Abstract
Normal human serum contains IgM antibodies that regulate the natural autoantibody activity of IgG in autologous serum. In the present study, we show that pooled normal human IgM (IVIgM) purified from plasma of more than 2,500 healthy donors and processed in a similar fashion to that of therapeutic preparations of pooled normal human IgG (IVIg) suppresses activity of IgG autoantibodies purified from the serum of patients with autoimmune diseases in vitro. The inhibitory effect of IVIgM was greater or equivalent to that of IVIg on a molar basis. We show that IVIgM contains anti-idiotypic antibodies directed against idiotypic determinants of autoantibodies, in particular by showing that Sepharose-bound IVIgM selectively retained F(ab′)2 fragments of IgG autoantibodies. The infusion of (Lewis × Brown-Norway) F1 rats with IVIgM protected the animals against experimental autoimmune uveitis induced by immunization with the soluble retinal S antigen, as evidenced by clinical scoring and histopathological analysis. The present findings provide a rationale for considering pooled IgM for immunomodulation of autoimmune disease.
NATURAL AUTOANTIBODIES of both the IgM and IgG isotypes reactive with a wide spectrum of membrane-associated, intracellular, nuclear, and circulating self antigens, including idiotypes of immunoglobulins, are present in normal serum.1-3 Natural IgM autoantibodies have been shown to be polyreactive, to exhibit variable affinity for target antigens, and to be encoded by unmutated germline Ig V genes.4-9 These antibodies represent a major fraction of the normal circulating IgM repertoire, particularly in neonates that are not exposed to exogeneous antigens except for idiotypes of maternal IgG.10 One of the functions of normal circulating IgM under physiologic conditions is to control the expression of IgG autoreactivity in whole serum through idiotypic complementarity with natural IgG autoantibodies.3,11 12
Pooled normal human polyspecific IgG (IVIg) is increasingly used in the treatment of autoimmune diseases.13,14 Several mutually nonexclusive mechanisms account for the immunomodulatory properties of IVIg, including the ability of IVIg to neutralize circulating autoantibodies, to inhibit the function of Fc receptors, to modulate cytokine production and complement activation, and to regulate the functions and select the repertoires of B and T lymphocytes.15-18 In the present study, we show that pooled normal IgM obtained from the plasma of more than 2,500 healthy donors and processed for potential therapeutic use (IVIgM) suppresses autoantibody activity of IgG purified from the serum of patients with autoimmune diseases through interactions with idiotypes of autoantibodies. IVIgM protected genetically susceptible rats from the onset of the soluble retinal S antigen (S Ag)-induced experimental autoimmune uveitis (EAU). Our observations provide a basis for therapeutic downregulation of autoaggressive immune responses by pooled normal IgM.
MATERIALS AND METHODS
Sources of antigens and antibodies. Normal IgM (a kind gift from the Laboratoire Français du Fractionnement et des Biotechnologies, Les Ulis, France) was processed from the pooled plasma of more than 2,500 healthy donors by using a modified Deutsch-Kistler-Nitschmann's ethanol fractionation procedure19-21 followed by octanoic acid precipitation and two successive ion-exchange chromatography steps. The IgM was greater than 90% pure, as assessed by enzyme-linked immunosorbent assay (ELISA) using isotype-specific antibodies, by gel-filtration chromatography on a Superose 6 column (Pharmacia Fine Chemicals, Uppsala, Sweden), by immunoelectrophoresis, and by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Although the IgM preparation has not been used clinically, the purification steps were those of a therapeutic Ig preparation for intravenous use. IgM was also purified from the serum of a single healthy donor and from sera containing rheumatoid factor activity by precipitation with saturated ammonium sulfate and gel-filtration on Sephacryl-S300 HR (Pharmacia), followed by the removal of residual IgG on protein G-Sepharose (Pharmacia). The concentration of purified IgM was determined by ELISA and by using the BCA Protein Assay (Pierce, Rockford, IL).
IVIg (Sandoglobulin) was a gift of the Central Laboratory of the Swiss Red Cross (Bern, Switzerland). F(ab′)2 fragments were prepared from IVIg by pepsin digestion (2.0% wt/wt; Sigma Chemicals, St Louis, MO) in acetate buffer, pH 4.1, for 18 hours at 37°C and chromatography on protein G-Sepharose. F(ab′)2 fragments were free of IgG and of Fc fragments as assessed by SDS-PAGE and ELISA. IgG was also purified from the serum of two healthy donors by chromatography on protein G-Sepharose. The concentration of IgG and F(ab′)2 fragments of IgG was determined spectrophotometrically at 280 nm and by ELISA.
Mouse monoclonal anti-idiotypic antibody, 20F2, directed against a cross-reactive idiotype of human anti-factor VIII (FVIII) autoantibodies was obtained as previously described.22 Rabbit anti-idiotypic antiserum directed against the disease-specific cross-reactive T44 α idiotype of anti-thyroglobulin (anti-TG) autoantibodies and mouse monoclonal anti-idiotypic antibodies to human anti-TG autoantibodies, 1B4 and 1C2, were prepared as previously described.23,24 Human monoclonal IgM antibody with rheumatoid factor activity was kindly provided by J.L. Pasquali (Strasbourg, France).25 Sera containing rheumatoid factor activity were provided by B. Weil (Paris, France).
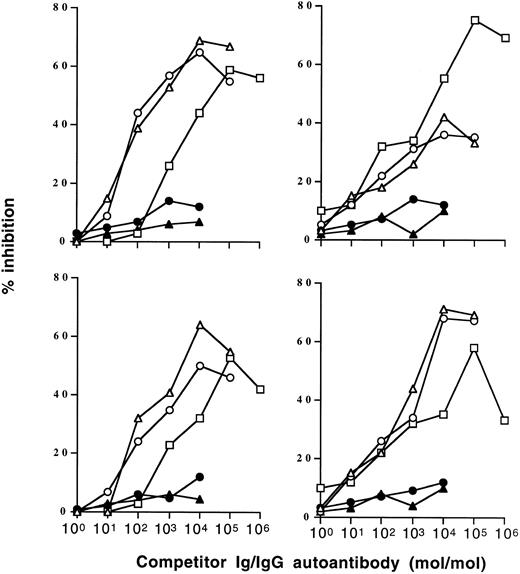
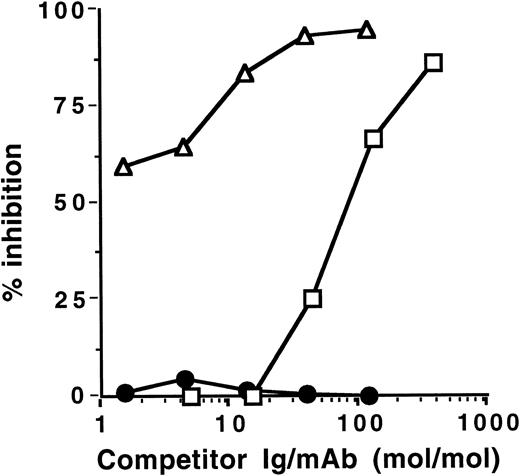
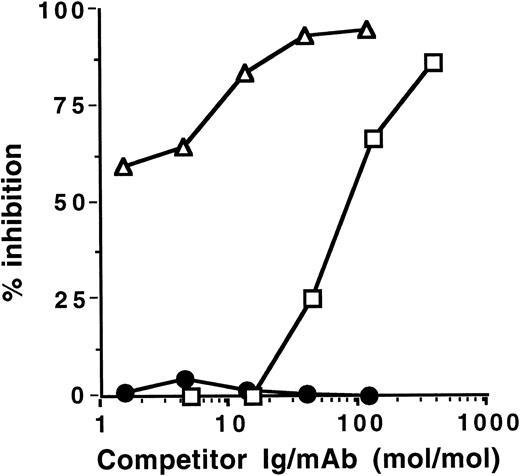
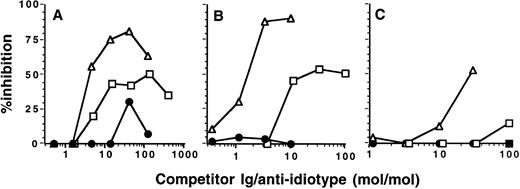
Inhibition of IgG autoantibody activity by IVIgM. Increasing amounts of IVIgM (▵), IVIgM depleted of its rheumatoid factor activity (○), IVIg (□), a human monoclonal IgM rheumatoid factor (•), or monoclonal IgM (▴) were coincubated with a fixed amount of 125I-labeled affinity-purified anti-TG or anti-DNA IgG from 2 patients with Hashimoto's thyroiditis and 2 patients with SLE overnight at 4°C. The binding of 125I-IgG to the corresponding antigen was then assessed. (Left panels) inhibition of anti-TG activity; (right panels) inhibition of anti-DNA activity. The abscissa indicates molar ratios between competitor Ig and 125I-IgG autoantibody.
Inhibition of IgG autoantibody activity by IVIgM. Increasing amounts of IVIgM (▵), IVIgM depleted of its rheumatoid factor activity (○), IVIg (□), a human monoclonal IgM rheumatoid factor (•), or monoclonal IgM (▴) were coincubated with a fixed amount of 125I-labeled affinity-purified anti-TG or anti-DNA IgG from 2 patients with Hashimoto's thyroiditis and 2 patients with SLE overnight at 4°C. The binding of 125I-IgG to the corresponding antigen was then assessed. (Left panels) inhibition of anti-TG activity; (right panels) inhibition of anti-DNA activity. The abscissa indicates molar ratios between competitor Ig and 125I-IgG autoantibody.
IgG autoantibodies were isolated from the serum of 2 patients with Hashimoto's thyroiditis, 2 patients with systemic lupus erythematosus (SLE), 2 patients with anti-von Willebrand factor (vWF ) autoimmune disease, 2 patients with anti-factor VIII autoimmune disease, and 2 patients with autoimmune uveitis by chromatography on protein G-Sepharose. Affinity-purified anti-TG and anti-DNA autoantibodies were obtained from sera of patients with Hashimoto's thyroiditis and from the sera of patients with SLE by chromatography on TG-Sepharose and DNA-cellulose (Sigma), respectively, as previously described.26 IgM purified from the plasma of a patient with Waldenström's macroglobulinemia (a gift of D. Hurez and A. Chevailler, Angers, France) was used as a source of monoclonal IgM. Fc fragments of IgG were provided by M.C. Bonnet (Institut Mérieux, Lyon, France).27
Human transferrin (TF ), calf thymus native double-stranded DNA, sheep skeletal muscle myoglobin (MG), spinach phosphoribulokinase (PK), and cabbage phospholipase D (PLD) were obtained from Sigma. Highly purified human procoagulant FVIII (Hemophil M) was from Hyland Baxter (Glendale, CA). Human TG was from UCB Bioproducts (L'Alleud, Belgium). Human vWF was from Rorer Biotechnology Inc (King of Prussia, PA). Human serum albumin (HSA) was from LFB (Les Ulis, France). Tetanus toxoid (TT) was a gift from L. Hänson (Göteborg, Sweden). Retinal S antigen was prepared as described previously.28
Assessment of autoantibody activity. For titration of antibody activity by ELISA, flat-bottomed 96-well polystyrene microtiter ELISA plates (Nunc, Roskilde, Denmark) were coated with S Ag (5 μg/mL), TG (10 μg/mL), MG (10 μg/mL), TF (10 μg/mL), PK (10 μg/mL), PLD (10 μg/mL), DNA (20 μg/mL), and FVIII (20 U/mL) in phosphate-buffered saline (PBS), pH 7.4, for 18 hours at 4°C. Uncoated sites were blocked with 1.0% bovine serum albumin in PBS (PBS-BSA) for 1 hour at 37°C. Antibodies to be tested were diluted in PBS-BSA and added to the wells for 2 hours at 20°C. After washing, bound antibody was shown using peroxidase-labeled goat antihuman Fcγ (Jackson Laboratories, Bar Harbor, ME) or antihuman Fcμ antibodies (Southern Biotechnology, Birmingham, AL).
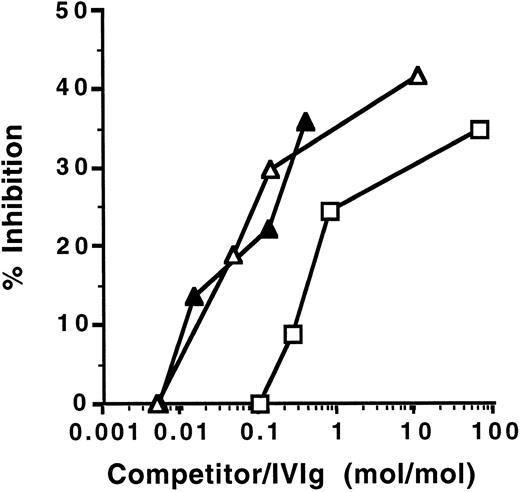
Inhibition of anti-TG activity of IVIg by IVIgM. Increasing amounts of IVIgM (▵), anti-TG–depleted IVIgM (▴), or IVIg (□) were coincubated with a fixed amount of 125I-labeled IVIg overnight at 4°C. The binding of 125I-IVIg to TG antigen was then asessed. The abscissa indicates molar ratios between competitor Ig and 125I-IVIg.
Inhibition of anti-TG activity of IVIg by IVIgM. Increasing amounts of IVIgM (▵), anti-TG–depleted IVIgM (▴), or IVIg (□) were coincubated with a fixed amount of 125I-labeled IVIg overnight at 4°C. The binding of 125I-IVIg to TG antigen was then asessed. The abscissa indicates molar ratios between competitor Ig and 125I-IVIg.
For solid-phase radioimmunoassays, IgG containing autoantibody activity and affinity-purified IgG autoantibodies were radiolabeled with 125I (Amersham, Les Ulis, France) using Iodogen (Pierce, Rockford, IL). Microtiter plates were coated with antigens, blocked with PBS-BSA for 1 hour at room temperature, and incubated with radiolabeled antibodies in the presence or absence of unlabeled competitor bound radioactivity.
Affinity chromatography of autoantibodies on IgM-Sepharose. IVIgM and IgM purified from the plasma of a patient with Waldenström's macroglobulinemia were coupled to CNBr-activated Sepharose (Pharmacia). IgG F(ab′)2 fragments of IgG containing autoantibody activity were then subjected to affinity chromatography on the IVIgM column equilibrated with PBS, pH 7.4. Bound proteins were eluted using 0.2 mol/L glycine-HCl at pH 2.8, were immediately brought to pH 7.0 using 3.0 mol/L Tris, and were dialysed against PBS.
Affinity chromatography of IVIgM on Fc-Sepharose. Fc fragments of IgG were coupled to CNBr-activated Sepharose. IVIgM was subjected to affinity chromatography on the Fc-Sepharose column equilibrated with PBS, pH 7.4, until complete depletion of rheumatoid factor activity from the IVIgM pool. Bound proteins were eluted as described above.
Binding of IVIgM to F(ab′)2 fragments of IgG autoantibodies. Microtiter plates were coated with F(ab′)2 fragments of affinity-purified anti-TG IgG from a patient with Hashimoto's thyroiditis (2 μg/mL) and with F(ab′)2 fragments of IgG purified from the plasma of a patient with anti-FVIII autoimmune disease for 18 hours at 4°C. Uncoated sites were blocked with 1.0% (wt/vol) PBS-BSA for 1 hour at 37°C. The plates were washed and then coincubated with IVIgM or with IgM purified from the plasma of a patient with Waldenström's macroglobulinemia used as irrelevant IgM for 2 hours at 37°C. After washing, bound IgM were detected using alkaline phosphatase-labeled goat antihuman IgM antibodies (Southern Biotechnology).
Competitive binding of IVIgM and heterologous anti-idiotypes to autoantibodies. Microtiter plates were coated with affinity-purified anti-TG IgG from a patient with Hashimoto's thyroiditis (2 μg/mL) and with IgG purified from the plasma of a patient with anti-FVIII autoimmune disease for 18 hours at 4°C. Uncoated sites were blocked with 1.0% (wt/vol) PBS-BSA for 1 hour at 37°C. The plates were washed and then coincubated with the corresponding mouse or rabbit anti-idiotypic antibodies in the presence of increasing amounts of IVIgM for 2 hours at 37°C. After washing, bound anti-idiotypes were shown using peroxidase-labeled rabbit antimouse IgG or donkey antirabbit IgG (Amersham).
Induction and clinico-pathologic assessment of EAU. (Lewis × Brown-Norway) F1 hybrid rats were bred in our animal facility from female Lewis (LEW) and male Brown-Norway (BN) rats initially obtained from the CSEAL (CNRS, Orléans La Source, France). Eight- to 13-week-old (LEW × BN) F1 males were used. The animals were cared and handled according to the principles expressed in the Declaration of Helsinki on the use of animals in research. Animals were immunized with purified bovine S Ag, as previously described.28 The severity of EAU was graded from 0 to 4 as follows: 0, normal iris dilation after instillation with a mydriatic drug, no cells in the aqueous humour or the vitreous body; 1, anterior uveitis with cell deposits in the pupil (hypopion); 2, total invasion of the pupil by the cellular infiltrates, no dilation under mydriatic drug; 3, severe inflammation associated with corneal oedema; and 4, ocular protrusion, hemorrhages in the anterior chamber. For histologic assessment of EAU, animals were ether-anesthetized and killed on day 35. Histologic lesions were graded from 0 to 7 as follows: 0, no destruction and no cell infiltration; 1 through 7, limited or total destruction of the various layers of rods and cones (1 and 2), of the outer nuclear layer (3 and 4), or of the inner nuclear layer (5 and 6) and destruction of the ganglion cell layer (7). For treatment with either IVIgM or IVIg, rats received one daily intravenous injection of 40 mg/100 g body weight of IVIgM or IVIg for 5 consecutive days starting from day 1 of immunization. Incidence of the disease was compared using the χ2 test. Clinical and histologic scores were compared using Mann-Whitney's U test.
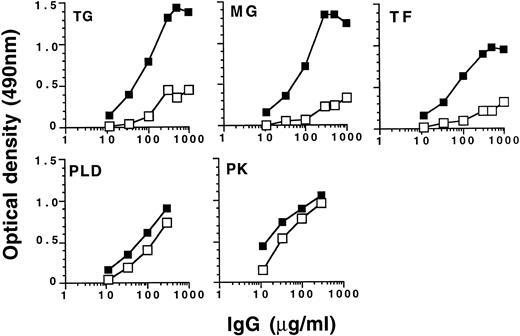
Selective retention of natural IgG autoantibody activity by IVIgM coupled to Sepharose. IVIg (50 mg) was subjected to affinity chromatography on 60 mg of IVIgM coupled to Sepharose. The acid-eluted fraction of the column (▪) and the loaded material (□) were then compared for reactivity with a panel of self (TG, MG, and TF ) and non-self (PLD and PK) antigens by ELISA.
Selective retention of natural IgG autoantibody activity by IVIgM coupled to Sepharose. IVIg (50 mg) was subjected to affinity chromatography on 60 mg of IVIgM coupled to Sepharose. The acid-eluted fraction of the column (▪) and the loaded material (□) were then compared for reactivity with a panel of self (TG, MG, and TF ) and non-self (PLD and PK) antigens by ELISA.
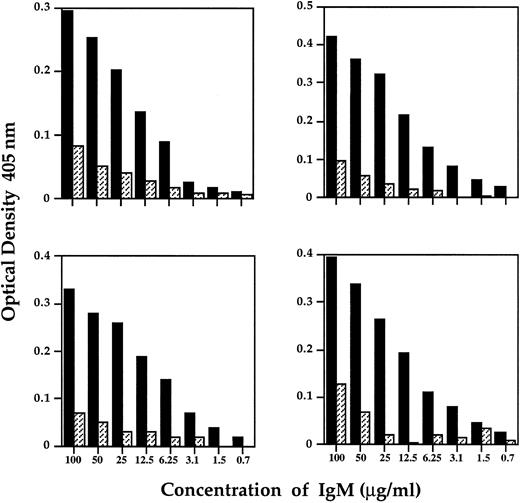
Binding of IVIgM to F(ab′)2 fragments of disease-associated IgG autoantibodies. Decreasing concentrations of IVIgM (▪) or IgM purified from the plasma of a patient with Waldenström's macroglobulinemia (▨) were incubated on plates coated with F(ab′)2 fragments of IgG of 2 patients with Hashimoto's thyroiditis (left panels) and F(ab′)2 fragments of IgG of 2 patients with anti-F.VIII autoantibodies.
Binding of IVIgM to F(ab′)2 fragments of disease-associated IgG autoantibodies. Decreasing concentrations of IVIgM (▪) or IgM purified from the plasma of a patient with Waldenström's macroglobulinemia (▨) were incubated on plates coated with F(ab′)2 fragments of IgG of 2 patients with Hashimoto's thyroiditis (left panels) and F(ab′)2 fragments of IgG of 2 patients with anti-F.VIII autoantibodies.
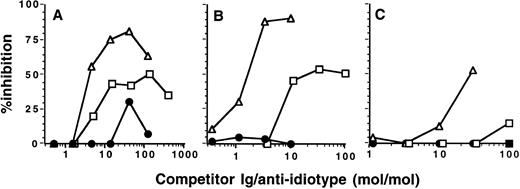
Competitive binding of IVIgM and heterologous anti-idiotypes to idiotypes of anti-F.VIII autoantibodies. Microtiter plates were coated with F(ab′)2 fragments of IgG purified from the plasma of a patient with anti-F.VIII autoimmune disease and incubated with 0.025 μg of anti-idiotypic monoclonal antibody 20F2 in the presence of increasing concentrations of IVIgM (▵), monoclonal IgM (•), or IVIg (▪). Bound anti-idiotype was then measured using peroxidase-labeled antimouse Ig antibodies. The abscissa represents the molar ratio between competitor Ig and monoclonal antibody 20F2.
Competitive binding of IVIgM and heterologous anti-idiotypes to idiotypes of anti-F.VIII autoantibodies. Microtiter plates were coated with F(ab′)2 fragments of IgG purified from the plasma of a patient with anti-F.VIII autoimmune disease and incubated with 0.025 μg of anti-idiotypic monoclonal antibody 20F2 in the presence of increasing concentrations of IVIgM (▵), monoclonal IgM (•), or IVIg (▪). Bound anti-idiotype was then measured using peroxidase-labeled antimouse Ig antibodies. The abscissa represents the molar ratio between competitor Ig and monoclonal antibody 20F2.
RESULTS
IVIgM inhibits autoantibody activity of patients with autoimmune disease. Coincubation of IVIgM with 125I-labeled IgG isolated from the serum of patients with autoimmune diseases resulted in dose-dependent inhibition of the binding of iodinated IgG to target autoantigens (Fig 1 and Table 1). Monoclonal IgM from a patient with Waldenström's macroglobulinemia did not have any inhibitory effect. On a molar basis, IVIgM was more effective than IVIg and than F(ab′)2 fragments of IVIg in inhibiting autoantibody activity. Figure 1 depicts dose-dependent inhibition by IVIgM, monoclonal IgM, and IVIg of the binding to TG and DNA of affinity-purified 125I-labeled anti-TG and anti-DNA IgG autoantibodies from patients with autoimmune thyroiditis and SLE, respectively. In addition and in a similar fashion, IVIgM inhibited the binding of IgG autoantibodies from a patient with Birdshot retinopathy to retinal S antigen, the binding of IgG autoantibodies from a patient with vWF syndrome to vWF antigen, and the binding of IgG autoantibodies from a patient with factor VIII autoimmune hempohilia to factor VIII. In these assays, pooled IgM was more potent in its inhibitory effect than both IVIg and F(ab′)2 fragments of IVIg at equimolar concentrations. IVIgM that was depleted of its rheumatoid factor activity by chromatography on an Fc-Sepharose column was tested for its ability to inhibit autoantibody activity. As shown in Fig 1, IVIgM that was free of its content in rheumatoid factor activity exhibited an inhibitory capacity equivalent to that of IVIgM. Monoclonal IgM from a patient with Waldenström's macroglobulinemia used as a control for pooled IgM did not have any inhibitory effect on the binding of various autoantibodies to the corresponding target self antigens. Furthermore, human monoclonal IgM rheumatoid factor and IgM purified from sera of patients with rheumatoid arthritis also did not inhibit the activity of autoantibodies tested (Fig 1 and data not shown).
Competitive binding of IVIgM and heterologous anti-idiotypes to idiotypes of anti-TG autoantibodies. Microtiter plates were coated with F(ab′)2 fragments of IgG expressing the T44, C2, and 1B4 idiotypes; purified from serum of a patient with Hashimoto's thyroiditis; and incubated with 0.1 μg of rabbit anti-T44 IgG (A) or with 0.1 μg of anti-idiotypic monoclonal antibodies 1B4 or 1C2 (B and C, respectively) in the presence of increasing concentrations of IVIgM (▵), monoclonal IgM (•), or IVIg (▪). Bound anti-idiotype was then measured using peroxidase-labeled antirabbit or antimouse Ig antibodies. The abscissa represents the molar ratio between competitor Ig and anti-idiotype.
Competitive binding of IVIgM and heterologous anti-idiotypes to idiotypes of anti-TG autoantibodies. Microtiter plates were coated with F(ab′)2 fragments of IgG expressing the T44, C2, and 1B4 idiotypes; purified from serum of a patient with Hashimoto's thyroiditis; and incubated with 0.1 μg of rabbit anti-T44 IgG (A) or with 0.1 μg of anti-idiotypic monoclonal antibodies 1B4 or 1C2 (B and C, respectively) in the presence of increasing concentrations of IVIgM (▵), monoclonal IgM (•), or IVIg (▪). Bound anti-idiotype was then measured using peroxidase-labeled antirabbit or antimouse Ig antibodies. The abscissa represents the molar ratio between competitor Ig and anti-idiotype.
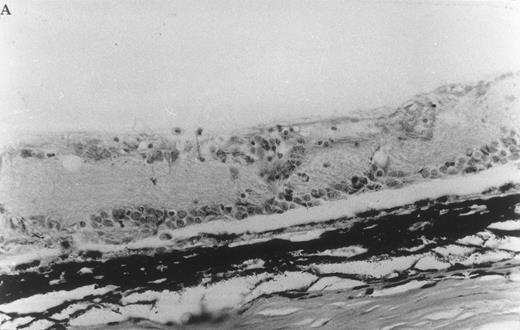
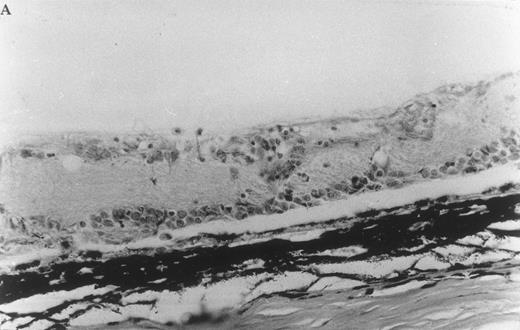
Histologic ocular changes observed in (LEW × BN) F1 rats 35 days after S Ag immunization. (A) In the control rat injected only with S Ag, there is destruction of the photoreceptor cell layer and most of the bipolar cell layer with the presence of inflammatory cells. (B) In a rat treated with IVIgM, there is complete integrity of the retinal structure (Hematoxylin eosin, original magnification × 270).
Histologic ocular changes observed in (LEW × BN) F1 rats 35 days after S Ag immunization. (A) In the control rat injected only with S Ag, there is destruction of the photoreceptor cell layer and most of the bipolar cell layer with the presence of inflammatory cells. (B) In a rat treated with IVIgM, there is complete integrity of the retinal structure (Hematoxylin eosin, original magnification × 270).
Because inhibition of the binding of autoantibodies to autoantigens by IVIgM could be due to interactions between IVIgM and the combining site of autoantibodies or to a competition between IgG autoantibodies and autoantibodies present in the IVIgM preparation, we investigated the ability of IVIgM depleted of natural anti-TG autoantibodies by affinity chromatography to inhibit anti-TG autoantibody activity. IVIgM and anti-TG–depleted IVIgM exhibited similar inhibitory capacity towards the binding of 125I-labeled IVIg to TG (Fig 2). The affinity chromatography-prepared IVIgM that was used was totally depleted in anti-TG activity, as assessed by ELISA (data not shown). On a molar basis, IVIgM was more effective than IVIg in inhibiting anti-TG activity present in IVIg.
Immobilized IVIgM selectively retains IgG autoantibody activity upon affinity chromatography. F(ab′)2 fragments of IgG isolated from the serum of patients with Hashimoto's thyroiditis and patients with anti-FVIII autoimmune disease were subjected to affinity chromatography on IVIgM-Sepharose and on Waldenström IgM-Sepharose, as described in the Materials and Methods. The amount of antibody in the acid-eluted fraction of the IVIgM-Sepharose column was 0.06% and 0.22% of loaded F(ab′)2 fragments of IgG of the patient with thyroiditis and of the patient with anti-FVIII autoantibodies, respectively. Specific autoantibody activity of acid-eluted F(ab′)2 fragments, as determined by ELISA and expressed as absorbance units per microgram, was 128 and 17 times higher than that of unchromatographed F(ab′)2 fragments in the case of anti-TG and anti-FVIII autoantibodies, respectively (Table 2). In contrast, Waldenström IgM-Sepharose affinity column retained only a negligible amount of F(ab′)2 fragments of IgG autoantibodies. Specific activity of eluted F(ab′)2 fragments from Waldenström IgM-Sepharose affinity column was only 1.7 and 0.08 times higher than that of loaded F(ab′)2 fragments in the case of anti-TG and anti-FVIII autoantibodies, respectively (Table 2).
We also subjected IVIg and F(ab′)2 fragments of IVIg to affinity chromatography on Sepharose-bound IVIgM. The amount of IVIg and F(ab′)2 fragments of IVIg in the acid-eluate represented 1.4% and 0.5% (wt/wt) of the loaded material, respectively. IVIg and F(ab′)2 fragments in the eluate expressed higher specific autoantibody activity than unchromatographed IVIg and F(ab′)2 fragments when tested for reactivity with TG, MG, and TF used as self antigens (Fig 3 and data not shown). Specific antibody activity did not differ between the eluate and unchromatographed IVIg in the case of external antigens such as cabbage PLD and spinach PK (Fig 3). The data indicate that IVIgM interacts through variable regions with autoantibodies more strongly than with antibodies to non-self antigens. Nonspecific retention of autoantibodies by the CNBr-activated affinity matrix alone was ruled out by the fact that CNBr-activated Sepharose to which IVIgM was not coupled failed to retain autoantibody activity (data not shown).
IVIgM interacts directly with F(ab′)2 fragments of IgG autoantibodies. The ability of IgM in the pooled preparation of IgM to bind to F(ab′)2 fragments of IgG autoantibodies was examined in ELISA. F(ab′)2 fragments of IgG autoantibodies from two patients with Hashimoto's thyroiditis and two patients with anti-FVIII disease were used as immobilized target antibodies. IVIgM bound in a dose-dependent manner to F(ab′)2 fragments of anti-TG and anti-FVIII IgG autoantibodies (Fig 4). IgM purified from the serum of a patient with Waldenström's macroglobulinemia failed to bind F(ab′)2 fragments of anti-TG and anti-FVIII IgG (Fig 4). These results indicate that the pooled IgM preparation may contain anti-idiotypes directed against idiotypic determinants of disease-associated autoantibodies.
IVIgM competes with heterologous anti-idiotypes for the binding to idiotypes of autoantibodies. To further establish the idiotypic nature of variable region-dependent interactions between IVIgM and IgG autoantibodies, we examined the ability of IVIgM to compete with heterologous anti-idiotypic antibodies for the binding to idiotypes expressed by autoantibodies to FVIII and TG. IVIgM inhibited the binding of monoclonal anti-idiotypic antibody 20F2 to F(ab′)2 fragments of anti-FVIII autoantibodies in a dose-dependent fashion (Fig 5). IVIgM also dose-dependently inhibited the binding of mouse and rabbit anti-idiotypes to F(ab′)2 fragments of Id + IgG from a patient with Hashimoto's thyroiditis (Fig 6). On a molar basis, IVIgM was 10- to 30-fold more effective in displacing heterologous anti-idiotypes than IVIg.
IVIgM prevents the onset of EAU. Six of nine (LEW × BN) F1 rats immunized with S Ag developed significant clinical ocular inflammation. Histologic examination of eyes from these animals showed a complete disappearance of the photoreceptor cell layer of the retina and a partial destruction of the bipolar and ganglion cell layers (Fig 7 and Table 3). From the 9 rats immunized with S Ag and treated with IVIgM, 6 were protected from EAU and the 3 remaining rats developed a clinical EAU significantly milder as compared with the control rats immunized only with S Ag. However, histologic examination showed in these 3 IVIgM-infused rats ocular inflammatory lesions with the same score as in untreated animals (Fig 7 and Table 3). All 5 rats immunized with S Ag and injected with IVIg were protected against EAU (Table 3). In rats immunized with S Ag and treated with either IVIgM or IVIg, infused IgM or IgG had a half life of 14 and 15 days, respectively (data not shown).
DISCUSSION
Administration of pooled normal polyspecific IgG (IVIg) has proven beneficial in the treatment of several autoimmune disorders.13 29 In the present study, we show that pooled normal human IgM purified from plasma of healthy donors (IVIgM) suppresses autoantibody activity of disease-related autoantibodies in vitro. The inhibitory effect of IVIgM was dependent, at least in part, on the presence in IVIgM of anti-idiotypes to the autoantibodies and was shown to be greater or equivalent to that of IVIg on a molar basis. Our findings provide a rationale for considering the use of IgM as immunomodulatory therapy of autoimmune diseases.
We observed that IVIgM dose-dependently inhibited the binding of IgG purified from the serum of patients with SLE, autoimmune thyroiditis, acquired von Willebrand disease, anti-FVIII autoimmune disease, and autoimmune uveitis to their respective target autoantigens. In the case of anti-DNA and anti-TG autoantibodies, IVIgM was shown to block the binding to the self antigens of patients' autoantibodies that had been affinity-purified on Sepharose-bound antigen. Although the amounts of IgM required for inhibition were relatively high under the experimental conditions used in vitro, the molar ratios of IgM to patients' IgG required for inhibition were in the same range as those necessary for inhibition with therapeutic IVIg. There is evidence that the ability of IVIg to inhibit autoantibody activity in vitro is directly correlated with the extent to which autoantibody titer is decreased in vivo after infusion of IVIg.30 31 No inhibition of autoantibody activity was observed when human monoclonal IgM was used, indicating that the inhibitory effect of IVIgM was mediated by variable regions of IgM. IVIgM that was depleted of its rheumatoid factor activity by affinity chromatography on Fc-Sepharose column strongly inhibited the binding of anti-Tg and anti-DNA autoantibodies to their corresponding target antigens. Furthermore, a human monoclonal IgM rheumatoid factor and IgM purified from sera of patients with rheumatoid arthritis did not exhibit inhibitory activity on the autoantibodies tested. The results suggest that the blocking effect of the pooled IgM preparation is not related to the presence of rheumatoid factor activity.
IVIgM that had been depleted in its content in natural anti-TG autoantibodies by affinity chromatography inhibited the binding of anti-TG IgG to TG to the same extent as did unfractionated IVIgM. The latter result showed that the inhibitory effect of IVIgM on autoantibody activity was not dependent on its ability to compete with IgG autoantibodies for the binding to the autoantigen but rather on an interaction between variable regions of IgG and IgM. These conclusions were further supported by the finding that F(ab′)2 fragments of IgG-containing autoantibody activity were selectively retained by IVIgM upon affinity chromatography.
Evidence suggesting the presence of anti-idiotypic antibodies in the pooled preparation of IgM came from the finding that IVIgM bind in a dose-dependent manner to F(ab′)2 fragments of disease-associated IgG autoantibodies from patients with autoimmune conditions. The occurrence of anti-idiotypes in pooled IgM prepared from plasma of large numbers of healthy donors was substantiated by the absence of binding of Waldenström IgM.
We then showed that IVIgM competitively inhibited the binding of mouse and rabbit anti-idiotypic antibodies to their corresponding idiotypes on anti-TG and anti-FVIII IgG autoantibodies, demonstrating that IVIgM contains anti-idiotypic antibodies directed against the autoantibodies. Idiotypic interactions provide a basis for the neutralization of circulating autoantibodies and for downregulation of autoantibody synthesis by pooled normal IgM, as we have previously shown in the case of IVIg.18 On a molar basis, IVIgM was found to be equivalent to or more efficient than IVIg in its ability to suppress autoantibody activity in vitro. The latter finding may relate to the higher extent of polyreactivity of natural IgM antibodies as compared with normal IgG.32
We further demonstrated immunomodulatory properties of IVIgM in vivo using the rat model of EAU. Indeed, as reported for IVIg,33 IVIgM administered at the time of immunization of (LEW × BN) F1 rats with the retinal S antigen prevented the occurrence of EAU. The underlying mechanism in such a protective effect of pooled IgM is currently being investigated.
Serum IgM has been shown to play an important role in controlling the expression of IgG autoreactivity.3,11 Thus, under physiologic conditions, autoreactive IgG autoantibodies, although present, are hardly detectable in whole serum of a healthy individual, whereas high levels of autoreactivity expressed by IgG become apparent when IgG is isolated from serum before being tested for autoantibody activity.3,11,12,34,35 The addition of purified IgM to autologous IgG suppresses IgG-associated autoreactivity.3 Regulation of IgG antibody activity by autologous IgM is preferentially operative in controlling reactivity with self antigens more than it is efficient in the case of antibody reactivities against foreign proteins.3 IgM-dependent regulation of IgG autoreactivity in serum is defective in autoimmune conditions, such as autoimmune thyroiditis and SLE.3,35 In addition, loss of detectable IgG autoantibody activity in the serum of patients in remission of antineutrophil cytoplasmic antigen-positive vasculitis was shown to be associated with the generation of IgM anti-idiotypes directed against the patient's acute-phase IgG autoantibodies, indicating that IgM may suppress pathogenic autoantibodies of the IgG isotype in remission of autoimmune disease.30,36 Testing the reactivity of IgM produced by several Epstein-Barr virus–transformed B-cell lines has shown a high degree of connectivity between normal IgM and variable regions of IgG autoantibodies.37 Variable region-dependent connectivity between Igs, eg, between IgG and autologous IgM or between IgG molecules within the IgG fraction of the serum of an individual, contributes to immune networks and to the maintainance of the homeostasis of autoreactivity under physiologic conditions.2,38 Infusion of relatively small amounts of homologous natural IgM antibodies prevents the development of diabetes in the insulin-dependent diabetes mellitus model of the nonobese diabetic mouse.39 The deviated pattern of use of the VH7183 gene that characterizes the Ig repertoire of adult NOD mice was also shown to be reversed by neonatal treatment with natural IgM monoclonal antibody.40 More recently, it has been shown that polyreactive monoclonal IgM antibodies generated from SJL/J mice injected with normal homogenized spinal cord promote central nervous system remyelination when passively transferred into syngeneic mice chronically infected with Theiler's virus-induced autoimmune murine encephalomyelitis.41 Sequence analysis showed that these monoclonal antibodies were encoded by identical germline Ig light chain and heavy chain genes with no definitive somatic mutations.41 An intravenous Ig preparation enriched in IgM has been shown to be beneficial in post–bone marrow transplantation infections42 and in patients with sepsis.43 Furthermore, IgM-enriched preparations of therapeutic Igs were found to exhibit higher opsonic activity than standard IVIg preparations.44
The present study indicates for the first time that pooled normal IgM shares with IVIg the ability to neutralize autoantibody activity through idiotypic interactions. Serum IgM mostly consists of natural germline-encoded autoantibodies, whereas serum IgG also contains significant amounts of immune antibodies to non-self antigens that may not be of direct relevance to network regulation of autoreactivity. Taken together, our observations provide a rationale for considering normal pooled IgM for immunomodulation of autoimmune disease.
ACKNOWLEDGMENT
The authors thank Nathalie Jouy and Emmanuelle Bonnin for technical assistance and Michel Paing for photography. The authors are greatful to Drs M.C. Bonnet, J.L. Pasquali, and B. Weil for providing us with Fc fragments of IgG, monoclonal IgM, and sera of patients with rheumatoid factor activity.
Supported by Institut National de la Santé et de la Recherche Médicale (INSERM), Laboratoire Français du Fractionnement et des Biotechnologies (LFB), Les Ulis, Centre National de la Recherche Scientifique (CNRS), France, and the Central Laboratory of the Swiss Red Cross (Bern, Switzerland).
Address reprint requests to Srini V. Kaveri, DVM, PhD, INSERM U 430, Hôpital Broussais, 96 rue Didot, 75674 Paris, France.