Abstract
One approach to developing safer and more efficacious agents for the treatment of thrombotic disease involves the design and testing of inhibitors that block specific steps in the coagulation cascade. We describe here the development of a mutant of human tissue factor (TF ) as a specific antagonist of the extrinsic pathway of blood coagulation and the testing of this mutant in a rabbit model of arterial thrombosis. Alanine substitutions of Lys residues 165 and 166 in human TF have been shown previously to diminish the cofactor function of TF in support of factor X (FX) activation catalyzed by factor VIIa (FVIIa). The K165A:K166A mutations have been incorporated into soluble TF (sTF; residues 1-219) to generate the molecule “hTFAA.” hTFAA binds FVIIa with kinetics and affinity equivalent to wild-type sTF, but the hTFAA⋅FVIIa complex shows a 34-fold reduction in catalytic efficiency for FX activation relative to the activity measured for sTF⋅FVIIa. hTFAA inhibits the activation of FX catalyzed by the complex formed between FVIIa and relipidated TF(1-243). hTFAA prolongs prothrombin time (PT) determined with human plasma and relipidated TF(1-243) or membrane bound TF, and has no effect on activated partial thromboplastin time, but is 70-fold less potent as an inhibitor of PT with rabbit plasma. The rabbit homologue of this mutant (“rTFAA”) was produced and shown to have greater potency with rabbit plasma. Both hTFAA and rTFAA display an antithrombotic effect in a rabbit model of arterial thrombosis with rTFAA giving full efficacy at a lower dose than hTFAA. Compared to heparin doses of equal antithrombotic potential, hTFAA and rTFAA cause less bleeding as judged by measurements of the cuticle bleeding time. These results indicate that TF⋅FVIIa is a good target for the development of new anticoagulant drugs for the treatment of thrombotic disease.
TISSUE FACTOR (TF ) plays a fundamental role in hemostasis by initiating the extrinsic pathway of blood coagulation.1,2 TF is the obligate cofactor for activation of zymogen coagulation factor VII (FVII) to the serine protease FVIIa3 and also for the activity of FVIIa on the physiological substrates factor IX (FIX) and factor X (FX).4 TF is a 263 residue membrane bound glycoprotein composed of a 219 residue extracellular domain, a single transmembrane sequence and a short cytoplasmic domain.5-7 TF is not normally expressed on cells of the vasculature, but is found on a variety of tissues, most notably the adventitial cell layer, which forms a hemostatic envelope surrounding blood vessels.8 Vascular damage exposes blood to TF which forms a high affinity, calcium dependent complex with circulating FVII resulting in triggering of the coagulation cascade.
Overexpression of TF has been linked to the pathophysiology of thrombotic disease and sepsis. TF expression on endothelial cells and monocytes is induced by exposure to inflammatory cytokines or bacterial lipopolysaccharide.9 Neutralizing anti-TF monoclonal antibodies (MoAbs) have been shown to prevent death in a baboon model of sepsis10 and block thrombus formation in a rabbit model of arterial thrombosis.11 Although MoAbs have great utility as reagents for concept testing, they are sometimes difficult to raise against a specific target and may only neutralize in a species-specific manner. In addition, MoAbs produced by immunization of rodents must be “humanized”12 to reduce their immunogenicity in humans. We describe here an alternative approach in which a soluble form of human TF, residues 1-219, is converted into an antagonist of membrane bound TF.
Lysine residues 165 and 166 have been shown previously13,14 to have an important role in the cofactor function of TF. Substitution of these residues by alanines does not affect binding to FVIIa, or the activity of the TF⋅FVIIa complex on peptide substrates, but greatly diminishes catalytic function for activation of FX. The structure of the sTF⋅FVIIa complex15 shows that Lys165,166 are not in contact with FVIIa and are located far from the protease active site. Because the complex of FVIIa with sTF has reduced activity for FX activation in the absence of high phospholipid concentration,16 and because sTF is a poor cofactor for activation of zymogen FVII,17 we reasoned that a good antagonist could be obtained by incorporating the K165A:K166A mutations into sTF. We find that this molecule, termed “hTFAA,” is a specific inhibitor of the extrinsic pathway of coagulation in vitro and displays an antithrombotic effect in a rabbit model of arterial thrombosis.
MATERIALS AND METHODS
Materials.Human FVIIa, FX, and FXa were purchased from Haematologic Technologies, Inc (Essex Junction, VT). Spectrozyme FXa was from American Diagnostica (Greenwich, CT) and Chromozym t-PA was from Boehringer Mannheim (Germany). DEAE-Sepharose-FF, S-Sepharose-FF, and Sephacryl S-200 HR were purchased from Pharmacia (Almeda, CA). Phenyl 650M hydrophobic interaction chromatography media was obtained from ToyoHaas (Philadelphia, PA). Membrane TF (mTF ) is a membrane fraction prepared by sonication of a human embryonic kidney cell line (293) expressing recombinant, full length (residues 1-263) TF22 and kindly provided by T. Lipari (Genentech, South San Francisco, CA). TF(1-243) is TF lacking the cytoplasmic domain that was constructed,18 purified, and formulated in detergent solution as previously described.19 sTF is the extracellular domain of TF comprising residues 1-219. Cephalin + activator (silica) and rabbit brain thromboplastin were purchased from Instrumentation Laboratory (Lexington, MA). Phosphatidylcholine (PC) from egg yolk and phosphatidylserine (PS) from bovine brain were purchased from Sigma Chemical Co (St Louis, MO).
TF(1-243) was relipidated with a 70/30 mixture of PC/PS by using the detergent dialysis protocol of Mimms et al20 as modified by Bach et al.21 Blank vesicles were prepared in a similar fashion except that 0.05 mg/mL bovine serum albumin replaced the TF(1-243). The final phospholipid concentration was estimated at 10 mmol/L with a calculated TF to phospholipid molar ratio of 1:6,800. TF concentration for both relipidated TF(1-243) and mTF was estimated by titration of FVIIa using the amidolytic assay (see below) to report complex formation. Both TF preparations were active in a prothrombin time measurement of coagulation but relipidated TF(1-243) gave a 10-fold higher specific activity in coagulation than mTF.
Expression and purification of hTFAA. Escherichia coli strain 27C7 transformed with pZTFAA, a phagemid vector designed for secretion of the 1-219 form of human K165A:K166A TF,22 was grown in a 10 L fermentor, obtained by centrifugation and the cell pellet (ca. 1,100 g wet weight) was frozen at −20°C. A typical protein preparation started from 200 g of cell paste. The cell paste was subjected to osmotic shock to release periplasmic contents by thawing in 5 volumes of 10 mmol/L Tris-HCl, pH 7.5, 1 mmol/L EDTA, 1 mmol/L phenyl methyl sulfonyl fluoride (PMSF), and 1 mmol/L benzamidine. After stirring on ice for 1 hour, the suspension was centrifuged at 18,000g for 20 minutes. The supernatant fraction was loaded onto a 5 × 20 cm column of DEAE-Sepharose-FF that had been equilibrated with 20 mmol/L Tris-HCl pH 8. Protein was eluted with a linear gradient formed from 2 L each of 20 mmol/L Tris-HCl pH 8 and 20 mmol/L Tris-HCl pH 8, 1.0 mol/L NaCl. Fractions containing hTFAA were identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and pooled. A one-fourth volume of 2 mol/L (NH4 )2SO4 , 80 mmol/L sodium acetate, pH 4 was added to this pool followed by chromatography on a 5 × 20 cm column of Phenyl-650M hydrophobic interaction media. The column was equilibrated and eluted with 0.5 mol/L (NH4 )2SO4 , 20 mmol/L sodium acetate pH 4. hTFAA eluted in the flow through fraction. This fraction was concentrated by ultrafiltration using an Amicon YM10 membrane and loaded onto a 5 × 90 cm Sephacryl S-200HR gel filtration column equilibrated and eluted with phosphate-buffered saline (PBS). The main peak from this column appeared to contain homogeneous (>95%) hTFAA by SDS-PAGE (not shown) and had a molecular weight (24,686) determined by electrospray-ionization mass spectrometry23 consistent with the sequence of hTFAA (expected molecular weight = 24683.5). Typical yields were 700 to 1,200 mg of hTFAA from 200 g of cells (wet weight). Residual endotoxin was removed by treatment with endotoxin-removal media (Cape Cod Associates, Wood's Hole, MA). The final pool had less than 1 EU/mg endotoxin as judged by crab hemocyte lysate assay.24
Cloning, expression, and purification of rTFAA.The rabbit TF cDNA encoding residues 1-21925 was PCR amplified by reverse transcription-polymerase chain reaction (RT-PCR) of rabbit brain mRNA. Oligo-dT primed cDNA was amplified with Stoffel fragment of Taq DNA polymerase (Perkin Elmer, Inc, Norwalk, CT) using the primers 5′-CCAATGCATCAGCAGACACTACAGGTAGAGCA-3′, and 5′-ACATGCATGCTTACTCCCTGGCCCTGCCCTGC-3′ incorporating Nsil and Sph I sites, respectively (shown underlined). Nsil and Sph I digested sTF cDNA was cloned into pZTFAA and sequenced. Oligonucleotide directed mutagenesis (Muta-Gene Kit; BioRad Corp, Hercules, CA) of K165 and K166 to Ala residues was performed with the primer 5′-GAGAGCTTCGAGCACAGGAGCCGCAACAGCCACGACAAAC-3′ (Ala codons underlined). Mutants were sequenced to confirm the double Ala substitutions in rTFAA.
rTFAA was expressed in E coli and an osmotic shock fraction was prepared as described above for hTFAA. The shockate was fractionated by (NH4 )2SO4 precipitation; rTFAA was contained in the 25% to 85% pellet. This pellet was resuspended in 10 mmol/L sodium acetate pH 5, dialyzed extensively against this same buffer, and loaded onto a 2.6 × 30 cm column of S-Sepharose FF (Pharmacia). Protein was eluted with a linear gradient of 0 to 0.5 mol/L NaCl containing 10 mmol/L sodium acetate pH 5. rTFAA containing fractions were identified by stimulation of the amidolytic activity of human FVIIa (see below). Active fractions were pooled and chromatographed on the Phenyl-650M column as described for hTFAA except that rTFAA binds to the column in the presence of 1 mol/L (NH4 )2SO4 and was eluted with PBS. The peak from this column was concentrated and chromatographed on the Sephacryl S-200 column as described for hTFAA. Residual endotoxin was removed by passage through a column of DEAE-Sepharose FF (Pharmacia). A typical preparation yielded 10 to 20 mg of rTFAA from 200 g of cell paste. This material appeared to be >90% homogenous by SDS-PAGE (not shown) and the molecular weight (24766.6) was confirmed by mass spectrometry.
BIAcore measurement of sTF⋅FVIIa interaction.Surface plasmon resonance measurements of sTF binding to immobilized FVIIa were performed as described previously.22
Coagulation assays.Measurements of the prothrombin time (PT) or activated partial thromboplastin time (APTT) were performed on an Instrumentation Laboratory (Lexington, MA) ACL300 coagulometer operated at 37°C. Unless noted otherwise, platelet-depleted, citrated human plasma pooled from multiple donors was used for these experiments. For measurements of the procoagulant function of TF (PT assay), coagulation was initiated by mixing equal volumes of plasma and a TF solution containing 25 mmol/L CaCl2 and the clotting time was determined. For inhibition experiments, hTFAA was diluted in plasma and then coagulation was initiated by mixing with either an equal volume of 5 nmol/L mTF containing 25 mmol/L CaCl2 or an equal volume of rabbit brain thromboplastin (Instrumentation Laboratory). In some experiments a standard curve of clot time as a function of TF concentration was prepared and used to evaluate apparent TF activity at varied inhibitor concentration. These experiments used relipidated TF (1-243) or rabbit brain thromboplastin to initiate clotting. A log-log plot of the clot time versus TF(1-243) concentration, or dilution of rabbit brain thromboplastin, was linear in the range of 0.01 to 10 mol/L TF (1-243) or 10- to 1,000-fold diluted rabbit thromboplastin. Data from these experiments were analyzed by nonlinear regression using equation 1:
where y is the apparent TF activity as a percentage of the activity observed with no inhibitor, [X] is the inhibitor concentration, A is the response at infinite inhibitor concentration, D is the response at zero inhibitor concentration, B is a curvature parameter, and C is the concentration giving 50% inhibition.
For APTT measurements, coagulation was initiated by mixing equal volumes of cephalin + activator (silica) (Instrumentation Laboratory), 25 mmol/L CaCl2 , and plasma containing wild-type sTF or hTFAA, and the clotting time was determined.
Amidolytic assay of FVIIa.The amidolytic activity of FVIIa was measured using either of the chromogenic substrates Spectrozyme FXa or Chromozym t-PA. FVIIa and TF in a solution containing 50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 5 mmol/L CaCl2 (TNC), were incubated for 30 minutes at 37°C. Substrate was added to a final concentration of 1 mmol/L and the absorbance at 405 nm was monitored on a SLT-Lab Instruments (Austria) EAR340AT plate reader operated at ambient temperature. Rates of substrate hydrolysis were determined by linear regression using software supplied by the manufacturer.
FX activation assay.Activation of FX by the TF⋅FVIIa complex was measured using a coupled, chromogenic assay. TF, FVIIa, and inhibitor were incubated in a solution containing 20 mmol/L HEPES pH 7.4, 150 mmol/L NaCl, 0.1% PEG-8000, 5 mmol/L CaCl2 (HBSC) for 1 hour at 37°C. FX was added to start the reaction, incubation at 37°C was continued, and aliquots were removed at varied times and quenched by mixing with an equal volume of 50 mmol/L EDTA. The amount of FXa generated was determined by measuring the rate of Spectrozyme FXa hydrolysis as described above except that the assay solution contained HBSC and 25 mmol/L EDTA. In the presence of 25 mmol/L EDTA, the TF⋅FVIIa complex dissociates and free FVIIa has negligible activity on Spectrozyme FXa. In some experiments, a standard curve was constructed using purified FXa (Haematologic Technologies, Essex Junction, VT) in order to convert the initial velocity of Spectrozyme FXa hydrolysis into the concentration of FXa generated. For experiments to determine the Km and Vmax values for FX activation, an excess of FVIIa over TF was used because preliminary experiments indicated that the FX had a 0.01% to 0.05% contamination with FVIIa. With TF as the limiting component no additional enzyme is formed as the substrate concentration is increased.
For experiments testing hTFAA inhibition of FX activation catalyzed by TF(1-243)⋅FVIIa, the concentration of FVIIa was quantitated by active site titration as previously described.26 The concentration of relipidated TF(1-243) was determined by titration of the amidolytic activity of active site-quantitated FVIIa.
Pharmacokinetic experiments.The pharmacokinetics of hTFAA was determined by IV injection of a 45 mg bolus to an anesthetized 3.6 kg New Zealand White rabbit. Blood samples (1 mL) were withdrawn at prespecified times from the left femoral artery via a catheter, anticoagulated by addition of a 1/10 volume of 3.8% trisodium citrate, centrifuged at 14,000 g for 6 minutes in a microcentrifuge, and the plasma procured. Samples were frozen at −20°C until analyzed. hTFAA concentration was determined from the initial rates of binding to the D3 antihuman TF MoAb27 measured on a Pharmacia BIAcore instrument. The D3 MoAb was immobilized through amine coupling and rates were determined under mass-transfer limiting conditions as described by Karlsson et al.28 The initial binding rates showed a linear dependence on hTFAA concentration in the range of 1 nmol/L to 1 μmol/L. PT and APTT were determined as described above with rabbit thromboplastin used to initiate clotting in the PT measurements.
Rabbit deep medial injury model of thrombosis.The left common carotid artery of an anesthetized rabbit was damaged with a 3F balloon embolectomy catheter. The balloon was inflated, dragged through a 2-cm stretch of artery, deflated, and returned to the initial position. This procedure was repeated 5 more times such that the endothelial and elastic lamina were breached exposing the medial and adventitial layers to the circulating blood. Before and for 40 minutes after the embolectomy procedure was initiated, arterial patency was monitored by flow measurements using an ultrasonic flow probe (Transonic Systems, Ithaca, NY). The total time for the balloon damage was typically 5 to 6 minutes. Bleeding tendency was assessed by determination of the cuticle bleeding time. Before reagent administration and vessel damage, the cuticle bleeding time was measured and blood samples were taken for baseline PT and APTT determination. Saline or test reagents were administered as a bolus IV injection 2 minutes before vessel damage. Patency rates are defined as the percentage of carotid arteries that were patent for at least 10 minutes during the 40 minute monitoring period. The cuticle bleeding time was determined, and blood samples were taken for PT and APTT measurements, at 1 or 10 minutes after the bolus injection.
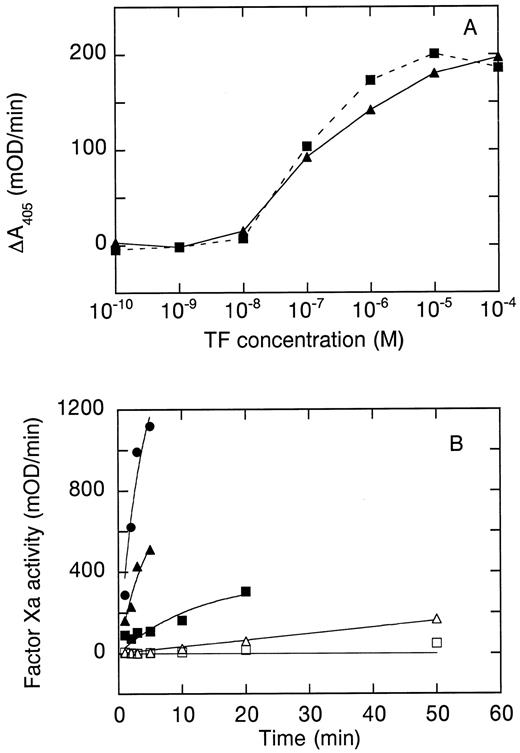
Cofactor function of hTFAA. (A) Stimulation of amidolytic activity of 100 nmol/L human factor VIIa on the chromogenic substrate Spectrozyme FXa by wild-type sTF (▴) or hTFAA (▪). (B) Activation of FX by FVIIa in complex with mTF (⋅), sTF (▴, ▵) or hTFAA (▪, □). For measurements with mTF, the mTF concentration was 25 nmol/L and the FVIIa concentration was 5 nmol/L. Experiments with sTF and hTFAA used 250 nmol/L sTF or hTFAA and 50 nmol/L FVIIa (▵, □) or 500 nmol/L FVIIa and 2.5 μmol/L sTF or hTFAA (▴, ▪). The substrate (FX) concentration in these experiments was 1 μmol/L.
Cofactor function of hTFAA. (A) Stimulation of amidolytic activity of 100 nmol/L human factor VIIa on the chromogenic substrate Spectrozyme FXa by wild-type sTF (▴) or hTFAA (▪). (B) Activation of FX by FVIIa in complex with mTF (⋅), sTF (▴, ▵) or hTFAA (▪, □). For measurements with mTF, the mTF concentration was 25 nmol/L and the FVIIa concentration was 5 nmol/L. Experiments with sTF and hTFAA used 250 nmol/L sTF or hTFAA and 50 nmol/L FVIIa (▵, □) or 500 nmol/L FVIIa and 2.5 μmol/L sTF or hTFAA (▴, ▪). The substrate (FX) concentration in these experiments was 1 μmol/L.
RESULTS
FVIIa binding.The affinity of hTFAA and wild-type sTF for binding to FVIIa was compared by surface plasmon resonance (SPR) measurements on a Pharmacia BIAcore instrument performed as previously described.22 29 As shown in Table 1, wild-type sTF and hTFAA displayed similar kinetics and affinity for binding to FVIIa. The capacity for hTFAA to stimulate the amidolytic activity of FVIIa was evaluated by using the chromogenic substrate Spectrozyme FXa. As shown in Fig 1A, hTFAA was equivalent to wild-type sTF for supporting cleavage of peptide substrates by FVIIa. These data indicate that hTFAA binds FVIIa and enhances amidolytic function with activity comparable to wild-type sTF.
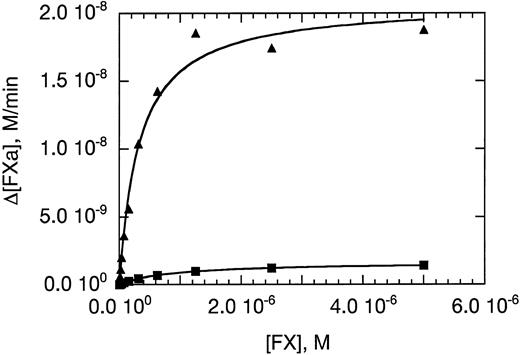
Activation of human factor X by the complex formed between 10 nmol/L FVIIa and 1 nmol/L wild-type sTF (▴) or hTFAA (▪) in the presence of phospholipid vesicles (0.5 mmol/L phospholipid) prepared from a 70/30 mixture of PC/PS. Initial rates of FXa formation were determined and are plotted versus substrate (FX) concentration. The solid lines are the result of nonlinear regression analysis by using the Michaelis-Menten equation.
Activation of human factor X by the complex formed between 10 nmol/L FVIIa and 1 nmol/L wild-type sTF (▴) or hTFAA (▪) in the presence of phospholipid vesicles (0.5 mmol/L phospholipid) prepared from a 70/30 mixture of PC/PS. Initial rates of FXa formation were determined and are plotted versus substrate (FX) concentration. The solid lines are the result of nonlinear regression analysis by using the Michaelis-Menten equation.
Cofactor function of hTFAA in support of FX activation.The cofactor function of hTFAA in cleavage of macromolecular substrates was evaluated by measuring the rate of FX activation by the complex formed between FVIIa and either mTF, wild-type sTF, or hTFAA. A fivefold excess of TF was used to drive complex formation. As shown in Fig 1B, 5 nmol/L FVIIa in complex with mTF gave a very robust rate of FX activation. In the absence of added phospholipid, 50 nmol/L FVIIa in complex with sTF or hTFAA gave a very slow rate of FX activation. Increasing the enzyme concentration by 10-fold to 500 nmol/L resulted in a rate of FX activation that was still slower than that measured for 5 nmol/L FVIIa in complex with mTF. At these concentrations of enzyme (500 nmol/L) and substrate (1 μmol/L), the apparent rate of FX activation was about threefold lower with hTFAA than with sTF as cofactor. Since the plasma concentration of FVII is about 10 nmol/L30 it is unlikely that the enzyme concentration would be high enough in vivo to obtain an appreciable rate of FX activation in the presence of sTF or hTFAA, and in the absence of added phospholipid.
The cofactor function of sTF and hTFAA was further compared by determining the substrate dependence of the initial rate of FXa generation. In these experiments, 0.5 mmol/L phospholipid was used to enhance the catalytic activity of FVIIa in complex with soluble TF. High concentrations of phospholipid have been shown to increase the kcat and lower the KM for FX activation catalyzed by sTF⋅FVIIa.16 Even in the presence of phospholipid vesicles, hTFAA gave a slower rate of FX activation than wild-type sTF (Fig 2). Analysis of these data using the Michaelis-Menten equation yielded Km and Vmax values of 0.32 ± 0.04 μmol/L and 21.0 ± 0.7 nmol/L/min for wild-type sTF, and 0.85 ± 0.02 μmol/L and 1.7 ± 0.02 nmol/L/min for hTFAA. Thus, hTFAA⋅FVIIa displays a 34-fold reduction in catalytic efficiency for FX activation relative to sTF⋅FVIIa that results from a 12.4-fold decrease in Vmax and 2.7-fold increase in Km . The Km and Vmax values determined for wild-type sTF are somewhat smaller than determined by Fiore et al,16 which we attribute to our use of a fourfold lower phospholipid concentration in the assay.
Procoagulant function of hTFAA.The potential for any procoagulant function of hTFAA was tested by measuring PT with human plasma. Clotting times observed for wild-type sTF, hTFAA and relipidated TF(1-243) are compared in Table 2. Addition of 10 μmol/L hTFAA did not decrease the clotting time from that measured for addition of calcium alone whereas 10 μmol/L wild-type sTF caused a nearly fourfold decrease in the clotting time. By comparison, 5 nmol/L TF(1-243) shortened the clotting time by nearly 20-fold relative to calcium alone. Addition of blank phospholipid vesicles to a concentration equivalent to that added with relipidated TF (50 μmol/L) resulted in a 1.7-fold decrease in the clotting time measured for wild-type sTF. Phospholipid addition had no effect on the clotting time determined with hTFAA. Increasing the phospholipid concentration by 10-fold did not further enhance the procoagulant function of wild-type sTF or hTFAA (data not shown). The APTT measured with human plasma was 30.9 ± 1.4 seconds. Addition of hTFAA (11 μmol/L) caused a small decrease in APTT (APTT = 29 ± 1 seconds) whereas wild-type sTF (12 μmol/L) shortened the APTT by twofold (APTT = 14.8 ± 1.5 seconds). These data show that at physiological concentrations of FVII, both in the presence and absence of added phospholipid, hTFAA is not procoagulant with human plasma.
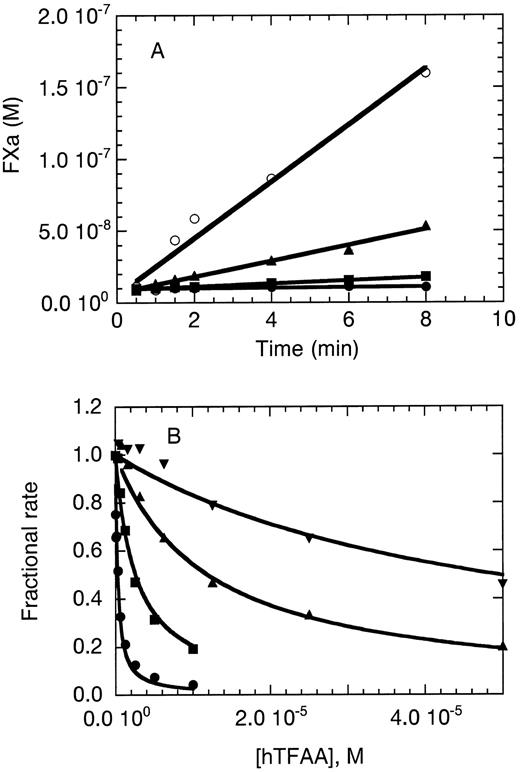
Inhibition of FX activation by hTFAA.The ability of hTFAA to function as an antagonist of the extrinsic pathway of coagulation was initially examined by using the FX activation assay. In these experiments, mTF (Fig 3A) or relipidated TF(1-243) (Fig 3B) was used as the cofactor for FVIIa. As shown in Fig 3A, hTFAA could inhibit FX activation catalyzed by a mixture of 1 nmol/L FVIIa and 10 nmol/L mTF. Partial inhibition was observed with 100 nmol/L hTFAA and complete inhibition was obtained with 10 μmol/L hTFAA.
hTFAA functions in vitro as an antagonist of membrane bound TF. (A) hTFAA inhibition of FX activation catalyzed by mTF⋅FVIIa. The concentrations of FVIIa, mTF, and FX were 1 nmol/L, 10 nmol/L, and 1 μmol/L, respectively. Rate of FXa generation is shown for 0 (○), 100 nmol/L (▴), 1 μmol/L (▪), and 10 μmol/L (⋅) hTFAA. (B) hTFAA inhibition of relipidated TF(1-243)⋅FVIIa catalyzed activation of FX. For these experiments, the FVIIa concentration was 0.05 nmol/L, the substrate (FX) concentration was 200 nmol/L, and the TF(1-243) concentration was 0.05 nmol/L (⋅), 0.2 nmol/L (▪), 1 nmol/L (▴), or 5 nmol/L (▾). The rate of FXa production in the presence of the indicated hTFAA concentration was measured and is reported as the fraction of the rate observed in the absence of hTFAA. Solid lines are the result of nonlinear regression analysis by using equation 2 yielding the binding constants shown in Table 3.
hTFAA functions in vitro as an antagonist of membrane bound TF. (A) hTFAA inhibition of FX activation catalyzed by mTF⋅FVIIa. The concentrations of FVIIa, mTF, and FX were 1 nmol/L, 10 nmol/L, and 1 μmol/L, respectively. Rate of FXa generation is shown for 0 (○), 100 nmol/L (▴), 1 μmol/L (▪), and 10 μmol/L (⋅) hTFAA. (B) hTFAA inhibition of relipidated TF(1-243)⋅FVIIa catalyzed activation of FX. For these experiments, the FVIIa concentration was 0.05 nmol/L, the substrate (FX) concentration was 200 nmol/L, and the TF(1-243) concentration was 0.05 nmol/L (⋅), 0.2 nmol/L (▪), 1 nmol/L (▴), or 5 nmol/L (▾). The rate of FXa production in the presence of the indicated hTFAA concentration was measured and is reported as the fraction of the rate observed in the absence of hTFAA. Solid lines are the result of nonlinear regression analysis by using equation 2 yielding the binding constants shown in Table 3.
Inhibition of TF(1-243)⋅FVIIa by hTFAA was quantitated by determining the fractional rate of FX activation in the presence of a varied concentration of hTFAA. Inhibition profiles were determined using 4 different concentrations of relipidated TF(1-243). As shown in Fig 3B, the inhibition profile was shifted to higher (hTFAA) with an increase in (TF[1-243]) as expected if the inhibitor and cofactor compete for binding to the same site on the enzyme. Data were analyzed according to the nonessential activator model of Scheme I.31
(Scheme I) where E is FVIIa, A is relipidated TF(1-243), I is the inhibitor hTFAA and S is the substrate FX. KS is the substrate binding constant, and Vmax the maximal velocity, for free FVIIa. α Is given by the ratio KappS ([A] → ∞) / KS . kp is the catalytic rate constant for free FVIIa and β is given by the ratio Vappmax([A] → ∞) / Vmax . If EI is not active then it can be shown31 that the fractional rate (vi /v0 , steady-state inhibited rate divided by the uninhibited rate) is given by equation 2
where [I] and [A] are the free concentrations of inhibitor and activator, respectively, and [S] is the total substrate concentration. FX activation was not detected in control experiments using 0.05 nmol/L FVIIa, 50 μmol/L hTFAA, 200 nmol/L FX and blank phospholipid vesicles at up to 50 μmol/L concentration, suggesting that the assumption of inactive EI is valid for the experimental conditions used. A phospholipid concentration of 50 μmol/L is equivalent to that present when 5 nmol/L relipidated TF(1-243) is used as the cofactor for FX activation.
The inhibition profiles observed for each concentration of TF(1-243) used were well described by equation 2 using the binding constants (Table 3) determined by nonlinear regression analysis. For these calculations, it was assumed that the free concentrations of I and A were equal to the total added. Since the enzyme concentration used (0.05 nmol/L) is 100-fold lower than the KD for hTFAA binding to FVIIa (Table 1), this assumption is valid for I; however, given the high affinity of FVIIa for relipidated TF31 this assumption is probably incorrect for low concentrations of TF(1-243). Thus, the calculated KA and Ki values were equivalent for the three highest cofactor concentrations tested (Table 3) but data from the lowest TF(1-243) concentration yielded larger values. The calculated Ki values agree well with the KD determined from SPR measurements and the KA values are similar to the KD (7.3 pmol/L) determined from pressure dissociation experiments32 for binding of human FVIIa to full-length TF relipidated with 100% phosphatidyl choline. These results suggest that scheme I provides an adequate description of the inhibition data. Because phosphatidyl serine is known to increase the affinity of bovine FVIIa for relipidated TF,21 it is not surprising that we calculated a threefold higher affinity for FVIIa binding to TF incorporated into phospholipid vesicles containing 30% phosphatidyl serine. [I]50 , the inhibitor concentration resulting in a 50% reduction in the rate of FX activation, was calculated from equation 3.
Given the high affinity of FVIIa for relipidated TF(1-243), the [I]50 value for hTFAA is about 10,000-fold higher than the TF(1-243) concentration.
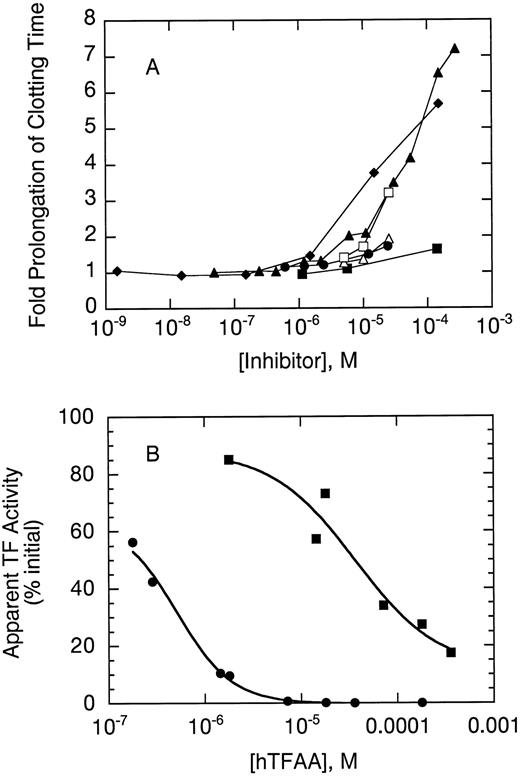
Inhibition of clotting by hTFAA.hTFAA was tested as an antagonist of mTF in a PT measurement of coagulation (Fig 4A). A hTFAA concentration of about 10 μmol/L resulted in a twofold prolongation of clotting time measured with 5 nmol/L mTF used to initiate coagulation. Higher concentrations of hTFAA further prolonged clotting time with a sevenfold prolongation obtained at 140 μmol/L hTFAA. hTFAA had a similar effect on clotting measured with rhesus plasma but was much less inhibitory when PT measurements were performed with rabbit plasma.
hTFAA inhibition of coagulation initiated by mTF. (A) Fold prolongation of clotting by hTFAA relative to no added antagonist is shown for human plasma (▴), rhesus plasma (♦) and rabbit plasma (▪). For comparison, effects of WT sTF on clotting with human plasma are also shown (⋅). Open symbols show the effect of rTFAA on clotting time with human (▵) and rabbit (□) plasma. Coagulation experiments with human plasma used human mTF to initiate clotting whereas measurements with rhesus and rabbit plasma used rabbit brain thromboplastin to initiate coagulation. In the absence of inhibitor the clotting times were 26.9 ± 1.7 seconds (human plasma), 14.2 ± 0.3 seconds (rhesus plasma), and 7.3 ± 0.5 seconds (rabbit plasma). (B) Comparison of hTFAA effects in human (⋅) and rabbit (▪) plasma. For these experiments, a standard curve relating clotting time to the concentration of relipidated human TF(1-243) (human plasma) or dilution of rabbit brain thromboplastin (rabbit plasma) was used to evaluate the apparent TF activity as a function of inhibitor concentration. The amount of initiator added was adjusted to give a clot time of 21 seconds in the absence of hTFAA. For human plasma, this clotting time was obtained with 1 nmol/L relipidated human TF(1-243). The solid lines are the result of nonlinear regression analysis using equation 1.
hTFAA inhibition of coagulation initiated by mTF. (A) Fold prolongation of clotting by hTFAA relative to no added antagonist is shown for human plasma (▴), rhesus plasma (♦) and rabbit plasma (▪). For comparison, effects of WT sTF on clotting with human plasma are also shown (⋅). Open symbols show the effect of rTFAA on clotting time with human (▵) and rabbit (□) plasma. Coagulation experiments with human plasma used human mTF to initiate clotting whereas measurements with rhesus and rabbit plasma used rabbit brain thromboplastin to initiate coagulation. In the absence of inhibitor the clotting times were 26.9 ± 1.7 seconds (human plasma), 14.2 ± 0.3 seconds (rhesus plasma), and 7.3 ± 0.5 seconds (rabbit plasma). (B) Comparison of hTFAA effects in human (⋅) and rabbit (▪) plasma. For these experiments, a standard curve relating clotting time to the concentration of relipidated human TF(1-243) (human plasma) or dilution of rabbit brain thromboplastin (rabbit plasma) was used to evaluate the apparent TF activity as a function of inhibitor concentration. The amount of initiator added was adjusted to give a clot time of 21 seconds in the absence of hTFAA. For human plasma, this clotting time was obtained with 1 nmol/L relipidated human TF(1-243). The solid lines are the result of nonlinear regression analysis using equation 1.
The relative potency of hTFAA as an antagonist in human and rabbit plasma was compared by performing coagulation experiments at the same level of apparent TF activity in the two plasmas. A log-log plot of clot time versus concentration of relipidated TF(1-243) or dilution of rabbit thromboplastin was used to calculate the percentage of initial TF activity as a function of antagonist (hTFAA) concentration. Analysis of these data (Fig 4B) by using equation 1 to determine the antagonist concentration giving 50% inhibition, suggests that hTFAA is 70-fold less potent in rabbit plasma as compared to human plasma.
Characterization of rTFAA.Since a rabbit model of arterial thrombosis would be used to test the antithrombotic potential of hTFAA, and given the lower potency of hTFAA in rabbit plasma, we chose to prepare the rabbit homologue of hTFAA, termed “rTFAA.” The extracellular domain of rabbit TF was PCR cloned into the E coli secretion vector. Rabbit TF also has lysine residues at positions 165 and 166; site-directed mutagenesis was used to mutate these two residues to alanines. rTFAA was expressed in E coli at about 50-fold lower levels than hTFAA and thus much smaller amounts of rTFAA could be purified.
Although purified rabbit FVIIa was not available, we could perform some initial characterization of rTFAA by using human FVIIa. rTFAA stimulated the amidolytic activity of human FVIIa on chromozym t-PA (data not shown). As shown in Table 1, rTFAA bound human FVIIa with affinity only slightly reduced from that measured for hTFAA. This result was expected since the residues on human TF that are important for FVIIa binding15,22,33,34 are conserved in rabbit TF.25 rTFAA gave a more potent inhibition of PT in rabbit plasma than hTFAA (Fig 4A) but gave a weaker inhibition in human plasma.
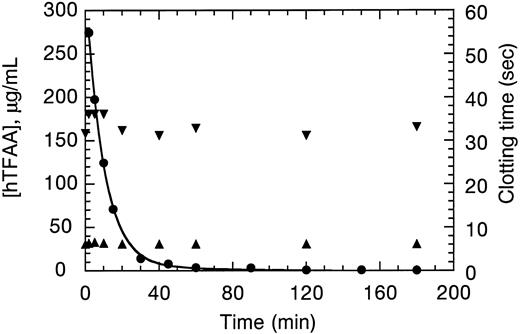
Pharmacokinetics of hTFAA.Pharmacokinetics experiments on hTFAA were performed in New Zealand White rabbits which were also used for testing of the antithrombotic potency (see below). Plasma concentrations of hTFAA and PT and APTT coagulation times observed after a 12.5 mg/kg bolus IV injection of hTFAA are shown in Fig 5. Analysis of the concentration data according to a two-compartment model35 indicated an initial volume of distribution (40.7 mL/kg) approximately equal to the plasma volume. There was a biphasic clearance of hTFAA with half-lives of 6.2 minutes and 41 minutes. The initial phase comprised 88% of the total area under the concentration time curve (AUC). The plasma clearance was 4.2 mL/min/kg with a mean residence time for hTFAA of 17.3 minutes. These values are typical for a protein of 25,000 molecular weight. Only small increases in the coagulation times were detected at the peak in hTFAA concentration and these parameters quickly returned to baseline values.
Pharmacokinetics of hTFAA in the rabbit. hTFAA concentration in plasma (⋅) was analyzed by nonlinear regression analysis using a two exponential decay (solid line). Samples were also measured for clotting time in PT (▴) and APTT (▾) assays.
Pharmacokinetics of hTFAA in the rabbit. hTFAA concentration in plasma (⋅) was analyzed by nonlinear regression analysis using a two exponential decay (solid line). Samples were also measured for clotting time in PT (▴) and APTT (▾) assays.
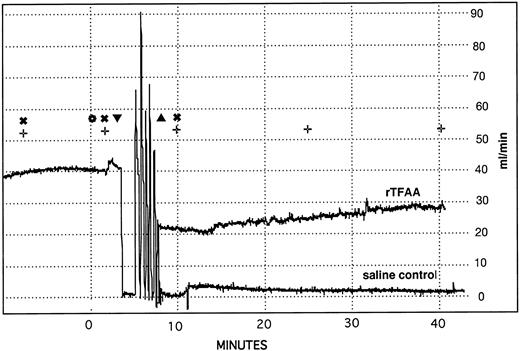
Superimposed recordings of carotid artery blood flow in representative rabbits treated with saline or rTFAA. Blood flow through the carotid artery of anesthetized rabbits was monitored with an ultrasonic flow probe (Transonics). The following procedures were performed at the times designated by the symbols in the figure: () IV bolus administration of test compounds, (▾) embolectomy catheter inserted, (▴) embolectomy catheter removed, (✖) cuticle bleeding time determined, (+) blood sample collected for APTT and PT determination. The rapid changes in blood flow that occur between catheter insertion and catheter removal are the result of inflating and deflating the balloon between six repeated passes through a 2-cm segment of artery.
Superimposed recordings of carotid artery blood flow in representative rabbits treated with saline or rTFAA. Blood flow through the carotid artery of anesthetized rabbits was monitored with an ultrasonic flow probe (Transonics). The following procedures were performed at the times designated by the symbols in the figure: () IV bolus administration of test compounds, (▾) embolectomy catheter inserted, (▴) embolectomy catheter removed, (✖) cuticle bleeding time determined, (+) blood sample collected for APTT and PT determination. The rapid changes in blood flow that occur between catheter insertion and catheter removal are the result of inflating and deflating the balloon between six repeated passes through a 2-cm segment of artery.
The low amount of rTFAA available and lack of suitable antibodies for concentration determination precluded a complete pharmacokinetics experiment with this protein. Preliminary experiments using the amidolytic assay to measure rTFAA concentration (data not shown), nonetheless, indicated that the clearance of rTFAA paralleled that observed with hTFAA.
TFAA in a rabbit model of thrombosis.The antithrombotic potential of hTFAA, rTFAA and heparin were compared in a deep medial injury model of arterial thrombosis in the rabbit. In this model, the carotid artery of an anesthetized rabbit was damaged with a balloon embolectomy catheter and arterial patency, as monitored by flow measurements (Fig 6), was determined. In control animals the balloon damage always (17 out of 17) results in immediate and sustained occlusion of the artery. Bleeding tendency was assessed by determination of the cuticle bleeding time. Given the fairly rapid clearance of hTFAA, cuticle bleeding time was usually determined at 1 minute after reagent administration.
As summarized in Table 4, occlusion was prevented in 5 out of 5 animals given 90 U/kg heparin. This dose of heparin resulted in a threefold increase in the cuticle bleeding time. APTT measured ex vivo was increased >18-fold and there was a 1.6-fold increase in the PT. Lowering the heparin dose resulted in a decrease in both the % patency and the cuticle bleeding time. Nonetheless, a dose of heparin (30 U/kg), which gave a 60% patency rate still yielded a twofold increase in cuticle bleeding time and threefold prolongation of APTT. The increase in cuticle bleeding time appeared to be correlated with the change in APTT. In contrast to these results, a 12.5 mg/kg dose of hTFAA gave a 62% patency rate (8 out of 13 animals) with an insignificant increase in cuticle bleeding time. hTFAA did not cause any increase in APTT or PT measured ex vivo. At a decreased dose of hTFAA (5 mg/kg) only 1 out of 6 animals had patent vessels. At doses of heparin (30 U/kg) and hTFAA (12.5 mg/kg) that gave equal antithrombotic effects, hTFAA gave a lower bleeding tendency and did not prolong APTT or PT.
rTFAA gave 100% efficacy at a dose of 2 mg/kg with a small increase in cuticle bleeding time but no increase in APTT or PT. By comparison, a heparin dose (90 U/kg) of equal antithrombotic potential gave a larger increase in cuticle bleeding time and had a large effect on APTT.
DISCUSSION
We have shown here that a potent and selective antagonist of the extrinsic pathway of blood coagulation can be obtained by incorporation of the K165A:K166A mutations into soluble tissue factor. hTFAA can be expressed at high levels by secretion from E coli and is easily purified in high yield (700 to 1,200 mg per 200 g cell paste) using conventional chromatography. This molecule is soluble at >50 mg/mL and binds FVIIa with affinity comparable to wild-type sTF. hTFAA is fully functional for supporting the amidolytic activity of FVIIa, but is severely impaired for FX activation both in the presence and absence of phospholipid. hTFAA is not procoagulant at physiological concentrations of FVII, both in the presence and absence of phospholipid, and slows clotting initiated by mTF. In a rabbit model of arterial thrombosis, both rTFAA and hTFAA had antithrombotic effects while causing smaller increases in bleeding tendency or coagulation parameters relative to heparin doses of equal antithrombotic potential. These results validate TF⋅FVIIa as a target for the development of new anticoagulant drugs in the treatment of thrombotic disease.
Role of Lys 165,166 in cofactor function.Lysine residues 165 and 166 are found on the C-terminal fibronectin type III domain of TF on the opposite surface of the molecule from residues found to be important for FVIIa binding.22 Both Lys residues are solvent accessible in the structure of free sTF.36,37 Lys 165,166 are not in contact with FVIIa in the structure of the sTF⋅FVIIa complex15 and are situated far from the protease active site of FVIIa. Because hTFAA is fully functional for stimulating the amidolytic activity of FVIIa, it seems unlikely that these lysine residues help form the active site of FVIIa. As suggested by Ruf et al,14 Lys 165 and 166 are important for macromolecular substrate recognition. These residues appear to help orient the substrate for cleavage, but do not provide a large binding energy for interaction with the substrate, since hTFAA⋅FVIIa has a 12.5-fold reduced Vmax but only 2.7-fold increased Km for FX activation relative to wild-type sTF⋅FVIIa. Huang et al38 have suggested that Lys 165,166 form part of a binding site for the gla domain of FX since K165,166 mutants are equal to wild-type for activation of gla-domainless FX.
hTFAA as an antithrombotic agent.The concentration (>10 μmol/L) of hTFAA required to cause a significant prolongation of PT in vitro is considerably larger than the KD for the hTFAA⋅FVIIa complex (KD = 0.9 to 4.2 nmol/L; Table 1). The analysis of the profiles observed for inhibition of FX activation (Fig 3B, Table 3) clearly shows that this is a consequence of the approximate 2,000-fold weaker affinity of FVIIa for soluble TF as compared to binding to relipidated TF. A high dose of hTFAA (12.5 mg/kg) was also required to produce an antithrombotic effect in the rabbit model of thrombosis. In addition, this dose was not effective in preventing occlusion in all animals. The incomplete efficacy in rabbits reflects the species dependence of coagulation inhibition observed in vitro (Fig 4A) as well as a fairly rapid clearance rate. Complete efficacy at a lower dose, however, was obtained by using rabbit TFAA. The species dependence of inhibition is somewhat surprising since human thromboplastin is an effective procoagulant with rabbit plasma.39 Residues of human TF shown to be important for FVIIa binding15,22,31,32 are conserved in rabbit TF.25 Given the correlation between potency and relative affinities observed with human components, one explanation for the species dependence is that rabbit FVIIa might display a larger difference in affinity for binding to soluble and membrane bound forms of TF. Support for this hypothesis requires determination of the relative affinities of purified rabbit FVIIa for soluble and membrane bound TF.
Further changes in hTFAA that improve its affinity for FVIIa, as well as slow its clearance rate, may lead to a more effective therapeutic agent for human thrombotic disease. A variety of protein engineering strategies could be attempted for increasing the affinity of hTFAA for FVIIa and thus enable more effective competition with mTF for binding FVIIa. A further refinement could incorporate other mutations known to diminish the coagulant function of TF without effect on FVIIa binding22 40 thereby further reducing any residual rate of FX activation observed with hTFAA⋅FVIIa.
ACKNOWLEDGMENT
We thank Jim Bourell for mass spectrometry of sTF, Terry Lipari for preparation of mTF, and Allison Blank for the gift of purified TF(1-243). We thank Dr Robert Lazarus for helpful discussions, encouragement, and critical reading of the manuscript. The continued support of Dr Tony Kossiakoff is gratefully acknowledged.
Address reprint requests to Robert F. Kelley, PhD, Department of Protein Engineering, Genentech, Inc, 460 Pt San Bruno Blvd, South San Francisco, CA 94080.