Abstract
Thiol-disulfide isomerization in thrombospondin may affect the function of this adhesive protein. Two assays were developed to analyze the determinants of thiol-disulfide exchange and to correlate this exchange with thrombospondin conformation. (1) A competitive immunoassay for the EDTA-conformation of thrombospondin was developed with monoclonal antibody D4.6. (2) The free thiol(s) in thrombospondin was labeled with [3H]N-ethylmaleimide (NEM) under various conditions (the presence or absence of calcium, temperature, and pH), and thrombin digests of the labeled protein were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Consistent with previous reports, thrombin digest fragments of 150, 120, 20, and 14 kD were observed, each with radioactivity under some condition, plus a 25-kD peptide that was not labeled. Sequence data for these fragments and comparisons of SDS-PAGE analyses under reducing and nonreducing conditions indicated that Cys974 was the free thiol. The appearance of thiol label in the 120-kD fragment was previously shown to be a consequence of thiol-disulfide exchange (J Biol Chem 265:17859,1990) and label was recovered in this peptide only under conditions (absence of calcium, 37°C and pH 8.4) that led to the appearance of the EDTA-conformation of thrombospondin. Additional evidence for the correlation of EDTA-conformation and thiol-disulfide exchange was the enhanced conversion of thrombospondin to its EDTA-conformation in the presence of protein disulfide isomerase and the inability of thrombospondin pretreated with NEM to attain the EDTA-conformation. Flow cytometry with antibody D4.6 revealed platelet-associated thrombospondin in the EDTA-conformation in the presence of calcium, suggesting that the EDTA-conformation is a physiological conformation that does not necessarily require EDTA.
THROMBOSPONDIN (TSP) is a large multifunctional protein released by activated platelets and by various cells in culture. It functions as an extracellular matrix protein that has been implicated in roles in cell growth and proliferation, in inflammation and in hemostasis and fibrinolysis. TSP binds other extracellular matrix proteins and proteins involved in hemostasis and fibrinolysis, such as fibrinogen, fibronectin, plasminogen,1 collagen,2,3 cathepsin G,4 and platelet-derived growth factor.5 For some proteins, binding is markedly dependent on conformational changes in TSP related to disulfide pairings5 or calcium (Ca2+) concentration.3,4 Since Zn2+ competes for Ca2+ binding sites on TSP but induces a different conformation than Ca2+, TSP may be present in one set of conformers in α-granules, where Zn2+ levels are high, and in a different set of conformers after release into the plasma, where Zn2+ levels are much lower.6
TSP also forms covalent complexes with itself and with other proteins, such as thrombin, through thiol-disulfide exchange7-9 in reactions that are accelerated by chelation of Ca2+, raising questions about the effect of Ca2+ on TSP thiols. TSP contains 69 cysteine residues in each of its three identical polypeptide chains. Speziale and Detwiler10 established that TSP has three free thiols (one equivalent/chain), but, surprisingly, the single equivalent/chain was distributed among at least 12 cysteine residues. They proposed isomerization of disulfide bonds. In contrast Sun et al,11 using different experimental conditions, concluded that Cys974 was the only cysteine with a free thiol in TSP.
We re-examined the localization of TSP thiols and their isomerization. Similar to our previous report10 we observed isomerization of disulfide bonds of TSP in the supernatant solution of activated platelets at pH 7.4 and room temperature when Ca2+ was chelated. After purification of TSP, there was no isomerization under those conditions, but isomerization of purified TSP occurred when protein disulfide isomerase was added, when the temperature was increased to 37°C or when the pH was increased to 8.4. Isomerization of thiols was correlated with conversion to the EDTA-conformation by a monoclonal antibody (MoAb) specific for the EDTA-conformation.
The effect of protein disulfide isomerase (PDI) on TSP is of special interest. PDI, an endoplasmic reticulum protein that facilitates protein folding, was only recently shown to be on the external surface of the platelet plasma membrane where it has catalytic activity against soluble substrates.12 PDI can be released from the platelet by vesiculation of the plasma membrane13 and the potential for release of a soluble PDI also exists.12 Platelets were initially hypothesized to release PDI on the basis of known intermolecular and intramolecular thiol-disulfide exchange reactions occurring in TSP in the supernatant solution of activated platelets.7-10 14 Therefore, we examined the effect of PDI on purified TSP.
MATERIALS AND METHODS
Materials.N-[ethyl-2-3H] maleimide (40 to 60 Ci/mmol) and En3Hance were from NEN Research Products, Dupont (Boston, MA). A23187 was from CalBiochem Corp (San Diego, CA). Thrombin was a generous gift of Dr J. W. Fenton II, New York State Department of Health (Albany, NY). Collagen was from Chrono-log Corp (Havertown, PA). Heparin-agarose was from Pierce (Rockford, IL). Leupeptin, PGE1 , phenylmethylsulfonyl fluoride (PMSF ), CM Sephadex, DEAE Sephacel, molecular weight markers, adenosine diphosphate (ADP) and MoAb MOPC 21 (in mouse ascites) were from Sigma (St Louis, MO). MoAbs D4.6, and A6.1 (in mouse ascites), which are specific for EDTA-treated TSP, were from GIBCO-BRL (Gaithersburg, MD). MoAb C6.7, which recognizes TSP equally in either Ca2+ or EDTA containing solutions was a generous gift of Dr W. Frazier (St Louis, MO). Polyacrylamide gel electrophoresis (PAGE) reagents, goat antimouse IgG alkaline phosphatase conjugate and alkaline phosphatase substrate kit were from BioRad Laboratories (Richmond, CA). Fluorescein-conjugated goat IgG to mouse IgG was from Organon Teknika Corp (Durham, NC). Ten percent to 20% gradient precast Tricine gels and a second set of molecular weight markers were from Novex (San Diego, CA).
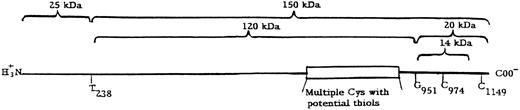
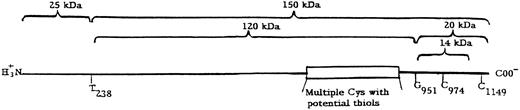
Structure of thrombospondin. The fragments are arranged about the main cleavage points at T238 and G951 and the additional cleavage site approximately 60 residues (6 kD) from the C-terminus. The multiple cysteines (Cys) with potential thiols are within the calcium binding region (type 3 repeats) of thrombospondin.10
Structure of thrombospondin. The fragments are arranged about the main cleavage points at T238 and G951 and the additional cleavage site approximately 60 residues (6 kD) from the C-terminus. The multiple cysteines (Cys) with potential thiols are within the calcium binding region (type 3 repeats) of thrombospondin.10
Partial thrombin digests of [3H]NEM-derivatized, Ca-TSP. One hundred twenty micrograms/milliliter Ca-TSP was derivatized in 6 mmol/L EDTA at 24°C. The label was removed by dialysis overnight as described in Materials and Methods. The labeled Ca-TSP was incubated in TBS containing 6 mmol/L EDTA with 120 nmol/L thrombin at pH 8.0 for 1 and 3 hours. The fragments were analyzed with a reducing, 5% to 20% gradient gel (A) or with a nonreducing, 5% to 15% gel (B). The gels were stained, destained, and prepared for fluorography. Fluorographs are shown.
Partial thrombin digests of [3H]NEM-derivatized, Ca-TSP. One hundred twenty micrograms/milliliter Ca-TSP was derivatized in 6 mmol/L EDTA at 24°C. The label was removed by dialysis overnight as described in Materials and Methods. The labeled Ca-TSP was incubated in TBS containing 6 mmol/L EDTA with 120 nmol/L thrombin at pH 8.0 for 1 and 3 hours. The fragments were analyzed with a reducing, 5% to 20% gradient gel (A) or with a nonreducing, 5% to 15% gel (B). The gels were stained, destained, and prepared for fluorography. Fluorographs are shown.
Preparation of platelets.Whole blood was collected from healthy volunteers into 0.1 volume of 3.8% sodium citrate. Platelets were prepared by differential centrifugation at room temperature. One micromolar PGE1 was added to the blood before centrifugation at 300g for 20 minutes. The platelet-rich plasma (PRP) obtained was further centrifuged at 1,000g for 20 minutes to pellet the platelets. Platelets were washed twice with HEPES buffer (150 mmol/L NaC1/10 mmol/L HEPES/0.5 mmol/L EDTA, pH 7.4) containing 300 nmol/L PGE1 and resuspended in Tris buffer (150 mmol/L NaC1/20 mmol/L Tris, pH 7.4) (TBS) with 2 mmol/L CaC12 . Platelets were also prepared from newly outdated platelet concentrates obtained from the hospital blood bank by the same procedure without PGE1 .
Preparation of supernatant from activated platelets.Platelets were activated by 2 μmol/L A23187 in the presence of 25 μg/mL leupeptin at room temperature. One millimolar PMSF was added before centrifugation at 1,000g for 20 minutes at 4°C to obtain the supernatant. The supernatant was further centrifuged at 10,000g at 4°C for 20 minutes to remove particulate materials.
TSP.TSP was prepared from activated platelet supernatant as described by Speziale and Detwiler.10 Supernatant was loaded onto a heparin-agarose affinity column equilibrated with TBS with 2 mmol/L CaC12 followed by step wise elution with 150, 325, and 550 mmol/L NaC1 containing 2 mmol/L CaCl2 . TSP was eluted at 550 mmol/L NaC1 and stored at −70°C. Thus, the TSP obtained (prepared with Ca2+ throughout) will be referred to in the text simply as Ca-TSP. It was shown not to react with an antibody specific for the EDTA-conformation (see Fig 5).15
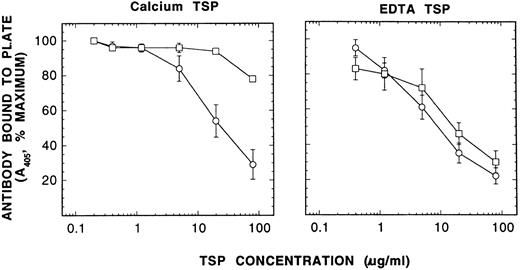
Characterization of Ca-TSP and EDTA-TSP by competitive immunoassay with MoAb D4.6. EDTA-TSP was prepared similarly to Ca-TSP (see Materials and Methods), except that 5 mmol/L EDTA was added to the activated platelet suspension after aggregation occurred, before the separation of the supernatant from platelets. EDTA-TSP was then purified from EDTA-containing supernatant by the same procedure as described for Ca-TSP using Ca2+ -containing buffers. The microtiter plate was coated with 10 μg/mL TSP containing 3 mmol/L EDTA at 4°C for overnight. After incubation of Ca-TSP or EDTA-TSP in either 2 mmol/L Ca2+ or 6 mmol/L EDTA for 1 hour at 37°C the TSP was diluted into solutions containing MoAb D4.6 with 2 mmol/L Ca2+ or 3 mmol/L EDTA. After 1 hour, the mixtures were added to the wells coated with TSP to determine the amount of antibody free to bind to the coated TSP. After washing four times, antibody bound was detected by the color reaction of p-nitrophenylphosphate with alkaline phosphatase coupled to a secondary antibody as described in Materials and Methods. The absorbance in the absence of competing TSP was taken as 100%. Competition by TSP is the reciprocal of antibody bound to plate, expressed as percent of maximum. Shown here are the competition curves using TSP (means ± SE of four separate experiments) that had been incubated for 1 hour in either 2 mmol/L Ca2+ (□) or 6 mmol/L EDTA (○). It shows that Ca-TSP can be converted to the EDTA-conformation, but that the reverse does not occur.
Characterization of Ca-TSP and EDTA-TSP by competitive immunoassay with MoAb D4.6. EDTA-TSP was prepared similarly to Ca-TSP (see Materials and Methods), except that 5 mmol/L EDTA was added to the activated platelet suspension after aggregation occurred, before the separation of the supernatant from platelets. EDTA-TSP was then purified from EDTA-containing supernatant by the same procedure as described for Ca-TSP using Ca2+ -containing buffers. The microtiter plate was coated with 10 μg/mL TSP containing 3 mmol/L EDTA at 4°C for overnight. After incubation of Ca-TSP or EDTA-TSP in either 2 mmol/L Ca2+ or 6 mmol/L EDTA for 1 hour at 37°C the TSP was diluted into solutions containing MoAb D4.6 with 2 mmol/L Ca2+ or 3 mmol/L EDTA. After 1 hour, the mixtures were added to the wells coated with TSP to determine the amount of antibody free to bind to the coated TSP. After washing four times, antibody bound was detected by the color reaction of p-nitrophenylphosphate with alkaline phosphatase coupled to a secondary antibody as described in Materials and Methods. The absorbance in the absence of competing TSP was taken as 100%. Competition by TSP is the reciprocal of antibody bound to plate, expressed as percent of maximum. Shown here are the competition curves using TSP (means ± SE of four separate experiments) that had been incubated for 1 hour in either 2 mmol/L Ca2+ (□) or 6 mmol/L EDTA (○). It shows that Ca-TSP can be converted to the EDTA-conformation, but that the reverse does not occur.
[3H]NEM derivatization of TSP.The procedure was performed as previously described.10 [3H]NEM stock solution was prepared by mixing the labeled NEM in pentane to a volume of 100 mmol/L bis-Tris-propane. Pentane was removed by a gentle stream of nitrogen. The concentration was adjusted to 500 to 700 μmol/L by adding cold NEM. Six millimolar EDTA was added to supernatant TSP or Ca-TSP before addition of 50 to 70 μmol/L stock [3H]NEM. Incubation was performed at room temperature for 45 minutes under nitrogen. The reaction was terminated by incubation with 40-fold excess of cold NEM for 20 minutes under the same conditions. Twelve millimolar CaCl2 was then added to the samples containing EDTA. When purified TSP was labeled, the noncovalently bound label was removed by overnight dialysis against TBS containing 2 mmol/L Ca2+. When TSP in the supernatant was labeled, the labeled TSP was subsequently purified by heparin-affinity chromatography as described above.
Limited thrombin digestion and sodium dodecyl sulfate-PAGE (SDS-PAGE) analysis.The pH of dialyzed TSP or TSP from the heparin column was adjusted to pH 8.0 with 0.1 mol/L Tris base. Thrombin was added to a final concentration of 120 nmol/L. Digestion was carried out using TBS in the presence of 6 mmol/L EDTA or 2 mmol/L CaCl2 at 37°C and was terminated by the addition of SDS-containing sample buffer. TSP and proteolytic fragments were analyzed with either 11%, 5% to 15%, or 5% to 20% Tris-glycine gels under reducing or nonreducing conditions. The stained gels were destained and treated with En3Hance for fluorography.
Peptide sequencing.After separation of the thrombin digest peptide by SDS-PAGE, the N-terminal sequences were determined by automated Edman degradation and phenylthiohydantoin (PTH)-amino acid analyses. The sequencing was performed by the Protein Sequencing Center at SUNY Health Science Center, using Applied Biosystems (Foster City, CA) model 470A Gas-phase protein sequencer/Model 900A Controller/Data Processor (Standard sequencing cycle, Run 470-1) connected on line to a microbore HPLC PTH-amino acid analyzer (Model 120A).
Effect of temperature and pH on derivatization of TSP with [3H]NEM. One hundred fifty micrograms/milliliter Ca-TSP was incubated at 37°C for 30 minutes or adjusted to pH 8.4 with 0.1 mol/L Tris base before derivatization with [3H]NEM. Limited thrombin digestion, SDS-PAGE on 5% to 20% gradient gels, and fluorography were as described in Materials and Methods. Shown here are fluorographs of undigested TSP (0 hours) and 3-hour thrombin digestion in 6 mmol/L EDTA.
Effect of temperature and pH on derivatization of TSP with [3H]NEM. One hundred fifty micrograms/milliliter Ca-TSP was incubated at 37°C for 30 minutes or adjusted to pH 8.4 with 0.1 mol/L Tris base before derivatization with [3H]NEM. Limited thrombin digestion, SDS-PAGE on 5% to 20% gradient gels, and fluorography were as described in Materials and Methods. Shown here are fluorographs of undigested TSP (0 hours) and 3-hour thrombin digestion in 6 mmol/L EDTA.
Effect of PDI on derivatization of TSP with [3H]NEM. One hundred microliters of purified platelet PDI (43 μg/mL) was incubated with 200 μmol/L GSH for 20 minutes before the addition of 0.9 mL 150 μg/mL Ca-TSP. After 30 minutes, TSP was derivatized, digested, and analyzed as described for Fig 3. Shown here are the fluorographs of the reduced gels of undigested TSP (0 hours) and a 3-hour thrombin digestion in 6 mmol/L EDTA.
Effect of PDI on derivatization of TSP with [3H]NEM. One hundred microliters of purified platelet PDI (43 μg/mL) was incubated with 200 μmol/L GSH for 20 minutes before the addition of 0.9 mL 150 μg/mL Ca-TSP. After 30 minutes, TSP was derivatized, digested, and analyzed as described for Fig 3. Shown here are the fluorographs of the reduced gels of undigested TSP (0 hours) and a 3-hour thrombin digestion in 6 mmol/L EDTA.
Purification of PDI.PDI was purified from newly outdated human platelets according to the procedure described by Hillson16 and modified by Chen et al.13 Briefly, 10 to 20 U of washed platelets were sonicated in the presence of 1% Triton X-100 (Sigma). After centrifugation at 18,000g for 40 minutes, the supernatant was heated at 55°C for 30 minutes. A 55% to 85% saturated ammonium sulfate pellet was prepared and dissolved in 25 mmol/L citrate buffer (pH 5.0), dialyzed against the same buffer overnight before loading onto a CM-Sephadex column, and eluted with the same buffer. PDI in the void volume of the eluate was precipitated by 100% saturated ammonium sulfate. The pellet was dissolved in 100 mmol/L phosphate buffer (pH 6.3) and dialyzed against the same buffer overnight followed by DEAE-Sephacel chromatography. Elution was with a gradient of 100 to 700 mmol/L NaC1. Absorbance at 280 nm and PDI activity (by the scrambled ribonuclease assay) were determined in the collected fractions and purity was assessed by SDS-(7.5%) PAGE.
Competitive immunoassays.Competitive immunoassays were designed to test the ability of different conformations of soluble TSP to compete with antibody for binding to TSP on the plate. The plate was coated with TSP containing 3 mmol/L EDTA overnight at 4°C. The plate was washed with TBS before blocking with 0.5% bovine serum albumin for 2 hours at room temperature. Three hundred twenty micrograms/milliliter Ca-TSP was incubated in the presence of 2 mmol/L Ca2+ or 6 mmol/L EDTA under specified conditions for 1 hour. In some experiments supernatant containing a similar amount of TSP was incubated in the presence or absence of 6 mmol/L EDTA. Sixty microliters of TSP was then added to 180 μL solution of MoAb D4.6 (1:20,000) or C6.7 (1:4,000) containing either 3 mmol/L EDTA or 2 mmol/L CaCl2 . TSP was serially diluted with the same antibody solution. After 1-hour incubation at room temperature, 80 μL of the TSP-antibody mixture was added in duplicate to the wells and was incubated for another hour at room temperature. The nonreacted antibody was removed by washing 4 times with TBS containing 0.05% Tween 20. The plate was incubated with goat antimouse IgG alkaline phosphatase conjugate for 1 hour. The amount of antibody bound was determined by absorbance at 405 nm due to hydrolysis of p-nitrophenylphosphate.
Flow cytometry analysis.Blood was collected from normal aspirin-free adult volunteer donors through a 19-gauge needle into sodium heparin anticoagulated tubes (Becton Dickinson, San Jose, CA) giving 14.3 USP U of heparin/mL of blood. PGE1 was added to a final concentration of 1 μmol/L to some of the samples. PRP free of erythrocytes and leukocytes was prepared by centrifuging the whole blood at 300g for 20 minutes. The platelets in the heparinized PRP, which did not contain PGE1 were activated with A23187 (2 μmol/L), ADP (20 μmol/L), or collagen (10 μg/mL) for 5 minutes after which a predetermined amount of the MoAb C6.7 (1:4,000), D4.6 (1:20,000), or A6.1 (1:160) to provide saturating concentrations was added for 20 minutes at room temperature. The platelets were pelleted at 1,000g for 20 minutes, resuspended in 50 μL of PBS filtered through a 0.2-μm filter and containing 0.1% sodium azide, and incubated with fluorescein isothiocyanate (FITC)-labeled goat antimouse IgG for 20 minutes at room temperature in the dark. An isotype specific MoAb MOPC 21 (raised against mineral oil) was used as a negative control for the primary antibodies with both nonactivated and activated platelets. The platelets were again pelleted, resuspended in 1 mL of filtered phosphate-buffered saline (PBS), and analyzed by flow cytometry using a Becton Dickinson FACstar plus flow cytometer as previously described.12,13 Results were expressed on histograms of log-platelet fluorescence on the abscissa and platelet number on the ordinate.
RESULTS
[3H]NEM labeling of free thiols in TSP.Digestion of TSP in EDTA with thrombin gave fragments of 150, 120, 25, 20, and 14 kD on SDS-PAGE. When free thiols of TSP were labeled with [3H]NEM in the supernatant solution, containing 6 mmol/L EDTA, all but the 25-kD peptide were labeled. This is essentially the same as previously described with [14C] iodoactamide-labeled TSP.10 It is consistent with multiple sites of free thiol. The N-terminal sequence of the 120-kD peptide, T238NYIGHKTKD, was identical to that of the 150-kD peptide, whereas the N-terminal sequence of the 14-kD peptide, G951 TSQNDPNWV, was identical to that of the 20-kD peptide. These data indicate that the 120-kD peptide was a fragment of the 150-kD peptide and that the 14-kD peptide was a fragment of the 20-kD peptide. These peptides are placed in a linear sequence of TSP in Fig 1. The positions are consistent with the assignments of similar peptides by Lawler et al17 based in part on identification with MoAbs. (Our peptides of 150, 120, 25, 20, and 14 kD presumably are equivalent to those of 160, 130, 30, 25, and 18 kD reported by Lawler et al.)
Because of apparent contradictions in the literature, we examined experimental conditions that might affect localization of thiols of TSP. As described here, the location of thiols depends on the conditions of labeling. When Ca-TSP (ie, purified in Ca2+-containing buffers) was labeled with NEM in the presence of 6 mmol/L EDTA at room temperature, pH 7.4, and then partially digested with thrombin (Fig 2), the radioactivity was recovered in fragments of 14-, 20-, and 150-kD, but not in the 120-kD fragment. By reference to Fig 1, it can be deduced that the label must have been in the 14-kD piece beginning with Gly951. That is, Cys974 must have been the predominant, if not the only, free thiol, consistent with the conclusion of Sun et al.11 Nonreduced electrophoresis (Fig 2B) showed that there was a labeled 14-kD fragment but not a labeled 20-kD fragment, indicating that Cys1149 was disulfide bonded to the remainder of the molecule.
When Ca-TSP was labeled in the presence of 6 mmol/L EDTA at a higher temperature (37°C) or higher pH (8.4), radioactivity was also recovered in a 120-kD fragment after digestion with thrombin (Fig 3). This is consistent with the conclusion of Speziale and Detwiler10 that the position of the thiol was randomized before or during labeling. The difference between the results reported here and those of Speziale and Detwiler10 is that they found label in the 120-kD peptide at room temperature, while we found it only at 37°C. The difference in experimental conditions is that they labeled in the supernatant solution, while we purified TSP before labeling. A possible explanation is the reported presence of protein disulfide isomerase in the supernatant solution of activated platelets.13 14 PDI-catalyzed isomerization of disulfide bonds in the supernatant solution would explain the discrepancy.
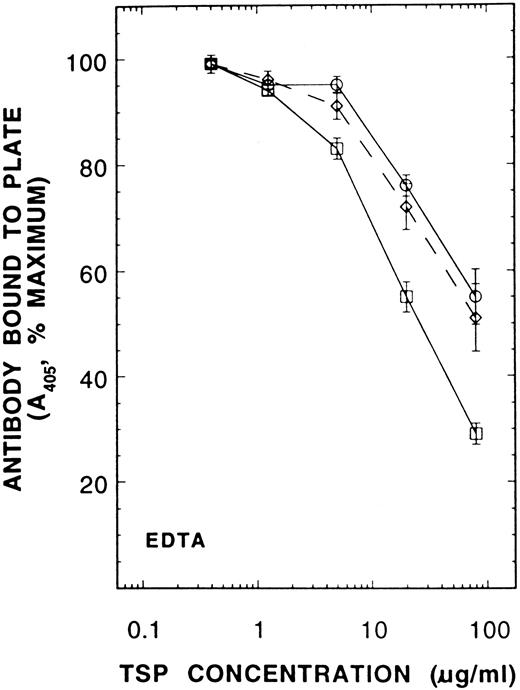
A competitive immunoassay showing the effect of PDI on conversion of Ca-TSP to the EDTA-conformation. Forty-three micrograms/milliliter PDI was incubated with 200 μmol/L GSH for 20 minutes before addition of Ca-TSP in 6 mmol/L EDTA. After 45-minutes incubation at room temperature, TSP was diluted into a solution containing antibody D4.6 and the competitive immunoassay was performed as described in Fig 5. The results are means ± SE of six separate experiments. (○), control TSP; (⋄), TSP with GSH; (□), TSP with PDI + GSH.
A competitive immunoassay showing the effect of PDI on conversion of Ca-TSP to the EDTA-conformation. Forty-three micrograms/milliliter PDI was incubated with 200 μmol/L GSH for 20 minutes before addition of Ca-TSP in 6 mmol/L EDTA. After 45-minutes incubation at room temperature, TSP was diluted into a solution containing antibody D4.6 and the competitive immunoassay was performed as described in Fig 5. The results are means ± SE of six separate experiments. (○), control TSP; (⋄), TSP with GSH; (□), TSP with PDI + GSH.
A competitive immunoassay showing the effect of prelabeling TSP with NEM on conversion to the EDTA-conformation. Supernatant TSP was derivatized with [3H]NEM in the presence of 2 mmol/L Ca2+. The labeled TSP was purified by heparin-affinity chromatography as described in Materials and Methods. Three hundred fifty micrograms/milliliter Ca-TSP or NEM-TSP was incubated at 37°C for 30 minutes before addition of MoAb D4.6 as described in Fig 5. The results are the means ± SE of 3 separate experiments. (□), 2 mmol/L Ca2+; (○), 6 mmol/L EDTA.
A competitive immunoassay showing the effect of prelabeling TSP with NEM on conversion to the EDTA-conformation. Supernatant TSP was derivatized with [3H]NEM in the presence of 2 mmol/L Ca2+. The labeled TSP was purified by heparin-affinity chromatography as described in Materials and Methods. Three hundred fifty micrograms/milliliter Ca-TSP or NEM-TSP was incubated at 37°C for 30 minutes before addition of MoAb D4.6 as described in Fig 5. The results are the means ± SE of 3 separate experiments. (□), 2 mmol/L Ca2+; (○), 6 mmol/L EDTA.
Incubation of Ca-TSP in the presence of 6 mmol/L EDTA with PDI at room temperature before derivatization led to recovery of label in the 120-kD fragment (Fig 4). This apparently was caused by the catalytic activity of PDI and not simply caused by a protein-protein interaction, because we were unable to reproduce this result using fibronectin, a protein known to bind to TSP, instead of PDI. Since the appearance of thiol in a new location was not associated with incorporation of additional radioactivity, it apparently was due to isomerization of disulfide bonds rather than to reduction of disulfide bonds. We conclude that the single equivalent of thiol is at Cys974 in freshly secreted TSP (representing the Ca2+-conformation of TSP), but that under certain conditions it can quickly become isomerized to positions in the putative Ca2+-binding domain with its many cysteines.
Competitive immunoassay to detect conformational changes in soluble TSP.The enhancement of the isomerization of disulfide bonds by elevated pH, physiological temperature, and incubation with PDI did not occur in media containing 2 mmol/L CaCl2 (data not shown). Thus, the changes could be due either to direct effects on disulfide bond isomerization or to indirect effects on the attainment of the EDTA-conformation,18 a poorly defined conformation of TSP that exists in TSP when Ca2+ is chelated, and, as shown in this report, in other conditions as well.
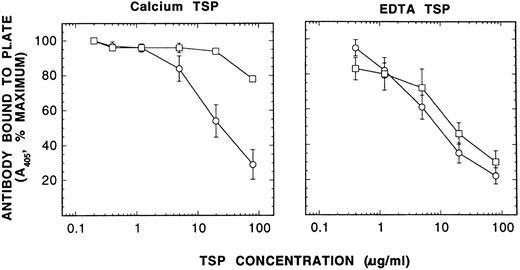
To assess the different conformations of TSP, we developed a competitive immunoassay using the MoAbs D4.6,15 which has been shown to react only with EDTA-treated TSP, and C6.7,19 which has been shown to react equally with TSP in either Ca2+ or the EDTA containing buffers. As expected Ca-TSP and EDTA-TSP competed equally well for the control antibody (C6.7) when the incubation was with either 2 mmol/L CaC12 or 6 mmol/L EDTA (data not shown). In contrast, Ca-TSP competed for antibody D4.6 only when the incubation was in the presence of excess EDTA, whereas EDTA-TSP competed when the incubation was in Ca2+ or in excess EDTA (Fig 5). These results are consistent with the idea that the Ca2+-conformation readily converts to the EDTA-conformation on removal of Ca2+, but the EDTA-conformation does not readily convert to the Ca2+-conformation when Ca2+ is restored.20 Thus, to study the conversion of one conformation to the other, EDTA was added to Ca-TSP.
In a similar experiment, Ca-TSP at pH 7.4 was adjusted to pH 6.4 or 8.4 and then incubated at room temperature or at 37°C for 45 minutes in 6 mmol/L EDTA. The competitive immunoassay with MoAb D4.6 was used (as described for Fig 5) to analyze TSP conformations. More Ca-TSP formed the EDTA-conformation in an EDTA buffer at room temperature when the pH was 8.4 than when it was 7.4 or 6.4, but at 37° the EDTA-conformation was formed independently of pH. In a Ca2+-containing buffer, there was no EDTA-conformation at any of those pHs. TSP in the supernatant solution (ie, before purification) formed the EDTA-conformation at room temperature when EDTA was added without the necessity of increasing the pH.
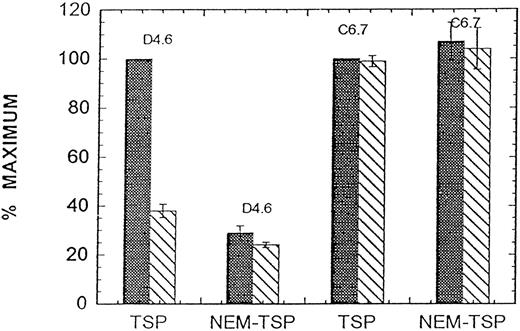
When purified Ca-TSP was incubated at room temperature in an EDTA-containing buffer at pH 7.4 (a condition that results in little EDTA-conformation), the addition of PDI caused an increased conversion to the EDTA-conformation (Fig 6). Our interpretation of this experiment is that PDI accelerates the conversion of TSP to the EDTA-conformation by catalyzing thiol-disulfide isomerization of internal disulfide bonds as suggested by Sun et al.11 To test this, we first blocked the presumed single thiol of supernatant TSP (from Fig 1, we consider this to be Cys974 ) by reaction with NEM. Ca-TSP and NEM-TSP competed equally for antibody C6.7 in either Ca2+ or EDTA-buffer (not shown), but NEM-TSP was not converted to a form that could react with D4.6 in an EDTA-buffer (Fig 7). Since previous studies provided evidence of different reactivities for TSP thiols10 the present results suggest that the most reactive thiol is an accessible Cys974 and that thiol-disulfide exchange involving this thiol is required for the EDTA-conformation.
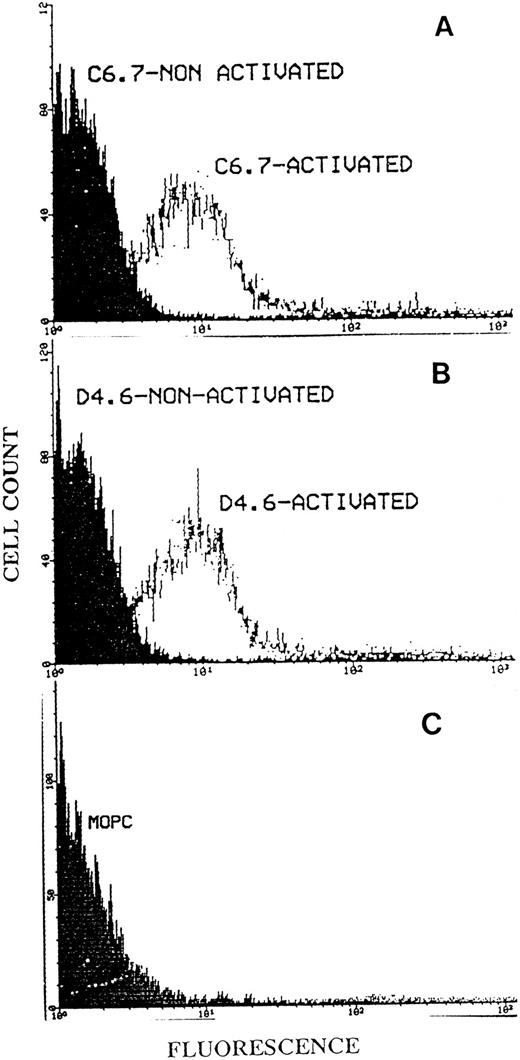
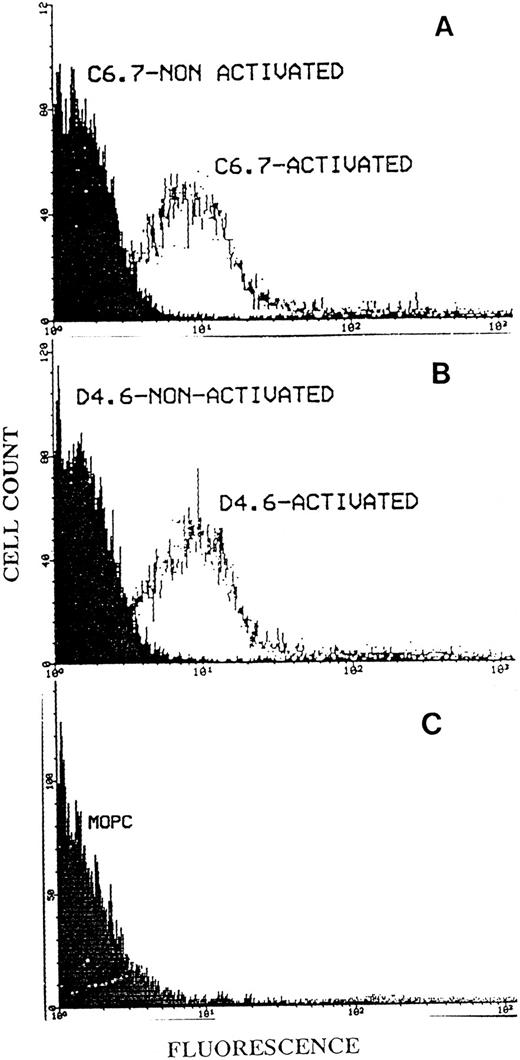
Binding of MoAbs C6.7 and D4.6 to platelets by flow cytometry.We initially found that gel-filtered platelets on activation with A23187 in the presence of Ca2+ showed substantially enhanced binding of D4.6 (which is specific for the EDTA-conformation of TSP) when compared with platelets not activated with A23187. To further study whether the EDTA-conformation of TSP could be detected on platelets in the presence of Ca2+, we incubated activated platelets in heparinized PRP with the antibody D4.6. Flow cytometry analysis of the platelets was performed as described in Materials and Methods. Figure 8 shows that there was binding of D4.6 to activated platelets with little binding to nonactivated platelets and that this binding was similar to that of antibody C6.7 (which recognizes TSP in either the Ca2+ or EDTA-conformation). An isotype specific control antibody bound to neither non-activated nor activated (Fig 8C) platelets. Experiments performed with another antibody specific for EDTA-treated TSP (A6.1) and with other platelet agonists (ADP and collagen) revealed similar results. Additional control experiments showed that D4.6 recognizes Ca-TSP much less well than Ca-TSP incubated in EDTA when TSP is bound to plastic surfaces (Fig 9). The relatively poor binding of D4.6 to Ca-TSP on a plastic surface, in contrast with the binding to TSP on the platelet surface in the presence of Ca2+, suggests that specific interactions on the platelet surface facilitate the conversion of TSP to a conformation recognized by D4.6.
Flow cytometry analysis of platelet TSP using the antibodies C6.7 and D4.6. Fifty microliters of PRP from heparinized blood was incubated at room temperature for 20 minutes with MoAb C6.7 (A) or D4.6 (B), with or without prior activation by A23187. (C) Shows the isotype specific control antibody, MOPC 21, which had been incubated with activated platelet. After a second incubation with FITC-labeled goat antimouse IgG the platelets were analyzed by flow cytometry as described in Materials and Methods. The log of fluorescence is indicated on the abscissa and cell count on the ordinate.
Flow cytometry analysis of platelet TSP using the antibodies C6.7 and D4.6. Fifty microliters of PRP from heparinized blood was incubated at room temperature for 20 minutes with MoAb C6.7 (A) or D4.6 (B), with or without prior activation by A23187. (C) Shows the isotype specific control antibody, MOPC 21, which had been incubated with activated platelet. After a second incubation with FITC-labeled goat antimouse IgG the platelets were analyzed by flow cytometry as described in Materials and Methods. The log of fluorescence is indicated on the abscissa and cell count on the ordinate.
Binding of antibodies D4.6 and C6.7 to solid phase TSP. Ten micrograms/milliliter Ca-TSP or NEM-TSP (prepared as in Fig 7) in 2 mmol/L Ca2+ (▧) or 3 mmol/L EDTA () was coated on the microtiter plate overnight at 4°C. MoAbs D4.6 or C6.7 in either Ca2+- or EDTA-containing buffers were added and the immunoassay was performed as described in Materials and Methods using a 1-hour incubation at room temperature. The maximum binding for TSP in the presence of EDTA was taken as 100%. The results (means ± SE of three separate experiments) are expressed as the percent of maximum.
Binding of antibodies D4.6 and C6.7 to solid phase TSP. Ten micrograms/milliliter Ca-TSP or NEM-TSP (prepared as in Fig 7) in 2 mmol/L Ca2+ (▧) or 3 mmol/L EDTA () was coated on the microtiter plate overnight at 4°C. MoAbs D4.6 or C6.7 in either Ca2+- or EDTA-containing buffers were added and the immunoassay was performed as described in Materials and Methods using a 1-hour incubation at room temperature. The maximum binding for TSP in the presence of EDTA was taken as 100%. The results (means ± SE of three separate experiments) are expressed as the percent of maximum.
DISCUSSION
In this study we have shown a correlation between the EDTA-conformation of TSP and the randomization of the single thiol/polypeptide chain among the 10 or so cysteines in the putative Ca2+ binding domain. We have also demonstrated for soluble TSP that while the Ca2+ conformation can be converted to the EDTA-conformation, the reverse reaction occurs slowly if at all. This is consistent with the finding of others for solid phase TSP,11,20 but differs from the findings of Dixit et al15 for TSP in solution. There remains the question whether disulfide bond isomerization is necessary for formation of the EDTA-conformation or whether the isomerization only stabilizes a conformation that otherwise would represent only a small fraction of the TSP molecules. The difference between these two mechanisms is, however, largely technical; without disulfide bond isomerization, there is little of the EDTA-conformation.
This shifts the question to the significance of the EDTA-conformation. The reactions of TSP with thrombin, collagen, and cathepsin G, as well as the isomerization of disulfide bonds, all require the absence of Ca2+, and it is possible that this is because they require the EDTA-conformation. Many proteins are known to undergo conformational changes upon binding to another protein or onto some surfaces. Fibronectin was shown to reveal a free thiol when bound to polystyrene beads.21 Upon binding to fibrinogen at the low affinity site, glycoprotein IIb/IIIa expressed a new high affinity site for fibrinogen.22 For TSP, binding to its putative receptor, CD36 (glycoprotein IV), also involved a two-step process. The low affinity binding to CD36 sequence 139-155 induced a conformational change in TSP, which exposed a high affinity site, for the CD36 sequence 93-110.23 This conformational change may or may not represent the conversion of TSP into its EDTA-conformation, but it raises the possibility that binding of TSP to a protein or a surface may allow TSP to achieve its EDTA-conformation in the presence of calcium. In flow cytometry studies we observed most TSP bound to the surface of activated platelets was recognized by antibodies specific for the EDTA-conformation suggesting that the EDTA-conformation is native for platelet-bound TSP. Since EDTA treatment of purified TSP has been shown to enhance TSP's binding to certain proteins3,4 24 this finding is of possible importance to the physiologic relevance of this conformation of TSP. This is also consistent with the notion that interactions of certain proteins with TSP may mimic the effect of EDTA on TSP, allowing PDI to facilitate the conformational change in TSP in the presence of Ca2+.
It is possible that isomerization of disulfide bonds may modify some TSP functions. Preliminary studies have noted that the binding of platelet-derived growth factor to TSP was markedly dependent on the specific disulfide conformation of TSP.5 TSP's inhibition of the proteases neutrophil elastase and cathepsin G also appears to be dependent on the disulfide conformation of TSP.25 Furthermore, reduction of TSP with DTT exposes the RGD sequence, substantially enhancing the cell adhesive activity of TSP.11 DTT concentrations required to enhance the adhesive activity of TSP were 10-fold lower when Ca2+ was chelated with EDTA. Since isomerization of disulfide bonds in TSP occurs primarily in the region of TSP surrounding the RGD sequence10 it seems likely that this process would also modulate TSP's adhesive function. While little is known about the effect of PDI on native proteins the evidence presented here suggests TSP may be a substrate and raises the possibility that other platelet or plasma proteins are regulated by this enzyme.
Supported by the US Department of Health and Human Services (Bethesda, MD), National Institutes of Health Grant No. HL37250.
Address reprint requests to David W. Essex, MD, Department of Biochemistry, SUNY Health Science Center at Brooklyn, 450 Clarkson Ave, Brooklyn, NY 11203.

![Fig. 2. Partial thrombin digests of [3H]NEM-derivatized, Ca-TSP. One hundred twenty micrograms/milliliter Ca-TSP was derivatized in 6 mmol/L EDTA at 24°C. The label was removed by dialysis overnight as described in Materials and Methods. The labeled Ca-TSP was incubated in TBS containing 6 mmol/L EDTA with 120 nmol/L thrombin at pH 8.0 for 1 and 3 hours. The fragments were analyzed with a reducing, 5% to 20% gradient gel (A) or with a nonreducing, 5% to 15% gel (B). The gels were stained, destained, and prepared for fluorography. Fluorographs are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/9/10.1182_blood.v89.9.3205/3/m_bl_0049f2.jpeg?Expires=1767696846&Signature=xm8Ru3QUTV4mU5Jz7p45MaCNiOYniJ7FHsIj3fZMaTk5f321pq1mCNMbQ5aVf0~xe52bN-LLTeo9H5XnJqy-Vn3UFyuw-oWJBR0hjCViSbf-x4ie~Hqih019GeJ~cpJJW5T0prXv5KKp~Ln3f1IQHzx6gN7E3hrf2PCzID8ShCtFz8noLk2jlRqj49Md0YgM5EhV06sp7LNlDeFxEpBH-I79yWmHZxz-td-xWd9D4HzPgaeW34HNMQ0zPK7L5Vd2VHBOLqbiNUG1k84Jg-RURU1FrKAzgTLt6CTteKUR0KaswNyYELK-XT49i-SQcoqUX4gS1lX~fYdF3ovS9VK48Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effect of temperature and pH on derivatization of TSP with [3H]NEM. One hundred fifty micrograms/milliliter Ca-TSP was incubated at 37°C for 30 minutes or adjusted to pH 8.4 with 0.1 mol/L Tris base before derivatization with [3H]NEM. Limited thrombin digestion, SDS-PAGE on 5% to 20% gradient gels, and fluorography were as described in Materials and Methods. Shown here are fluorographs of undigested TSP (0 hours) and 3-hour thrombin digestion in 6 mmol/L EDTA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/9/10.1182_blood.v89.9.3205/3/m_bl_0049f3.jpeg?Expires=1767696846&Signature=qoAyRbM1IAfE6dYoFCrvbGk962MvdU~Ui~HPOlA7W621KRqBT33hfO9beKgXhmFfZJQBLzDVApR-tsljiuHkp4x40YyRC9UhqrbbtWSAvV-L6UWBc8gnpyXOl-agWWvBy9ge8-sEWL0FiumHKNTxQC8NM4vzRx4ZvDciCYJcqnpRAYF8fj1pLh2472-TTxtK~1aPM6ouH-qcRxzGGU~MgrOyIT5DjWLewzAtONng8LKAYFN24~qUuk4nZ~5op-W-QeF4ebInIRELGDbRDyJz1NoxRgwOA5Y7Ei-Go4EdFn8W6nIKMyN5e773xaLpb1OWLgFNOgI2i93WnimLi-Enpg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of PDI on derivatization of TSP with [3H]NEM. One hundred microliters of purified platelet PDI (43 μg/mL) was incubated with 200 μmol/L GSH for 20 minutes before the addition of 0.9 mL 150 μg/mL Ca-TSP. After 30 minutes, TSP was derivatized, digested, and analyzed as described for Fig 3. Shown here are the fluorographs of the reduced gels of undigested TSP (0 hours) and a 3-hour thrombin digestion in 6 mmol/L EDTA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/9/10.1182_blood.v89.9.3205/3/m_bl_0049f4.jpeg?Expires=1767696846&Signature=hz331n6Blt6QHR31oLSpivJS8GJCIXnPE0HpeIXVE7nwq25L16vTx3-0iXI9iSKM1KUJr-uSNa5cCG64~7XKz0e0MYlwaGokbYnz-nKstxHQYWGfSvmhiO9378Sr7INBgiXx1wQNfZPTF8uVD8KVt~X4dccg6mbY4qGptfq9EPfvwQWHa8pjN7ZAJLk6BnkA9V-XdBtN8~MZQ4mZ~kvNHzko66pIqlkZ8aE5ehA4I42hUNxj4AUxjZ8W0tceb4eQoFbmc2m178tWxCwRh6NRY6m5F1Gtj4dPlGowRu7pfFxKNs3un8nsthCZy56u1NhUVwFQq2B2DiRJ5XDR~NPQfQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. A competitive immunoassay showing the effect of prelabeling TSP with NEM on conversion to the EDTA-conformation. Supernatant TSP was derivatized with [3H]NEM in the presence of 2 mmol/L Ca2+. The labeled TSP was purified by heparin-affinity chromatography as described in Materials and Methods. Three hundred fifty micrograms/milliliter Ca-TSP or NEM-TSP was incubated at 37°C for 30 minutes before addition of MoAb D4.6 as described in Fig 5. The results are the means ± SE of 3 separate experiments. (□), 2 mmol/L Ca2+; (○), 6 mmol/L EDTA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/9/10.1182_blood.v89.9.3205/3/m_bl_0049f7.jpeg?Expires=1767696846&Signature=aVmiPd6fTeB~S071Nlfq5BXruQkLykoElYu~efYtTVgoQy3dTAnQxvz-pogUdv2v1rgPWVAXivibUYAGutYLbV1zmH1kB9Ug56ar8TfPXvbJ3szboulYgamARjiNuEZlhsv7dINlmpy5EHHMN4XnOv4B64m98uuLktzT~L09Hq29VIAFMM2j3pSzouWQBbFz1VV34~ocKW8HIJnqZsVUvXbenAcV5PiLAebZf4BKcKOocUOhexPIO7qlJ4HHwYPO6SX~sCMsXBjSRMnD-FLfXZP295wWHOh9KalceUVxMamjBwCITobNFza0xDFhrfVkTSChEGrWDTaVt-5S6d-SvQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. Partial thrombin digests of [3H]NEM-derivatized, Ca-TSP. One hundred twenty micrograms/milliliter Ca-TSP was derivatized in 6 mmol/L EDTA at 24°C. The label was removed by dialysis overnight as described in Materials and Methods. The labeled Ca-TSP was incubated in TBS containing 6 mmol/L EDTA with 120 nmol/L thrombin at pH 8.0 for 1 and 3 hours. The fragments were analyzed with a reducing, 5% to 20% gradient gel (A) or with a nonreducing, 5% to 15% gel (B). The gels were stained, destained, and prepared for fluorography. Fluorographs are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/9/10.1182_blood.v89.9.3205/3/m_bl_0049f2.jpeg?Expires=1767696847&Signature=XdLgYRsFoqUzUteJbzLkwrmYJELDjxqsXyc4BTz1PHerUyFvtY11YbBb3iGF7~c6GHD~J7jmcxeKQ84uKZICJ5DeRUiWQPYH~8s9zNmMuaS90w~Sg0SEGmsvbS4-5ifkQ~wpNoVILEZb5CXiv2xj9wIy83uQOdwE0IFF0-fVKrLF4dWzJvCH01S142PEHgHsMvI4diFWBREBH7QazaArcDY4yaZr8u2Ff7E~4cib1grqPXhg9nrQXxW1XzmzHBTZ7il~Kgn9ajV8tRZ6DxR7bMbsitKGtjXwStQ1T51GqfqMC3e9H9zj7bMSKaaqhrrLW3Vw-3CXp8wQ3GtTdW0CIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Effect of temperature and pH on derivatization of TSP with [3H]NEM. One hundred fifty micrograms/milliliter Ca-TSP was incubated at 37°C for 30 minutes or adjusted to pH 8.4 with 0.1 mol/L Tris base before derivatization with [3H]NEM. Limited thrombin digestion, SDS-PAGE on 5% to 20% gradient gels, and fluorography were as described in Materials and Methods. Shown here are fluorographs of undigested TSP (0 hours) and 3-hour thrombin digestion in 6 mmol/L EDTA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/9/10.1182_blood.v89.9.3205/3/m_bl_0049f3.jpeg?Expires=1767696847&Signature=QInXZjWz-a5rdsZFLAPzO4-pAwAkBPC70c~oEF-loxi9lpaYAGeMrRuQ76XNoVtzWE78P3VOlacTp-qUk36ZUB4EidAR15ha6RXsYdgTk8JYzlrOaDgVm67D-RIDOykvujUOqTOicy1Y5I9L7CYWCTpBGFgeQRKRhyfMmaTCHBmFDDEYPAIBQ8WqFaED9koPj0d8sxZeRREWgJL2UwxBCeSZTGUP4uMYhGenCXIQc~gcwQ2zotQ6uzZnTrlehtjAkKauRcwURWvH9pLgVzBLm1hO7WirF~gQB0A~0akkAvWGh7F85QBs4k-WjSsCYBtLYki2ec~bAT3oSnvkMdHNiQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of PDI on derivatization of TSP with [3H]NEM. One hundred microliters of purified platelet PDI (43 μg/mL) was incubated with 200 μmol/L GSH for 20 minutes before the addition of 0.9 mL 150 μg/mL Ca-TSP. After 30 minutes, TSP was derivatized, digested, and analyzed as described for Fig 3. Shown here are the fluorographs of the reduced gels of undigested TSP (0 hours) and a 3-hour thrombin digestion in 6 mmol/L EDTA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/9/10.1182_blood.v89.9.3205/3/m_bl_0049f4.jpeg?Expires=1767696847&Signature=uev3g4VM~A8T1R08VMFErEkHXT4b5cu-S5SDk7RNCmQTmTUnwqUS4JyTntIMlnMeo12uJSpiNqUCB6HXfqOe-cWHKp7WFbPGgmJvEMs7JjaKq56rmJKBGZVFEklOt0FTdSoOJtR3DNq1jbdmwCb-PX2XnqTAxHKtsH6vSha0GZDbjDD8WgfIbbqpchmGdEJHCbTuBvVRv3bW799JeG2ubAcV2S4Ablu6SsUSHItFDXkzNAZZRNb6PWq00NbdOZZZChrrcHj2PWrDxs1o0wN~dPQGbIsS7qLThWJ6ou5NraU-~3E9YjxLtjfqO~zqEWXoN3CKaFhZwfFtvHGhP-Oweg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
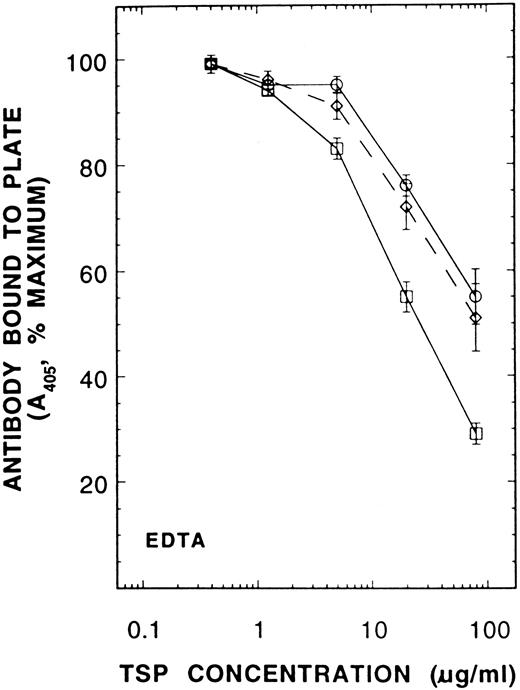
![Fig. 7. A competitive immunoassay showing the effect of prelabeling TSP with NEM on conversion to the EDTA-conformation. Supernatant TSP was derivatized with [3H]NEM in the presence of 2 mmol/L Ca2+. The labeled TSP was purified by heparin-affinity chromatography as described in Materials and Methods. Three hundred fifty micrograms/milliliter Ca-TSP or NEM-TSP was incubated at 37°C for 30 minutes before addition of MoAb D4.6 as described in Fig 5. The results are the means ± SE of 3 separate experiments. (□), 2 mmol/L Ca2+; (○), 6 mmol/L EDTA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/9/10.1182_blood.v89.9.3205/3/m_bl_0049f7.jpeg?Expires=1767696847&Signature=BS7pZp4XR-DhP~McJkACCgK6AEyqdqL7GOdm-368uiNv~-oRq7bC4z9H~21dglfyvHaWF6aJbOki9giELLpR~TX0LQl8vpzbKjcOiLpdo4cdlm-6YUxvCAwuyMF7834y~C706IknC1zidqYtW-dSIqeO4e1dsx5~MXku2gaEeqNnzNUFtgLymEyHl747HplagztgyVDrwfVxUB9kqQ0-ImFTy0yy7W10RTcHXJum733TturotIqZr9eRS21~aKZsfL6km3XyXscA97IQP1TYGcoyS8qhFdZvVYX8ANgfva191dulQMDTJd-doHRbqPVe6beqF2Ef40D4QVKXJUEQoQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)