Abstract
Leukemic cells from a significant number of children with acute lymphoblastic leukemia (ALL) express protein antigens characteristic of both lymphoid and myeloid cells, yet the clinical significance of this immunophenotype has remained controversial. In the current study, we have determined relationships between myeloid antigen expression and treatment outcome in a large cohort of children with newly diagnosed ALL. A total of 1,557 children enrolled on risk-adjusted Children's Cancer Group studies were classified as myeloid antigen positive (My+) or myeloid antigen negative (My−) B-lineage ALL (BL) or T-lineage ALL (TL), according to expression of CD7, CD19, CD13, and CD33 antigens on the surface of their leukemic cells. My+ patients in both BL and TL groups were more likely than My− patients to have favorable presenting features. Induction therapy outcome was similar for My+ and My− patients in both the BL and TL categories. Importantly, 4-year event-free survival (EFS) was similar for My+ BL (77.0%, standard deviation [SD] = 4.0%) versus My− BL (75.9%, SD = 1.8%) and for My+ TL (72.7%, SD = 7.1%) versus My− TL (70.1%, SD = 5.7%). An overall relative hazard rate (RHR) of 0.89 (P = .49) was determined by a cross strata analysis for My+ versus My− patients. Moreover, similar EFS and RHR also were found when My+ and My− BL patients were compared according to National Cancer Institute risk classification. Thus, patients with My+ ALL have similar treatment outcomes as My− ALL patients. In contrast to previous studies, this result was independent of treatment risk category, demonstrating that myeloid antigen expression was not an adverse prognostic factor for childhood ALL.
ACUTE LYMPHOBLASTIC leukemia (ALL) is an immunophenotypically heterogeneous group of diseases. Leukemic cells from the majority of patients with ALL express on their surface a variety of protein antigens that are found at discrete stages of maturation on normal B- or T-lymphocyte precursors.1-6 Thus leukemic clones from ALL patients are thought to originate from normal lymphoid progenitor cells arrested at early stages of B- or T-lymphocyte ontogeny.7-9 Recent improvements in immunofluorescence and flow cytometry, as well as the availability of monoclonal antibodies that recognize lineage-associated cell surface molecules, have motivated more detailed investigations of immunophenotypic heterogeneity in childhood ALL. It is now clear that leukemic cells from a 5% to 20% of children with ALL also express myeloid differentiation antigens.5,10-20 The expression of myeloid antigens by ALL cells is speculated to reflect either lineage infidelity due to aberrant gene expression, neoplastic transformation of rare bilineage lymphoid/myeloid progenitor cells, or transformation of a multipotent lymphohematopoietic precursor cell.6 21-23
The clinical significance of myeloid antigen expression in pediatric ALL has remained controversial. Several studies5,14,15,20 have reported poor outcome for children with ALL of mixed myeloid/lymphoid phenotype, whereas others have found similar induction and treatment outcomes for patients with myeloid antigen negative and myeloid antigen positive ALL.10,11,13,16-19 24 Because of these conflicting reports regarding prognosis and treatment outcome, there has been no consensus among pediatric oncologists regarding assignment of patients to risk-directed ALL chemotherapy protocols, employment of therapies directed at acute myelogenous leukemia (AML), or necessity for bone marrow transplantation in first remission.
Herein, we report the results of a prospective study of myeloid antigen expression in a large cohort of 1,557 children with newly diagnosed ALL who were enrolled on risk-adjusted treatment protocols of the Children's Cancer Group (CCG). The presenting features and treatment outcomes of both myeloid antigen positive (My+) B-lineage (BL) and My+ T-lineage (TL) ALL patients were compared with those of myeloid antigen negative (My−) BL and My− TL controls. Our results provide new insights regarding the clinical relevance of myeloid antigen expression in childhood ALL by demonstrating that regardless of treatment protocol, My+ ALL and My− ALL patients have similar treatment outcomes.
MATERIALS AND METHODS
Study patients.The sample for these analyses included pediatric patients (<21 years of age) with newly diagnosed ALL enrolled between January 1, 1989 and December 31, 1993 on risk-adjusted treatment protocols of the CCG for whom a complete immunophenotyping profile of specified lymphoid and myeloid antigens (see below) was obtained. Diagnosis of ALL was based on morphological, biochemical, and immunological features of the leukemic cells, including lymphoblast morphology on Wright-Giemsa stained bone marrow smears, positive nuclear staining for terminal deoxynucleotidyl transferase (TdT), negative staining for myeloperoxidase, and cell surface expression of two or more lymphoid differentiation antigens (see below). Degree of organomegaly (moderate or marked enlargement) was as defined previously.25 CCG risk-adjusted ALL protocols were as follows: CCG 1881 (low-risk protocol for children age 2 to 9 years and white blood cell count [WBC] < 10,000/μL); CCG 1882 (high-risk protocol for patients 1 to 9 years of age with WBC ≥ 50,000/μL or age ≥ 10 years); CCG 1883 (protocol for infants less than 1 year of age), 1891 (intermediate risk protocol for children aged 2 to 9 years and WBC 10,000 to 49,999/μL or age 1 year and WBC < 50,000/μL) and CCG 1901 (high-risk protocol for patients with lymphomatous features). Lymphomatous features are essentially as described by the revised criteria of Steinherz et al.25 Each protocol was approved by the National Cancer Institute (NCI), as well as the Institutional Review Boards of the participating CCG-affiliated institutions. Informed consent was obtained from parents, patients, or both, as deemed appropriate, according to Department of Health and Human Services guidelines. For comparisons of presenting features, antigen expression, and therapy outcomes, patients were classified as myeloid antigen positive (My+) B-lineage leukemia (BL), myeloid antigen negative (My−) BL, My+ T-lineage leukemia (TL) and My− TL, as described below. A small number of patients (24 BL and 3 TL) were excluded from the current analyses because they failed to meet the criteria given by the algorithm. Analyses performed using these 27 patients indicated similar presenting characteristics and outcome compared with the patients included in this report. Thus, there appears to be no selection bias associated with the removal of these patients. B-lineage My+ and My− patients were also grouped according to recently published NCI risk classification criteria.26 These criteria classify patients age 1 to 9 years and WBC < 50,000/μL as standard risk and all other patients as high-risk.
Immunophenotyping.Highly blast-enriched mononuclear cell fractions containing ≥90% leukemic cells were isolated from pretreatment bone marrow aspirate samples by centrifugation on Ficoll-Hypaque density gradients. Immunophenotyping was performed centrally in the CCG ALL Biology Reference Laboratory by indirect immunofluorescence and flow cytometry using monoclonal antibodies reactive with B-lymphoid–associated (CD19, CD20, CD21, CD22, CD72), T-lymphoid–associated (CD1, CD2, CD3, CD4, CD5, CD7, CD8), myeloid-associated (CD13, CD33), and nonlineage-associated (CD9, CD10, CD24, CD34, CD40) differentiation antigens, as previously described.27 28 Antigen expression data are presented as the mean ± standard error (SE) and median percentages of leukemic cells scored positive for expression of a given antigen. Cells were scored positive based on increased immunofluorescence observed with an antigen-specific monoclonal antibody compared with that observed with an irrelevant antibody. The term “expression frequency” is used throughout to indicate the percentage of leukemic cells expressing a given antigen. Patients were classified as BL if ≥30% of the isolated leukemic cells were positive for CD19 and < 30% were positive for CD2, CD5, and CD7. Likewise, patients were classified as TL if ≥30% of the isolated blasts were positive for CD2, CD5, or CD7 and <30% were positive for CD19. For patients exceeding 30% positivity for both criteria, the immunological surface marker results were examined further and classified according to the lineage marker of higher expression frequency, as well as the composite immunophenotype (ie, expression frequencies of other lineage-restricted antigens). BL and TL patients were classified as My+ if ≥30% of the isolated leukemic cells were positive for CD13 or CD33, or both. The majority of the 1,557 patients (85.4%) had BL, whereas 14.6% had TL. Overall, 13.9% patients were classified as My+ BL, 71.5% were My− BL, 2.8% were My+ TL, and 11.8% were My− TL.
Statistical methods.My+ BL and My+ TL patients were compared with their respective My− BL and My− TL controls for similarity of clinical, demographic, and laboratory features, as well as induction therapy outcome using global chi-square tests for homogeneity of proportions. Comparisons of antigen expression frequency distributions were performed using the Kruskal-Wallis nonparametric rank test.29 Most of the outcome analyses used life table methods and associated statistics. The primary endpoint was event-free survival (EFS) from the date of study entry. An event was defined as induction failure (no response to therapy or death during induction), leukemic relapse at any site, death during remission, or the development of a second malignant neoplasm, whichever occurred first. Patients not experiencing an event at the time of analysis were censored in the EFS analysis at the time of their last contact. Data analysis was performed in July 1996.
Life-table estimates were calculated by the Kaplan-Meier (KM) procedure, and the standard deviation (SD) of the life table estimate was obtained using Greenwood's formula.30 To indicate precision, the KM estimate of EFS and its SD were given for selected follow-up time points. An approximate 95% confidence interval can be obtained by using the life-table estimate ± 1.96 SDs. Life-table comparisons of EFS outcome pattern for patient groups used the log-rank statistic.31,32 Stratified log-rank tests were sometimes used to adjust for the possible modifying effects of other factors on the comparison of interest.32,33P values for life-table comparisons are based on the pattern of outcome across the entire period of patient follow-up, although EFS estimates at specific time points may be given for comparative purposes. Estimates of the life-table relative hazard rate (RHR) for a particular event were calculated by the O/E method for log-rank analyses.34
RESULTS
Immunophenotypic features of primary leukemic cells from children with My+ and My− ALL.In accordance with the algorithm used for immunophenotypic classification, all BL patients showed high expression frequency for CD19 and all TL patients showed high expression frequency of CD7 (Table 1). Leukemic cells from BL patients were negative for T-lineage differentiation antigens and leukemic cells from TL patients were negative for B-lineage differentiation antigens (data not shown). The median expression frequencies of CD13 and CD33 were greater for My+ BL and My+ TL patients compared with My− BL and My− TL patients.
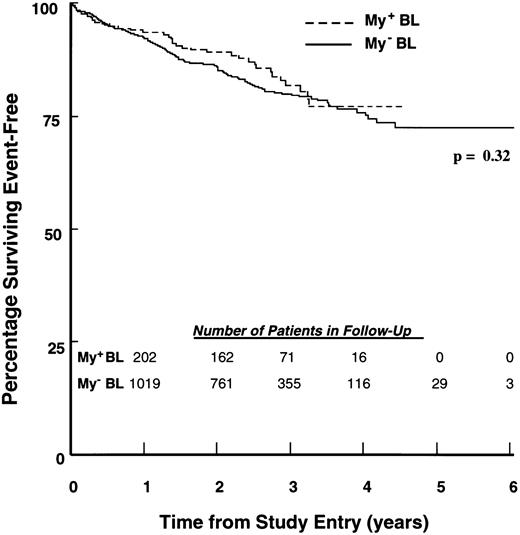
EFS of children with ALL according to BL immunophenotype. Percentages of 217 My+ BL (hatched line) and 1,113 My− BL (solid line) patients achieving EFS during 6 years of follow-up were calculated as described in Materials and Methods. The number of patients in each group remaining in follow-up at the indicated time points is shown in the inset.
EFS of children with ALL according to BL immunophenotype. Percentages of 217 My+ BL (hatched line) and 1,113 My− BL (solid line) patients achieving EFS during 6 years of follow-up were calculated as described in Materials and Methods. The number of patients in each group remaining in follow-up at the indicated time points is shown in the inset.
Within the BL group, My+ and My− patients had identical 88% median expression frequencies of CD10. Median expression frequencies for the CD34 and CD40 antigens were significantly higher in the My+ BL group than in the My− BL group (P < .0001 for both comparisons). Similar immunophenotypic comparisons were performed for My+ TL and My− TL patients (Table 1). Expression frequencies of CD2 and CD10 were similar for both groups. In contrast, the median expression frequency of CD5 was significantly lower for My+ TL patients compared with My− TL patients (78% v 93%, P = .001). As was observed for BL patients, the median expression frequency of CD34 was significantly higher for the My+ TL patients compared with the My− TL control group (60% v 8%, P = .0002).
Presenting features of children with My+ and My− BL ALL.Clinical and laboratory features of My+ BL and My− BL patients were compared by a global chi-square statistic (Table 2). WBC differed significantly between the two groups due to a higher percentage of My+ BL patients presenting with a low (<20,000/μL) WBC (68.7% v 58.1%; P = .006). The median WBC counts for My+ BL and My− BL patients were 9,900 (range, 800 to 507,800) and 14,400 (range, 300 to 1,000,000), respectively. My+ BL patients were less likely than My− BL patients to present with splenomegaly (47.0% v 57.2%; P = .0002) or lymphadenopathy (36.4% v 48.6%; P = .004). Platelet count also was significantly different (P = .01) between the two groups: My+ BL patients less often had low (<50,000/μL) and more often had high (≥150,000/μL) platelet counts at presentation. Centrally reviewed cytogenetic analysis was performed on leukemic cells from a subset of 71 My+ BL and 386 My− BL patients. Within this subset, there were two significant differences between the groups. First, chromosome number differed significantly (P = .003) due both to the greater frequency of My+ BL patients presenting with a normal diploid karyotype (43.7% v 24.1%) and to the lower frequency of My+ BL patients presenting with high hyperdiploid (>50 chromosomes) karyotype (12.7% v 29.0%). Second, chromosomal aberrations were more frequent in the My− BL group (75.9% v 53.6%, P = .001).
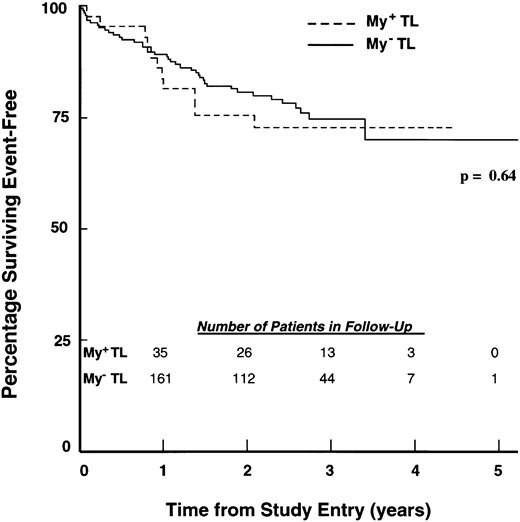
EFS of children with ALL according to TL immunophenotype. Percentages of 43 My+ TL (hatched line) and 184 My− TL (solid line) patients achieving EFS during 5 years of follow-up were calculated as described in Materials and Methods. The number of patients in each group remaining in follow-up at the indicated time points is shown in the inset.
EFS of children with ALL according to TL immunophenotype. Percentages of 43 My+ TL (hatched line) and 184 My− TL (solid line) patients achieving EFS during 5 years of follow-up were calculated as described in Materials and Methods. The number of patients in each group remaining in follow-up at the indicated time points is shown in the inset.
Presenting features of children with My+ and My− TL ALL.Clinical and laboratory features of My+ TL and My− TL patients were compared in a similar manner (Table 3). Age distribution was significantly different (P = .003) for the My+ TL versus My− TL groups largely due to a higher percentage of My+ TL patients (55.8% v 31.0%) presenting with ≥ 10 years of age. A higher percentage of My+ TL patients than My− TL patients presented with a normal liver and spleen; however, these differences did not reach statistical significance. My+ TL patients were less likely than My− TL patients to present with lymphadenopathy (58.2% v 76.1%, P = .05), a mediastinal mass (32.5% v 58.1%, P = .008), or high (≥11 g/dL) hemoglobin values (20.9% v 41.1%, P = .02). Cytogenetic data was available for only a small subset of patients (17 My+ TL and 82 My− TL patients), and within this subset, there were no significant differences between the My+ TL and My− TL patients.
Treatment outcomes for children with My+ and My− ALL.Induction therapy outcomes were similar for My+ and My− controls. At the end of induction chemotherapy, 98.6% of My+ BL patients and 98.3% of My− BL patients achieved a remission (P = .97). Similarly, 97.6% of My+ TL patients and 96.6% of My− TL patients achieved remission (P = .85). EFS outcomes of My+ BL and My+ TL patients were compared with those of My− BL and TL patients using life-table methods, as described in Materials and Methods. Follow-up for event-free survivors ranged from 1 to 73 months (median, 32 months). My+ and My− BL patients had similar outcomes (P = .32; Fig 1), with 4-year EFS estimates of 77.0% (SD = 4.0%) and 75.9% (SD = 1.8%), respectively. Similarly, the My+ TL and My− TL groups had similar outcomes (P = .64; Fig 2 ), with 4-year EFS estimates of 72.7% (SD = 7.1%) and 70.1% (SD = 5.7%), respectively. The estimated RHR values for My+ BL versus My− BL and My+ TL versus My− TL were 0.83 and 1.18, respectively (Table 4). An overall RHR estimate of 0.89 (P = .49) was determined for My+ patients compared with My− patients by a stratified analysis across lineage groups (Table 4).
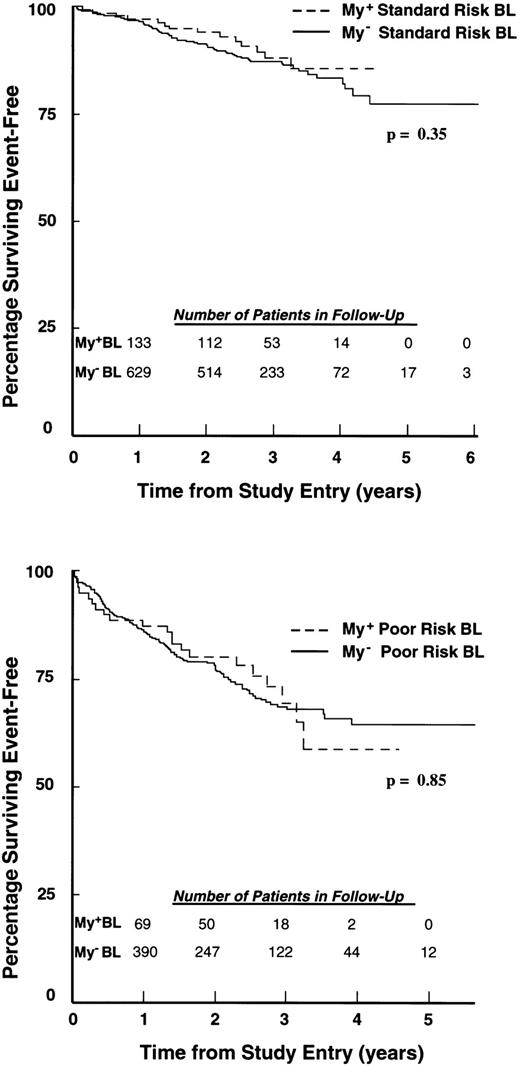
In addition, outcomes remained similar when BL patients were compared across NCI standard and poor risk group categories (P = .35 and P = .85, respectively; Fig 3). By this analysis, 4-year EFS estimates for My+ and My− patients were 85.7% (SD = 3.9%) and 83.2% (SD = 2.1%), respectively, within the standard risk group, and 58.7% (SD = 9.1%) and 64.5% (SD = 3.2%), respectively, within the poor risk group. Estimates of RHR for My+ versus My− patients in standard and poor-risk groups were 0.77 and 0.95, respectively. A stratified risk analysis was also performed to compare patients on CCG protocols with less intensive therapy (CCG 1881 and CCG 1891) with those on protocols with more intensive therapies (CCG 1882, CCG 1883, and CCG 1901). This analysis also showed similar outcome for My+ and My− patients in low and high intensity treatment categories (P = .44 and P = .94, respectively; data not shown).
EFS of children with My+ and My− BL ALL according to NCI risk classification. (Top) Standard risk: 138 My+ BL (hatched line) and 657 My− BL (solid line) patients were followed for 6 years. (Bottom) Poor risk: 79 My+ BL (hatched line) and 456 My− BL (solid line) were followed for 5 years. Percentages of patients achieving EFS during follow-up were calculated as described in Materials and Methods. The number of patients in each group remaining in follow-up at the indicated time points is shown in the inset.
EFS of children with My+ and My− BL ALL according to NCI risk classification. (Top) Standard risk: 138 My+ BL (hatched line) and 657 My− BL (solid line) patients were followed for 6 years. (Bottom) Poor risk: 79 My+ BL (hatched line) and 456 My− BL (solid line) were followed for 5 years. Percentages of patients achieving EFS during follow-up were calculated as described in Materials and Methods. The number of patients in each group remaining in follow-up at the indicated time points is shown in the inset.
DISCUSSION
We have examined the clinical importance of myeloid antigen expression in a large cohort of children enrolled in risk-adjusted treatment protocols of the CCG. In general, children with My+ ALL compared with My− ALL had similar or more favorable presenting features, including low WBC levels and normal karyotypes, as well as absence of splenomegaly, lymphadenopathy, and mediastinal mass. Importantly, we observed that remission induction rates, as well as EFS outcomes, were virtually identical for the My+ patients and My− patients, demonstrating that myeloid antigen expression was not an adverse risk factor in this cohort.
Patients analyzed herein were defined according to coexpression of the myeloid differentiation antigens CD13 and CD33 together with either B (CD19) or T-(CD7) lymphoid–associated antigens. CD13, CD33, CD14, CD15, and CDw65 are the myeloid-associated antigens most frequently expressed on the surface of leukemic cells from ALL patients.35 Moreover, Drexler and Ludwig35 found that among ALL patients from numerous studies, similar percentages were positive for each of the individual myeloid antigens. Also, previous studies have documented the expression of various combinations of these myeloid-associated antigens in 5% to 20% of pediatric ALL patients,5 10-20 and differences in outcome do not appear to be related to the choice of antigens examined. Thus, analysis of CD13 and CD33 should be representative of overall myeloid antigen expression.
My+ ALL patients in the current study showed higher expression frequency of both CD34 and CD40 than My− ALL patients. Similarly, Borowitz et al36 and Guyotat et al37 observed that in pediatric and adult patients, CD34 expression was correlated with myeloid antigen expression. Saeland et al38 reported that CD40 is also present on CD34+ immature myeloid progenitor cells, but is lost on interleukin-3 (IL-3) induced myeloid differentiation. CD34 is a 110 kD integral membrane protein thought to be expressed normally by immature hematopoietic progenitor cells,39,40 and CD40, a member of the nerve growth factor receptor superfamily, plays a role in proliferation and differentiation of normal B-lineage lymphoid cells.41-44 Therefore, expression of the CD34 and CD40 antigens by My+ ALL cells further supports the hypothesis that My+ ALL arises via transformation of an immature progenitor cell.
The clinical significance of myeloid antigen expression in children with ALL is controversial. In a single institution study involving 53 children with My+ ALL and 183 children with My− ALL, Wiersma et al20 reported 3-year EFS estimates of 84% for My− patients with WBC <50,000/μL, 57% for My− patients with WBC ≥50,000/μL, 47% for My+ patients with WBC<50,000/μL, and 26% for My+ ALL patients with WBC ≥50,000/μL. These differences were statistically significant and multivariate analysis indicated that myeloid antigen expression was the most important predictor of a poor EFS outcome. Wiersma's study concurs with reports by Cantu Rajnoldi et al,14 Kurec et al,5 and Fink et al.15 Interestingly, these results also were consistent with preclinical observations that leukemic cells from My+ ALL patients were more resistant to glucocorticoid-induced killing than cells from My− ALL patients.45
Although the studies described above demonstrated a poor outcome associated with myeloid antigen expression in childhood ALL, numerous investigators have reported conflicting results. Bradstock et al13 and Mirro et al24 reported that myeloid antigen expression in pediatric ALL was not correlated with induction outcome, and other studies later showed no effect of myeloid antigen expression on treatment outcome.10,11,16,19 Likewise, Pui et al17 reported that myeloid antigen expression in 61 of 372 children with newly diagnosed ALL treated at the St. Jude Children's Hospital had no effect on either induction outcome or EFS. In a follow-up study, myeloid antigen expression again lacked prognostic significance in 25 children with My+ ALL.18 Subsequently, Pui et al46 reported that the estimated 3-year EFS estimates for 50 children with My+ ALL and 260 children with My−ALL were 85% and 75%, respectively. The St Jude researchers concluded that myeloid antigen expression in childhood ALL is not associated with poor outcome if intensive chemotherapy regimens are used. Our results are generally consistent with these studies in showing that myeloid antigen expression does not correlate with poor outcome for children with ALL. In conclusion, this study provides new insight on the clinical significance of myeloid antigen expression in childhood ALL and shows that regardless of risk classification, ALL patients who are My+ have treatment outcomes similar to those who are My−.
Supported in part by research grants including CCG Chairman's Grants No. CA-13539, CA-51425, CA-42633, CA-42111, CA-60437, and CA-27137 from the National Cancer Institute, National Institutes of Health, Bethesda, MD. F.M.U. is a Stohlman Scholar of the Leukemia Society of America.
Address reprint requests to Fatih M. Uckun, MD, Children's Cancer Group ALL Biology Reference Laboratory, Hughes Institute, 2657 Patton Rd, St Paul, MN 55113.