Abstract
Recent analyses of circulating blood B cells in myeloma have generated controversy concerning the exact levels of these cells and whether they may represent circulating clonal tumor B cells. Previous reports suggested that CD19+ B cells are markedly increased in myeloma patients and that this population shares clonotypic rearrangements with the malignant plasma cell. We studied the numbers of CD19+ B cells by flow cytometry in previously untreated newly diagnosed myeloma patients in Eastern Cooperative Oncology Group (ECOG) phase III trial E9486. There were 628 patients who were eligible for the clinical protocol E9486, but of these 521 were also entered on the companion laboratory study (E9487) and had CD19 data. In comparison with normal controls, the myeloma patients exhibited a marked heterogeneity in the number of circulating CD19+ B cells as detected by flow cytometry. Approximately 20% of patients had significantly increased levels of circulating CD19+ B cells. However, the total CD19+ blood population from myeloma was not significantly different from the median of age-matched, normal controls. Analysis of CD19+ blood cells in relationship to circulating clonal cells was done in 13 myeloma patients using a clonotypic, quantitative allele-specific oligonucleotide-polymerase chain reaction (PCR) assay. No correlation was found between the numbers of CD19+ B cells (range, 5% to 51%) and PCR estimates of the number of clonal cells in the peripheral blood (range, .009% to 3.6%). Low CD19+ B-cell level (<125 μL) was associated with clinical stage III (P = .033). A significant relationship exists between higher levels (≥125/μL) of CD19 cells and longer overall survival (P < .0001). In addition, high CD19 levels also predicted a clinical response and longer event-free survival. There was a strong inverse association between the level of CD19 values at diagnosis and infections within the first 2 months of diagnosis. Importantly, the number of deaths related to infections was significantly greater in the low versus high CD19 group (P < .0202). Also, CD19 is an independent prognostic factor in addition to plasma cell labeling indices, β2 -microglobulin, hemoglobin, and plasmablastic morphology. Patients with infections were more likely to have low levels of CD19+ cells. In summary, higher CD19+ cell levels are a favorable prognostic sign with no apparent relationship to circulating tumor cells. In addition, this analysis strongly suggests that low peripheral blood levels of CD19+ cells are an adverse prognostic sign in myeloma. The CD19+ cell levels in myeloma patients is an important parameter in the overall assessment of these patients.
CIRCULATING blood B cells in multiple myeloma (MM) are a complex of normal polyclonal populations and presumably neoplastic cells. The latter cells may consist of plasma cells and, perhaps, CD19+ expressing B lymphocytes.1-3 Expanded B-cell clonal populations can be recognized through detection of clonotypic IgH gene rearrangements.4,5 Polyclonal B-cell populations in MM blood can be identified by reactivity for CD19 surface antigen and lack of common Ig gene rearrangements.6 7 The precise characterization of both normal polyclonal and clonal B cells is of critical importance in understanding their relationship to the clinical course of MM. The circulating clonal lymphocytes may represent clones that can repopulate tissue sites and contribute to disease dissemination. Therefore, it is important to determine the relative balance between normal polyclonal B-cell populations and a B-cell population clonally related to the plasma cell tumor. If certain MM patients have a very high proportion of circulating clonal to polyclonal B cells, it might be a valuable indication of more progressive disease. A very low proportion of cells clonally related to the tumor could suggest a relatively intact polyclonal B-cell population and a more normal immune responsiveness.
CD19 is a member of the Ig superfamily and is a pan B-cell molecule possibly involved in signal transduction as an accessory molecule.8,9 The presence of CD19-reactive cells as determined by monoclonal antibodies (MoAbs) specific for CD19 is now a standard approach for determining the numbers of circulating B cells in human blood. Although CD19 is expressed on normal mature B cells and B-cell precursors, it is frequently found on many B-cell tumors such as B-chronic lymphocytic leukemia (B-CLL), prolymphocytic leukemia, small cleaved follicular center cell lymphoma, and hairy cell leukemia.10
In an attempt to clarify the relative proportions of normal, polyclonal B cells and the levels of circulating clonal B cells in patients with MM, we prospectively evaluated the peripheral blood B-cell pool in the large patient cohort entered on the Eastern Cooperative Oncology Group (ECOG) phase III randomized chemotherapy trial E9486 for newly diagnosed MM. In this study we analyzed peripheral blood lymphocytes for the presence of CD19+ lymphocytes and assessed the quantitative levels of the CD19+ B cells in relationship to a variety of clinical parameters. In a smaller cohort of patients we compared the levels of circulating clonal B cells to circulating CD19+ lymphocytes.
MATERIALS AND METHODS
Isolation of blood lymphocytes from MM patient cohort.The patients used for analysis of blood lymphocyte phenotype included the newly diagnosed MM patients (N = 521) who were entered and eligible for both ECOG clinical protocol E9486, and a companion laboratory protocol E9487. All patients signed informed consent. The protocol was reviewed by the National Cancer Institute and was approved by the investigational review committees of the participating institutions. All bloods were shipped overnight to the flow cytometry laboratory for subsequent processing described in this report. Anticoagulated peripheral blood (PB) was removed by venipuncture from MM patients just before their initiation of therapy on the ECOG protocol. This blood was then subject to Ficoll-Hypaque (Sigma, St Louis, MO) centrifugation and the PB mononuclear cells (PBMCs) were obtained by sterile technique from the interface of Ficoll-Hypaque gradient. The PBMCs were then used for analysis of PB lymphocyte (PBL) phenotype (CD19 reactivity) by flow cytometry as described below. Control age-matched populations were processed in a similar fashion and assessed by flow cytometry.
Flow cytometric detection of human blood lymphocytes reactive for CD19.Reactivity of PBMC for the MoAb CD19 (Becton Dickinson, San Jose, CA) was determined by incubating 5 × 105 to 1 × 106 cells in 50 μL of RPMI 1640 with 10% fetal bovine serum, pH 7.2. The latter cells were cocultured with a saturating dilution of phycoerythrin (PE)-conjugated anti-CD19 (a pan B-cell MoAb) for 30 minutes at 4°C in the dark. These incubated cells were then washed with 2 mL of phosphate-buffered saline–bovine serum albumin (PBS-BSA)-azide containing buffer and analyzed by a FACstar flow cytometer (Becton Dickinson). Fluorescent levels of CD19 binding were detected by analysis of at least 10,000 cells using multi parameter flow cytometry and subsequent data analysis through a Simulset version 2.3.3 and Lysis version 1.0.2 consort 40 system (Becton Dickinson). Blood levels of CD19+ cells were then calculated using the percentage of CD19+ cells determined by flow cytometry times the absolute level of PBLs. The latter was determined by multiplying the total white blood cell (WBC) count by the percentage of lymphocytes as determined on a WBC differential.
Detection and quantitation of clonal circulating cells by allele-specific oligonucleotide-polymerase chain reaction (PCR). The method used to determine levels of clonal circulating cells and the PBL% PCR+ values shown in Table 1 have been previously reported.11,12 Briefly, genomic DNA was isolated from the bone marrow (BM) of each myeloma patient and the tumor burden determined by DNA blot hybridization. From this sample the CDR3 sequence of the Ig heavy chain was determined and an ASO primer was designed as described.11 Allele-specific dilution curves were generated from analysis of the patient marrow to establish the linear range for quantitation of ASO-PCR amplified PB samples. PB samples were analyzed in triplicate. In each case the patient specific primer was shown to be specific by its amplification on only patient tumor DNA and not a panel of unrelated tumors or normal BM controls.
Plasma cell labeling indices (PCLI).PCLI were determined by immunofluorescence microscopy using a previous technique.13 Fluorescein conjugated antiserum to kappa and lambda light chain was applied to alcohol fixed cytospin slides of BM mononuclear cells to identify clonal plasma cells. Antibody to 2-bromodeoxyuridine (BrdU) followed by a rhodamine-conjugated antimouse Ig antisera was used to identify cells in S-phase of the cell cycle. A kappa: lambda ratio greater than 4:1 or less than 0.5:1 was considered abnormal. Cells stained with the light chain of the nondominant isotype were considered to be nonclonal and not included in the labeling index determination. The labeling index was measured by determining the percent of BrdU-labeled cells among 500 plasma cells (Pcs) on a slide stained with the antikappa or antilambda reagent that identified the dominant Pc isotype.
Determination of C-reactive protein (CRP), β2 -microglobulin (β2M), and soluble interleukin-6 receptor (sIL-6R).CRP measurement was performed using immunophelometry (Beckman Instruments, Inc, Brea, CA). The normal value is <0.8 mg/dL. The β2M measurement was done using a microparticle enzyme immunoassay on Abbott Diagnostics Instrumentation (Abbott Laboratories, Abbott Park, IL). The normal value is ≤2.7 mg/mL. These analyses were done on fresh serum stored no longer than 2 weeks at 4°C. sIL-6R levels were performed by radioimmunoassay in the laboratory of Dr Bernard Klein in Nantes, France.14 Normal value is ≤300 ng/mL.
Statistical methods.Associations between categorical patient characteristics and quantitative CD19 values were tested with the Mann Whitney-Wilcoxon test. Fisher's Exact test was used to compare groups (high CD19 v low CD19) with respect to a dichotomous endpoint such as response versus nonresponse. Survival was computed from the time of study registration to the date of death or date last known alive. Event-free survival was calculated from the time of study registration to the date of progression, relapse, death, or date last known to be progression free. Survival curves were calculated using the method of Kaplan-Meier and compared by the log-rank test. All P values are two-sided.
To dichotomize CD19, classification trees were performed to select a cutoff point for the quantitative CD19 values. With this method, survival times through martingale residuals are repeatedly divided into subgroups at levels that identify the biggest differences in survival.15 The first “split” represents the point where the difference is the greatest. In this case, the first split was at 125 cells/μL.
RESULTS
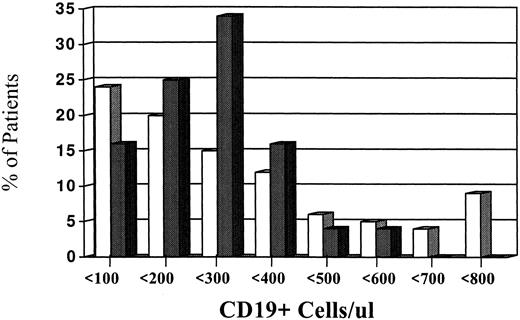
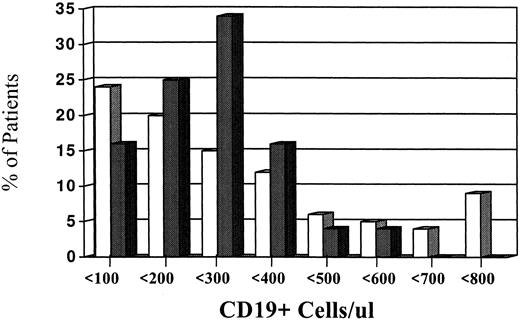
Levels of CD19 blood B-cell in myeloma patients.Figure 1 illustrates the levels of CD19+ cells in patients compared to controls. When all eligible MM patients were analyzed and compared with our control population, a marked heterogeneity of CD19 values were observed. The range of CD19 values for the myeloma patients was 0% to 79% and 0 to 3,196 cells/μL. This was in contrast to the control group (N = 24) who had ranges of 4% to 17% and CD19 cells of 41 to 530 cells/μL. Despite the marked heterogeneity, the MM patients did not have significantly larger CD19 values in contrast to the controls. In fact, a median value of 228 cells/μL was observed in our patient population, which was comparable to a median value of 215 cells/μL for the control group. Figure 1 also shows that approximately 20% of the patients had CD19 values that were greater than 500 cells/μL, whereas only 4% of controls had CD19+ greater than 500 cells/μL.
Relationship of blood CD19 levels to the percent frequency of myeloma patients. This figure illustrates the levels of blood CD19 levels (absolute numbers) in MM patients (□) and normal controls (). The levels of CD19+ cells are divided into incremental subgroups of 100 up to greater than 700. Each tick mark on the horizontal axes represents groups of 100.
Relationship of blood CD19 levels to the percent frequency of myeloma patients. This figure illustrates the levels of blood CD19 levels (absolute numbers) in MM patients (□) and normal controls (). The levels of CD19+ cells are divided into incremental subgroups of 100 up to greater than 700. Each tick mark on the horizontal axes represents groups of 100.
Relationship of blood CD19 cells to circulating clonal cells.To compare the levels of blood CD19+ B cells to clonal cells, we studied a smaller cohort of myeloma patients (N = 13) for their blood CD19+ cells and levels of clonal cells using the ASO-PCR method. Table 1 shows the analysis of this myeloma cohort. The mean level of CD19+ B cells was at a value of 16.6% whereas the mean level of clonal cells as determined by ASO-PCR analysis was only 0.5%. Table 1 shows the relationship between blood CD19+ B cells and clonal cells in 13 myeloma patients who were analyzed for both flow cytometry and ASO-PCR reactivity. Myeloma patients have widely disparate values in both the CD19+ cells and levels of cells clonally related to the tumor. For example, two myeloma patients (MM8 and MM9) had CD19 percentages of 15% and 13%, respectively, yet have very different levels of circulating PCR+ clonal cells (.03% v 1.6%) (Table 1). Thus, we conclude there is no clear association between the level of circulating CD19+ cells and clonally related cells. Moreover, circulating clonal cells typically are present in much smaller numbers than total CD19+ cells.
Analysis of clinical parameters in relationship to CD19 blood levels.We evaluated the association of clinical parameters with levels of blood CD19 cells in the 521 patients eligible for this analysis. No significant association was detected between CD19+ cell levels and several clinical parameters including, age, sex, infection status in last 6 months before study entry, and paraprotein isotype. In addition, there was no association of CD19 levels with the serum Ig (ie, noninvolved Ig) levels of the myeloma patients (data not shown).
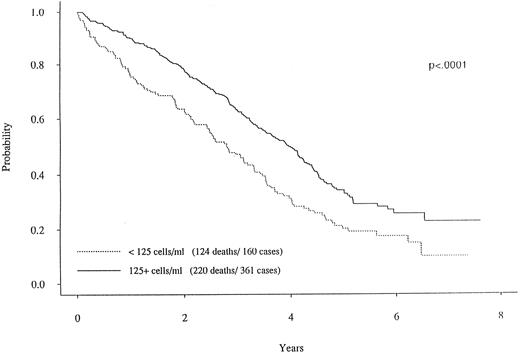
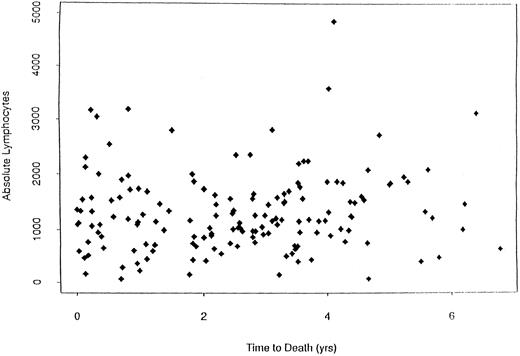
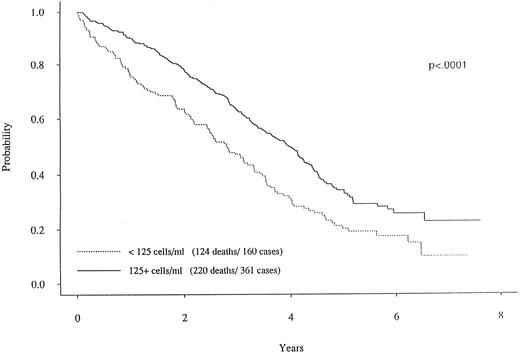
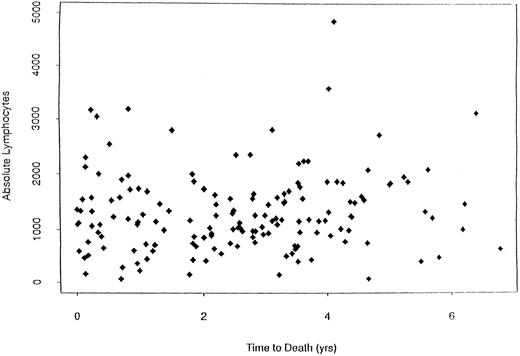
When dichotomizing CD19 into high (≥125) or low (<125), there was a statistically significant longer survival (P < .0001) for patients with high CD19 (Fig 2). The overall survival was 2.8 years for the low (<125) CD19 group while the high (≥125) CD19 group survival was 4.0 years (P < .0001). Event-free survival was also significantly different, with medians of 2.7 years and 2.0 years for high and low CD19 patients, respectively (P < .0001). To determine if the unfavorable prognosis associated with low levels of CD19 was a reflection of low lymphocyte levels, we compared the total blood lymphocyte amount for patients with low CD19 values to the patient's time to death (ie, no censoring). Figure 3 illustrates no obvious relationship between total blood lymphocyte values and patient's time to death. Finally, while we did not see significant differences in time to response or time to complete response between low and high CD19 levels, there was a significant difference in the incidence of response. Patients with low CD19 levels achieved a 60% response rate (95 of 158) whereas 71% of high CD19 (≥125) patients responded (256 of 363 [P = .025]). In addition to its prognostic significance unvariately, when CD19 is considered in a multivariate model with other known prognostic factors it retains its significance. This survival model includes the following independent prognostic factors: CD19, β2M, plasma cell labeling index, hemoglobin, sIL-6R, and plasmablastic morphology.
Survival curves for two MM subgroups. One curve with the solid line represents the MM patients with CD19 cells greater than 125.0/μL. In contrast, the patients with less than 125.0 CD19 cells (dotted curve) had a markedly worse survival curve (P < .0001).
Survival curves for two MM subgroups. One curve with the solid line represents the MM patients with CD19 cells greater than 125.0/μL. In contrast, the patients with less than 125.0 CD19 cells (dotted curve) had a markedly worse survival curve (P < .0001).
Plot of the absolute lymphocyte counts versus survival for MM patients (N = 124) with low CD19 values. This plot shows that there is no relationship between total blood lymphocyte values and survival in these MM patients.
Plot of the absolute lymphocyte counts versus survival for MM patients (N = 124) with low CD19 values. This plot shows that there is no relationship between total blood lymphocyte values and survival in these MM patients.
There was also an association between stage and CD19 level(s). Fifty-two percent of high CD19 patients were stage III, whereas 65% of the low CD19 patients were stage III (P = .033). Because B cells produce Ig we were particularly concerned about the relationship of CD19+ B cells to infection in these myeloma patients. We found an association between the number of infections and the patient CD19 values within the first 2 months of treatment. Thus, 35% of the low CD19 patients experienced at least one documented infection during the first 2 months whereas 24% of high CD19 patients had at least one infection (P = .011). The severity of infections was also significantly affected by the CD19 levels. Severity of infections were graded on a 0 to 5 scale, with 0 = no infection and 5 = death.16 Thirteen percent of the low CD19 patients had at least one severe or worse (grade 5) infection while only 4% of high CD19 patients experienced at least a severe (grade 3) infection (P = .0002). The association of infections with CD19 levels was not significant when analyzed over the first year of treatment. The number of infections ranged from 1 to 9, with a median of 1 for both the low and high CD19 groups. Finally, the number of deaths over the first 2 months attributable to infection was greater in the low CD19 versus high CD19 group (5.6% v 1.7%, P = .0202).
DISCUSSION
We have conducted a detailed analysis of blood CD19 cells in newly diagnosed MM patients immediately before these patients being placed on therapy. Our analysis of blood CD19 cells in untreated patients has yielded several new insights. First, in most patients the levels of blood CD19 cell counts at diagnosis are not significantly higher than control levels, but a cohort of myeloma patients appear to have elevated levels of CD19 cells. Second, clonal cells do circulate in the blood of myeloma patients but appear to be present at very low levels when compared with the numbers of CD19+ B cells and do not correlate to CD19 levels. Third, low blood CD19 cell levels may be associated with stage of disease and are strongly associated with a patient's propensity for infection and for deaths attributable to infection during the first 2 months of treatment. Importantly, the initial CD19 blood levels have a significant correlation with survival and the incidence of clinical response.
Previous studies have analyzed blood lymphocytes in myeloma patients. These studies have found that circulating blood lymphocytes may contain antigens that exhibit typical features of plasma cells.7 In addition, several studies have documented that patients with MM have circulating CD19+ blood B cells with phenotypic features, suggesting that some of these B cells may be monoclonal. Evidence for monoclonality has included restriction to one light chain expression,17,18 clonal Ig gene rearrangements,19 and clonotypic sequences that are similar to the clonal Ig rearrangements seen in the malignant plasma cells of myeloma patients.12,18 One report has indicated that CD19+ B cells in MM patients are always above the control values.20 In our study of newly diagnosed MM patients, there is a relatively wide distribution of CD19+ B cells in myeloma patients, with approximately 80% of patients having CD19+ cells equal to or less than 500 cells/μL. In contrast, a smaller cohort of MM patients (20%) had CD19+ B cells greater than 500 cells/μL.
Our study adds confirmatory evidence that there are very small numbers of clonal cells in myeloma blood. In particular, we have added more information about the numbers of clonal cells in newly diagnosed untreated patients. The exact percentage or proportion of clonal versus polyclonal B cells in the PB of myeloma patients has been controversial. Our previous studies have indicated that the vast majority of myeloma patients will have circulating clonal cells in the blood11,21; however, an important issue is the level of clonal cells that may by present within the PB B-cell pool of myeloma patients. A prior study of myeloma patients detected only 6% B cells in the blood mononuclear cells with a small percent of those having clonal identity with the myeloma clone.22 However, one analysis has detected from 2 to 8 times the level of CD19+ cells in myeloma blood versus normal individuals.20 In our study the relative level of clonal B cells using the ASO-PCR technique in comparison to the numbers of CD19+ B cells determined by flow cytometry indicates a very low percentage of circulating clonal cells related to the malignant plasma cells in newly diagnosed myeloma patients. This relatively low level of clonal cells was not associated with the proportion of CD19+ cells in our myeloma patients. Thus, patients with both high and low levels of CD19+ cells in their PB had similar levels of clonal cells. It is unclear if the variations in these low levels of clonal cells may be important in the ultimate prognosis of myeloma patients.
Precise quantification of blood B (CD19+) cells can provide valuable information about the host immune system. Deficiencies of the total level of B cells would suggest that the immune system has an altered ability to deal with infectious agents and that the overall capacity of the immune system is impaired. Therefore, it was relevant to determine if CD19+ levels correlated with clinical parameters of the MM cohort. Indeed, we were able to show several striking associations with the levels of CD19+ cells. First, low CD19+ patients had a significantly higher proportion of stage III patients than did high CD19 patients. Second, the patients with high CD19+ cells, above a level of 125, had a very positive association with prolonged survival. The favorable survival for high CD19+ patients was accompanied by a higher response rate compared to patients with low CD19+ cells. Taken together, this data analysis provides initial compelling evidence that a high CD19 value is associated with a more favorable clinical outcome. The multivariate analysis comparing CD19 to other known prognostic factors indicated that CD19 was still an independent prognostic factor. The reason for this may be that the other prognostic factors measure separate aspects of the myeloma process. For example, β2M and hemoglobin reflect renal function and tumor burden, PCLI and plasmablastic morphology reflect tumor proliferation whereas CD19 cell detection measures a component of the immune system.
One potential explanation for this positive relationship is that patients with higher levels of CD19+ cells have an intact or less disordered immune system. This could then result in a subgroup of patients having a more favorable clinical course when treated with chemotherapy. Evidence that these patients do indeed have a more functional immune system is the association between the level of CD19+ cells and the reductions in both the incidence, severity of infection, and deaths related to infection shown in our study. It is not clear if the difference in susceptibility to infections is also associated with host resistance to the tumor. One explanation for low numbers of CD19+ cells in myeloma blood is that the myeloma tumor is able to suppress B-cell maturation. Recent evidence that myeloma plasma cells can increase apoptosis of pre-B cells following interaction with marrow stromal cells support this possibility.23
Future clinical studies of myeloma patients should be accompanied by a detailed analysis of their immune system, including quantification of circulating CD19+ cells. A current phase three clinical and laboratory ECOG protocol (E5A93 and E3A93) for untreated myeloma will continue to explore the relationship between CD19 cells and survival/relapse.
This study was conducted by the Eastern Cooperative Oncology Group and supported in part by Public Service Grants No. CA 15947, CA 23318, CA 18653, CA 21076, and CA 21115 from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Address reprint requests to Neil E. Kay, MD, University of Kentucky, Department of Hematology/Oncology, 800 Rose St, CC 405, Lexington, KY 40536-0093.