified DNA was analyzed on a 10% polyacrylamide gel, stained with ethidium bromide, and examined under UV light. CD34+, CD4+, and CD8+ cells were analyzed before DLI and PBMNC before and in weekly intervals after DLI. The sensitivity of our PCR method allowed the detection of 5% to 10% of donor cells in the presence of recipient cells and vice versa.
Immunological staining and cell sorting.Recipient BM and PBMNC were separated on Ficoll-Hypaque (Seromed; Biochrom KG, Berlin, Germany), washed, and resuspended in Iscoves modified Dulbecco's medium. (IMDM; GIBCO, Paisley, Scotland) supplemented with 2% fetal calf serum (FCS; Sigma, St Louis, MO). For immunological staining CD34+, CD3+, CD4+, and CD8+ cells were incubated at a concentration of 107 mononuclear cells (MNC)/mL in appropriately diluted monoclonal antibodies (MoAbs) as described.11 Flow cytometric cell sorting was performed on a FACStar Plus (Becton Dickinson, Sunnyvale, CA) using an argon laser adjusted to an excitation wave length of 488 nm. Thirty thousand to 50,000 cells were sorted for each target cell population. The purity of each sort was assessed by reanalyzes and ranged from 96% to 100%.
Fluorescence in situ hybridization (FISH).In sex-mismatched transplantations (patients 3 to 7) differentially labeled X and Y chromosome-specific centromere probes (Vysis Inc, Downers Grove, Woodcreek, USA) and, in Ph+ disease (patients 1 to 4), differentially labeled BCR and ABL probes (Vysis Inc) were used. FISH analyses were performed on cytospin preparations, each containing up to 10,000 sorted CD34+, CD4+, and CD8+ cells according to the manufacturer's instructions. The cut-off level (median plus 3 × SD) which was determined on normal controls was 1.6% for the X- and Y-specific probes and 3.5% for the BCR- and ABL-specific probes. At least 200 interphase nuclei per slide were analyzed in a blinded fashion. In conjunction with the progenitor cell assays at least 12 individual colonies from both the nonadherent and adherent cell fractions were picked after 1 and 5 weeks, respectively, and analyzed with FISH using the X- and Y- as well as the BCR- and ABL-specific probes in appropriate cases.
Clonal assay for hematopoietic progenitor cells in methylcellulose.BMMNC were plated in duplicate 35 mm dishes (Falcon) at a density of 105 cells/mL in 0.9% methylcellulose (Fluka Chem. AG, Buchs, Switzerland), IMDM supplemented with 20% FCS (Sigma), 1% bovine serum albumin (Sigma), thioglycerol, and sodium bicarbonat (Sigma). The maximum colony formation was obtained by adding 25 ng/mL of interleukin-3 (IL-3; Peprotech, London, UK), 50 ng/mL of granulocyte-macrophage colony-stimulating factor (GM-CSF; Sandoz, Basel, Switzerland), and 10 ng/mL of stem cell factor (SCF; Peprotech). The numbers of colonies were evaluated after 14 days incubation at 37°C in 100% humidified air with 5% CO2 .
Long-term BM culture (LTBMC).Cultures were established according to a method described previously.12 Briefly, 2 × 106 BMMNC/mL were inoculated in multiwell dishes (Falcon, Becton Dickinson, New Jersey) with 4 mL of IMDM supplemented with 12.5% FCS (Sigma), 12.5% horse serum (GIBCO), 1 × 10−6 mol/L hydrocortisone, and 5 × 10−4 mol/mL thioglycerol. At initiation of the culture and during the weekly feeding procedure IL-3 at 25 ng/mL, GM-CSF at 50 ng/mL, and SCF at 10 ng/mL were added. These cytokines showed optimal stimulation of colony-forming unit cells' (CFU-C) proliferation without exhausting the proliferative capacity of LTBMC. Cultures were incubated for 3 to 4 days at 37°C and then moved to 33°C in 100% humidified air with 5% CO2 . At weekly intervals the medium was completely replaced without detatching the adherent layer. The establishment of stromal cell layers in the culture was monitored by weekly examination under an inverted microscope. After 5 weeks cells from the adherent layer were mechanically detached after 2 minutes exposure to 0.25% trypsin (Sigma). Immediately after the harvest pure FCS was added and the single cell suspension was washed in IMDM with 20% FCS for abrogation of the trypsin action. The nonadherent and adherent cells were counted, tested for viability by trypan blue dye exclusion, and then plated in methylcellulose to assess hematopoietic progenitor cell content. Additionally, they were analyzed by VNTR-PCR and BCR-ABL PCR.
RESULTS
Chimerism of CD34+, CD4+, and CD8+ cells in relapse before donor leukocyte infusion.In Table 2 analyses of the CD34+ cells are shown. In Patient 1 who suffered from Ph+ ALL 90% and 85% of CD34+ BM cells were BCR-ABL− before first and second DLI, respectively. VNTR-PCR showed an exclusive donor genotype before first DLI and donor and recipient cell chimerism before second DLI. In patients 2, 3, and 4 who experienced relapses of CML after BMT, 45% to 72% of CD34+ cells were BCR-ABL− as seen by FISH. VNTR-PCR showed that these cells were of recipient origin in patients 2 and 3. More specifically, FISH analyses with X and Y chromosome–specific centromere probes revealed that in patients 3 and 4 5% and 41% of CD34+ cells were of donor origin. Patient 5 had only CD34+ recipient cells in relapse after BMT. After first DLI disease progression enforced conventional chemotherapy inducing a complete remission with 97% CD34+ donor cells. Patients 6 and 7 with relapse of AML received chemotherapy and afterward had 98% CD34+ donor cells before DLI (Table 2). FISH and VNTR-PCR showed that the CD4 and CD8+ cells were of donor origin in all patients (data not shown). These cells were also BCR-ABL− in patients with Ph+ leukemias.
Aplasia.In patients 2, 3, and 5a, BM aplasia occurred 6 to 8 weeks after DLI (Table 2). Because patient 2 remained aplastic for 3 weeks BM from his sibling donor was given. However, aplasia persisted for another 10 weeks. After intensive immunosuppression with cyclophosphamide and antithymocyte globulin and infusion of G-CSF–stimulated PB stem cells from his sibling, donor hematopoietic regeneration was achieved 22 weeks after DLI as previously reported.13 Patient 3 recovered spontaneously from aplasia within 2 weeks. Patient 5 experienced BM aplasia 6 weeks after first DLI and recovered hematologically after infusion of G-CSF–stimulated PB stem cells from her sibling donor. The second DLI which was given 24 weeks after the first one did not induce pancytopenia.
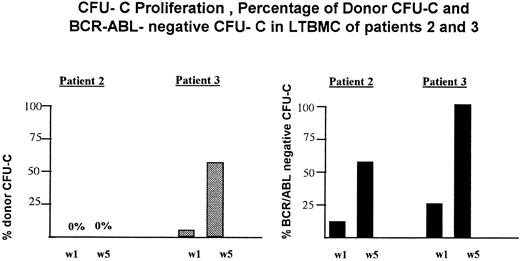
Proliferation and maintenance of residual donor hematopoiesis in LTBMC before donor leukocyte infusion.LTBMC were set up from BM of patients 2, 3, and 5 before DLI. As shown by VNTR-PCR both the nonadherent cells that were analyzed weekly and the adherent cells that were obtained after 5 weeks were exclusively of recipient origin in patient 2 (Fig 1). From the first to the fifth week BCR-ABL− cells increased from 10% to 64% in LTBMC but were of recipient origin. At initiation of LTBMC no CFU-C of donor origin were detected in patient 3 (Fig 1). After 1- and 5-week culture periods donor CFU-C increased to 5% and 60%, respectively. BCR-ABL− colonies increased from 25% after 1 week to 100% of colonies after 5 weeks in LTBMC (Fig 1). In patient 5 no LTBMC could be established.
Proliferation of residual donor cells and BCR-ABL− cells in LTBMC before DLI in patients 2 and 3. w1, CFU-C obtained from the nonadherent cells after 1 week in LTBMC; w5, CFU-C obtained from the adherent cell layer after a 5-week culture period. (), CFU-C from donor origin; (▪), BCR-ABL− CFU-C.
Proliferation of residual donor cells and BCR-ABL− cells in LTBMC before DLI in patients 2 and 3. w1, CFU-C obtained from the nonadherent cells after 1 week in LTBMC; w5, CFU-C obtained from the adherent cell layer after a 5-week culture period. (), CFU-C from donor origin; (▪), BCR-ABL− CFU-C.
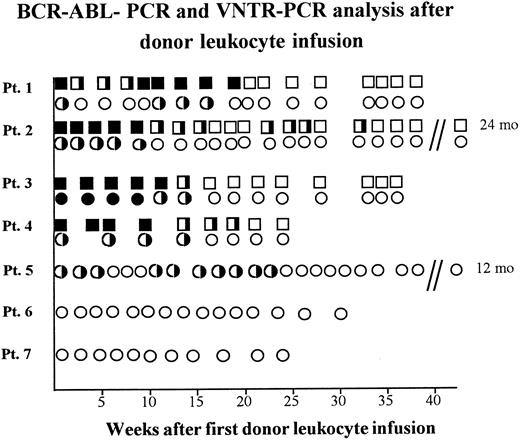
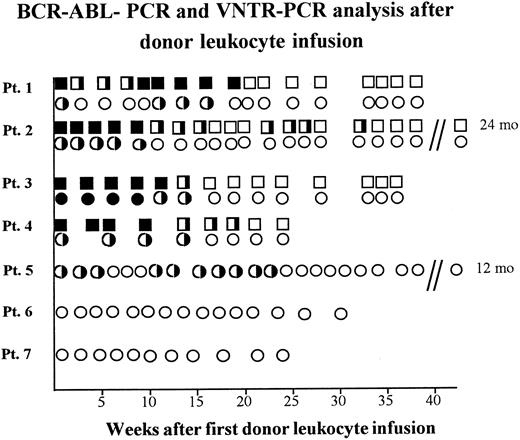
Antileukemic effect of donor leukocyte infusions.After first DLI, patient 1 developed leukemic BM infiltrations and, as shown with RT-PCR, BCR-ABL+ cells in PB increased (Fig 2). Therefore, a second course of donor leukocytes was administered 13 weeks later, which resulted in a continuous BCR-ABL negativity in the two-step RT-PCR three weeks later. In line with this result, BM was morphologically leukemia-free and of donor genotype. As demonstrated by RT-PCR, the three patients with CML and the patient with Ph+ ALL achieved molecular genetic remissions 14 to 32 weeks after DLI (Fig 2). Patients 5, 6, and 7 who received DLI after conventional chemotherapy are still in hematologic remission 12, 8, and 6 months after DLI with donor-derived hematopoiesis as seen in VNTR-PCR (Fig 2).
BCR-ABL-positivity and chimerism analyzed in PB of five patients relapsing after BMT receiving donor leukocyte infusions. BCR-ABL-PCR of PB samples: (▪), one-step-positivity: one leukemic cell in 104 cells; (╞), two-step-positivity: one leukemic cell in 106 cells; (□), BCR-ABL-PCR-negativity with less than one leukemic cell in 106 cells. VNTR-PCR of PB samples: (•), recipient banding in VNTR-PCR; (◑), chimerism demonstrated by donor and recipient banding in VNTR-PCR; (○), donor banding in VNTR-PCR.
BCR-ABL-positivity and chimerism analyzed in PB of five patients relapsing after BMT receiving donor leukocyte infusions. BCR-ABL-PCR of PB samples: (▪), one-step-positivity: one leukemic cell in 104 cells; (╞), two-step-positivity: one leukemic cell in 106 cells; (□), BCR-ABL-PCR-negativity with less than one leukemic cell in 106 cells. VNTR-PCR of PB samples: (•), recipient banding in VNTR-PCR; (◑), chimerism demonstrated by donor and recipient banding in VNTR-PCR; (○), donor banding in VNTR-PCR.
GVHD.GVHD occurred in all patients. In patients 2, 4, 5, 6, and 7 it was mild, self-limited, and even without the necessity of immunosuppressive treatment (Table 2). Patient 1 experienced mild GVHD after the first cycle of DLI, but developed grade 3 GVHD of skin and liver 3 weeks after the second course of DLI. He neither responded to cyclosporine nor to steroids and died because of bacterial infection 38 weeks after the first DLI. In patient 3 mild skin GVHD developed 5 weeks after DLI and evolved into chronic GVHD with keratoconjunctivitis, liver and lung involvement, and chronic wasting syndrome. Despite treatment with cyclosporine, steroids, and azathioprine, pulmonary function deteriorated and spontaneous pneumothorax occurred. The patient died because of an acute respiratory distress syndrome 36 weeks after DLI.
DISCUSSION
Severe myelosuppression is a well-known and often lethal complication after DLI for relapsed leukemia. About 20% of patients who achieve remissions after DLI die because of severe myelosuppression and GVHD.4,6 Although Kolb et al4 have shown that DLI in complete remission of acute leukemia or in cytogenetic relapse of CML produces less severe aplasia than in advanced disease stages, neither the severity of the resulting myelosuppression nor the time for recovery from aplasia has been predictable so far. Our in vitro data explain why patients in early stage of disease do not experience aplasia after DLI.
Patients 1, 5b, 6, and 7 who were in hematologic remission and patient 4 who was in cytogenetic relapse, but nevertheless had a high proportion of CD34+ donor cells, did not experience myelosuppression. This shows that donor leukocyte infusions do not induce prolonged BM aplasia in patients with residual donor hematopoiesis. As seen in patient 3, even 5% CD34+ donor cells were sufficient for hematopoietic recovery within 2 weeks. In contrast, VNTR-PCR, FISH, and LTBMC did not show residual donor-derived hematopoietic cells before DLI in patients 2 and 5a who experienced a prolonged BM aplasia after DLI.
In line with previous reports on CML patients,14 we also detected CD34+ recipient cells that were BCR-ABL− in patients 2 and 3. Because these patients nevertheless developed BM aplasia after DLI, these residual, presumably normal, recipient cells were unable to prevent BM aplasia. Moreover, as their BCR-ABL+ counterparts they seem to be eradicated after DLI. This effect may be due to minor histocompatibility antigen-specific (MiHA) cytotoxic T cells that play an important role in GVHD and graft-versus-leukemia effects.15 Because these T cells do not discriminate between leukemic and nonleukemic recipient cells, they could also suppress normal hematopoietic stem cells by direct cell contact.15 16 A stem-cell–confined expression of MiHA might induce alloreactivity of infused donor cytotoxc T cells against BCR-ABL+ and BCR-ABL− hematopoietic stem cells of recipient origin. Our data suggest that, at least for CML, graft-versus-leukemia effect may be part of a more generalized GVHD reaction.
Interestingly, in all patients with relapse T cells were exclusively derived from the donor. Obviously, these T cells could not prevent the expansion of the leukemic cell population, whereas infusion of fresh donor T cells eradicated the leukemic cells and led to complete and continuous remissions even on the molecular genetic level. Currently, the inertness of the original donor T cells is difficult to explain, but may be induced or triggered by standard immunosuppressive treatment after BMT.
In summary, our in vitro data and previous clinical reports4 17 strongly support treatment of early stage relapse by DLI when the amount of donor-derived hematopoiesis allows unimpaired hematologic reconstitution with excellent disease eradication. In case of advanced disease determination of the origin of CD34+ BM cells before DLI is recommended. If residual donor hematopoiesis persists in relapse, DLI-induced long-lasting BM aplasia is unlikely to occur. However, in the absence of CD34+ donor cells, prolonged aplasia must be expected and an early infusion of donor BM or PB stem cells seems to be necessary to reduce DLI-associated morbidity and mortality.
ACKNOWLEDGMENT
We thank the dedicated nurses of our bone marrow transplantation program, our fellows and house staff, the medical technicians, and the staff of the blood bank for their technical assistance in bone marrow harvesting and their support with blood products; and Dr R. Hawliczek for performing the total body irradiation. We thank Karin Winkler, Margit König, Dieter Prinz, and Petra Buchinger for excellent technical assistance.
Supported by grants from the “Fonds zur Förderung der wissenschaftlichen Forschung” (P09044-MED, P09549-MED, and P10454-MED) and the “Jubiläumsfonds der Österreichischen Nationalbank (No. 5025”) and the “Österreichische Kinderkrebshilfe.”
Address reprint requests to Felix Keil, MD, AKH Vienna, Klinik fuer Innere Medizin I, Knochenmarktransplantation, Waehringer Guertel 18-20, A-1090 Vienna, Austria.