Abstract
Thrombopoietin (TPO) is implicated as a primary regulator of megakaryopoiesis and thrombopoiesis through binding to the cytokine receptor c-Mpl (the product of the c-mpl proto-oncogene). In an effort to determine the pathophysiological role of TPO-c-Mpl system in essential thrombocythemia (ET), we have examined the levels of serum TPO and the expression and function of platelet c-Mpl in 17 patients with ET. In spite of extreme thrombocytosis, serum TPO levels were slightly elevated or within normal range in most, if not all, patients with ET (mean ± SD, 1.31 ± 1.64 fmol/mL), as compared with normal subjects (0.76 ± 0.21 fmol/mL). Flow cytometric and Western blot analyses revealed that the expression of platelet c-Mpl was strikingly reduced in all patients with ET. Furthermore, the expression of platelet c-mpl mRNA was found to be significantly decreased in the ET patients tested. In contrast, almost identical levels of GPIIb/IIIa protein and mRNA were expressed in platelets from ET patients and normal controls. In addition to expression level, activation state of platelet c-Mpl was investigated in ET patients. Immunoblotting with anti-phosphotyrosine antibody showed that no aberrant protein-tyrosine phosphorylation was observed in platelets of ET patients before treatment with TPO, and the levels of TPO-induced protein-tyrosine phosphorylation, including c-Mpl-tyrosyl phosphorylation, roughly paralleled those of c-Mpl expression, suggesting that c-Mpl–mediated signaling pathway was not constitutively activated in platelets of ET patients. These results suggested that the TPO-c-Mpl system may not be directly linked to pathogenesis of ET, and that gene(s) mutated in ET may be important in regulating the levels of c-mpl gene expression in addition to the growth and differentiation of multipotential hematopoietic stem cells.
ESSENTIAL THROMBOCYTHEMIA (ET) is a chronic myeloproliferative disorder characterized by a persistent increase in circulating platelet count and by the bone marrow hyperplasia with excessive proliferation of megakaryocytes.1-4 In common with the other myeloproliferative disorders such as polycythemia vera and idiopathic myelofibrosis, ET is a clonal disorder of the multipotential hematopoietic stem cells.5 It has been reported that, as compared with normal subjects or patients with secondary thrombocytosis, the culture of peripheral blood or bone marrow of patients with ET gives rise to increased numbers of colony-forming units composed of megakaryocytes (CFU-Meg), suggesting that the abnormal clone may have the preferential responsiveness to regulatory factors that favor its differentiation into the megakaryocyte-platelet lineage.6-8 Furthermore, it is suggested that, although most patients with ET have no cytogenetic abnormalities, the mutation may occur in a single multipotential hematopoietic stem cell, thereby leading to its clonal expansion accompanied by differentiation primarily into platelets.5 In addition to the quantitative change, a variety of functional abnormalities have been described in platelets of ET patients. In a substantial fraction of ET patients, platelets show the reduced or defective aggregation in response to collagen, ADP, and/or epinephrine.1,9-11 By contrast, some patients exhibit platelet hyperaggregation or spontaneous aggregation in vitro.1,11-14 However, none of these abnormalities are specific for ET, and they have not been found to be pathogenically coupled with bleeding and/or thrombotic complications that are major causes of morbidity and mortality in the patients with ET.1-4 Thus, the molecular mechanisms responsible for thrombocythemia and abnormal platelet function are not known.
Thrombopoietin (TPO) is a novel hematopoietic growth factor that regulates megakaryopoiesis and platelet production through binding to its cell surface receptor encoded by the c-mpl proto-oncogene.15 The c-mpl proto-oncogene is expressed in hematopoietic tissues, particularly in CD34+ hematopoietic progenitor cells, megakaryocytes, and platelets.16 The daily infusion of TPO into mice or nonhuman primates has been reported to induce a marked increase in the counts of platelets, megakaryocytes, and megakaryocytic progenitor cells.17,18 In addition, the c-mpl– or TPO-deficient mice generated by homologous recombination have been demonstrated to exhibit a striking reduction in the numbers of platelets, megakaryocytes, and megakaryocytic progenitor cells.19-21 These findings indicate that the TPO-c-Mpl (c-Mpl: c-mpl protein) system is a physiological regulator of megakaryocyte and platelet production. Furthermore, it has recently been suggested that the TPO-c-Mpl system may influence the function of platelets. For example, the pretreatment of platelets with TPO was shown to augment their aggregation induced by various agonists such as ADP, thrombin, collagen, and adrenaline, although TPO by itself had little or no effect on platelet aggregation.22-26 Because of the potential activity of the TPO-c-Mpl system in regulating both production and function of platelets, it is important to determine if the TPO-c-Mpl system is involved in some aspect of abnormal growth and function of platelets in the patients with ET. In this study, therefore, we examined the serum concentration of TPO and the expression and function of platelet c-Mpl in a series of patients with ET.
MATERIALS AND METHODS
Reagents and antibodies. Highly purified rhTPO was provided by Kirin Brewery Company Ltd (Tokyo, Japan). Prostaglandin E1 (PGE1) was purchased from Sigma (St Louis, MO). Rabbit anti–c-Mpl IgG was purified by using a protein A Sepharose (Pharmacia Biotech, Uppsala, Sweden) from antiserum against extracellular domain of c-Mpl.27 The specificity of this antibody was confirmed by examining its binding to 32D/c-mpl cells (32D cells stably transfected with a human c-mpl expression vector) but not to parental 32D cells (a murine IL-3 dependent myeloid cell line lacking c-mpl expression) on flow cytometric analysis. Anti-phosphotyrosine, a murine monoclonal antibody (MoAb) generated against phosphotyramine, was supplied by Dr B. Drucker (Oregon Health Science University, Portland, OR). AP2 MoAb that recognizes GPIIb/IIIa complex and rabbit anti-GPIIb/IIIa antiserum were generously provided by Dr T. Kunicki (Scripps Research Institute, La Jolla, CA).28
Patients and platelet preparation. Peripheral blood samples were obtained from 17 patients with ET, one patient with Ph1-positive chronic myelogenous leukemia (CML) and five healthy volunteers after informed consent was given. The diagnosis of ET was made according to the clinical and laboratory criteria established by Polycythemia Vera Study Group.29 The CML patient was accompanied with thrombocytosis (967 × 103/μL). Washed platelets were prepared as previously described.30 In brief, 5 vol of freshly drawn venous blood was mixed with 1 vol of acid/citrate/dextrose (ACD)-A solution (2.2% [wt/vol] sodium citrate, 0.8% citric acid, and 2.2% glucose, pH 4.5-5.5) and 50 ng/mL PGE1, and was centrifuged at 250g for 10 minutes to obtain platelet-rich plasma (PRP). The PRP was spun at 800g to form a soft platelet pellet, and the platelets were washed twice by citrate buffer (0.05 mol/L sodium citrate, 0.1 mol/L NaCl, and 0.14 mol/L glucose, pH 6.2) and resuspended in a modified HEPES-Tyrode buffer (137 mmol/L NaCl, 2.7 mmol/L KCl, 11.9 mmol/L NaHCO3, 0.42 mmol/L NaH2PO4, 1.0 mmol/L MgCl2, 556 mmol/L glucose, 5.0 mmol/L HEPES, and 1 mmol/L CaCl2, pH 7.35) at a concentration of 2.22 × 106/μL.
Enzyme-linked immunosorbent assay (ELISA). Serum concentrations of TPO in the patients with thrombocytosis and healthy volunteers were measured by a solid-phase “sandwich” ELISA, as previously reported.31 32
Flow cytometry. Surface antigens of platelets were examined with the indirect immunofluorescent method, as previously described.33 Briefly, platelets were incubated first with 0.8 μL of anti–c-Mpl IgG or AP2 MoAb at 4°C for 30 minutes, rinsed, and developed with fluorescein isothiocyanate (FITC)-conjugated F(ab)′2 goat antirabbit IgG or antimouse IgG (Organon Teknika Corp, West Chester, PA, and Sigma, respectively). The platelets were then rinsed and analyzed on FACScan (Becton Dickinson). The log fluorescence intensity was examined by reference to rabbit control IgG or nonspecific isotype control MoAb.
Immunoblotting. The platelets were lysed in lysis buffer (20 mmol/L Tris-HCl pH 8.0, 137 mmol/L NaCl, 10% glycerol, 1% NP-40, 10 mmol/L EDTA, 100 mmol/L sodium fluoride) containing protease and phosphatase inhibitors, as described previously.33 Insoluble material was removed by centrifugation at 10,000g for 20 minutes at 4°C. In some experiments, platelets were stimulated with various concentrations of rhTPO for 10 minutes at 37°C or with 200 ng/mL of rhTPO for 0 to 240 minutes, and then lysed in lysis buffer. The procedures of gel electrophoresis and immunoblotting were performed according to the methods described previously.33 Briefly, the lysates (15 μg per each lane) were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then electrophoretically transferred onto a polyvinylidene difluoride membrane (Immobilon, Millipore Corp, Bedford, MA). After blocking residual binding sites on the filter by incubation in TBS (10 mmol/l Tris-HCl pH 8.0, 150 mmol/l NaCl) containing 1% gelatin (Bio-Rad Laboratories, Richmond, CA), immunoblotting was performed with anti–c-Mpl or anti-GPIIb/IIIa antiserum, or with anti-phosphotyrosine MoAb. Immunoreactive proteins were visualized with the enhanced chemiluminescence detection system (DuPont NEN, Boston, MA).
Detection of c-mpl mRNA by Northern blot and RT-PCR analyses. Isolation of total cellular RNA and RT-PCR analysis was performed as previously described.34 For Northern blot analysis, equal amounts of RNA (4 μg) were electrophoresed through 1.0% formaldehyde agarose gels. After blotting to the nylon membranes, the filters were prehybridized and then hybridized with random 32P-labeled probe in rapid hybridization buffer (Amerciam, Tokyo, Japan) for 2 hours at 65°C. The probe for c-mpl was 170 bp of SphI-NcoI fragment (nucleotides 253 to 423) obtained from PCR product,34 and the probe for GPIIb was full-length of cDNA kindly provided by Dr Mortimer Poncz (The Childrens Hospital of Philadelphia, Philadelphia, PA). The filters were washed and autoradiographed at −70°C with two intensifying screens.
For RT-PCR analysis, each of the total cellular RNA samples were subjected to reverse-transcription in batched and parallel reactions. In short, equal amounts of total cellular RNA (2.5 μg) were reverse-transcribed with oligo dT primers (Pharmacia, Piscataway, NJ) at 37°C for 60 minutes in a final volume of 50 μL with 200 U of Moloney leukemia virus reverse transcriptase (GIBCO BRL, Gaithersburg, MD) according to the manufacturer's instruction. The cDNA product (1.5 μL) was resuspended in a total volume of 30 μL containing 0.75 U of Taq Gold DNA polymerase (Perkin Elmer, Foster City, CA), 2 mmol/L MgCl2, 200 μmol/L dNTP mix, 15 pmol of forward and reverse primers, and 1× reaction buffer provided by the manufacturer. To amplify P-form of c-mpl cDNA,35 we used sense primer (5′-TGGCTGCAGCTGCGCAGCGAACCT-3′, nucleotides 757 to 780) in combination with reverse primer (5′-TCAAGGCTGCTGCCAATAGCTTAG-3′, nucleotides 1885 to 1908).34 GPIIb cDNA was amplified with a sense primer (5′-GAGCTGCAGATGGACGCAGCCAAC-3′, nucleotides 1988 to 2011) and a reverse primer (5′-CAGGAAGGCCAGCACCGTGACCATG-3′, nucleotides 2797 to 2821).36 The samples were denatured for 5 minutes at 94°C, followed by 20 to 34 cycles of amplification (94°C, 30 seconds for denaturation; 55°C, 30 seconds for annealing; 72°C, 90 seconds for extension). Aliquots (15 μL) were size fractionated on 1.0% agarose gels containing ethidium bromide to examine the size and amount of PCR products.
RESULTS
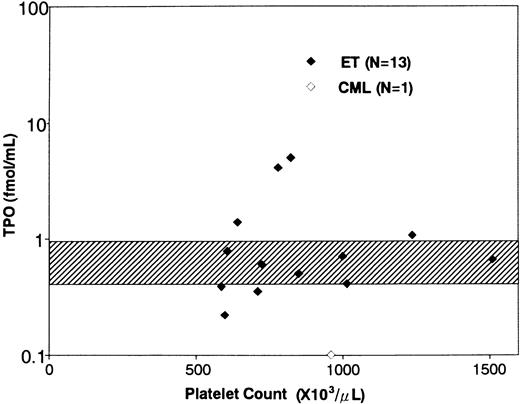
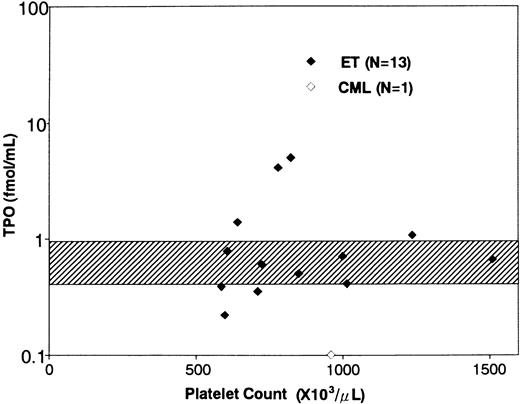
Clinical characteristics and serum TPO levels of the patients with ET. The clinical characteristics and serum TPO levels of the patients enrolled in this study are shown in Table 1. In a total of 17 ET patients, the platelet counts ranged from 539 to 2,000 × 103/μL (mean ± SD, 960 ± 378 × 103/μL). The duration of the disease ranged from 0.5 to 24 years (mean ± SD, 5.34 ± 5.79). Six patients (patient no. 5, 7, 9, 13, 14, and 17) had thrombotic episodes such as angina, acute myocardial infarction, and transient ischemic attack, while two (patient no. 12 and 15) manifested hemorrhagic symptoms. The other nine patients were free from symptom during the disease period. Fourteen patients were prophylactically treated with anti-platelet agents and/or hydroxyurea, and the remaining three were not medicated. Serum TPO levels in ET patients ranged from 0.22 to 5.20 fmol/mL (1.31 ± 1.64 fmol/mL, mean ± SD); the mean value of ET patients was slightly higher than that of normal subjects (0.76 ± 0.21 fmol/mL, n = 21, mean ± SD),31 although there was no statistically significant difference between the two groups (Table 1 and Fig 1). In addition, the TPO levels did not positively or negatively correlate with the platelet counts in ET patients (Fig 1), whereas serum TPO level in a patient with CML was extremely low (0.10 fmol/mL). In a preliminary experiment, furthermore, we noted that serum TPO level of an ET patient increased after treatment with hydroxyurea (before treatment: platelet count 78.1 × 103/μL, serum TPO level 2.24 fmol/mL; after treatment: platelet count 39.2 × 103/μL, serum TPO level 4.61 fmol/mL).
Relationship between serum TPO levels and platelet counts. The diamonds indicate the serum TPO levels of ET patients (♦) and a CML patient (⋄). The shaded area indicates serum TPO levels in normal subjects (mean ± SD).
Relationship between serum TPO levels and platelet counts. The diamonds indicate the serum TPO levels of ET patients (♦) and a CML patient (⋄). The shaded area indicates serum TPO levels in normal subjects (mean ± SD).
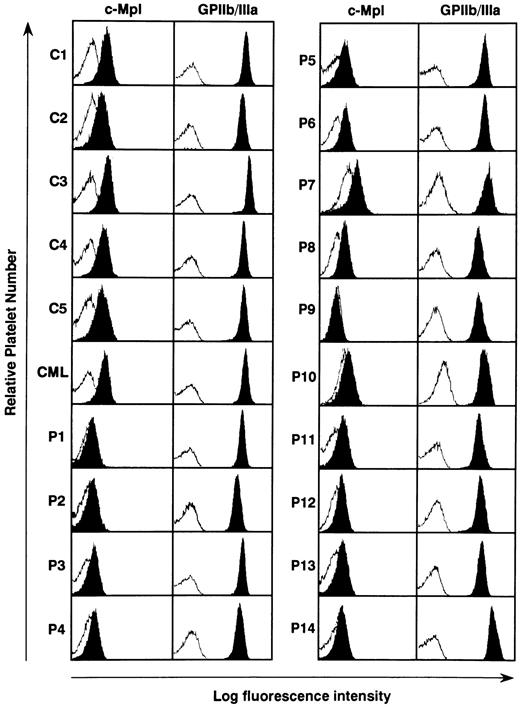
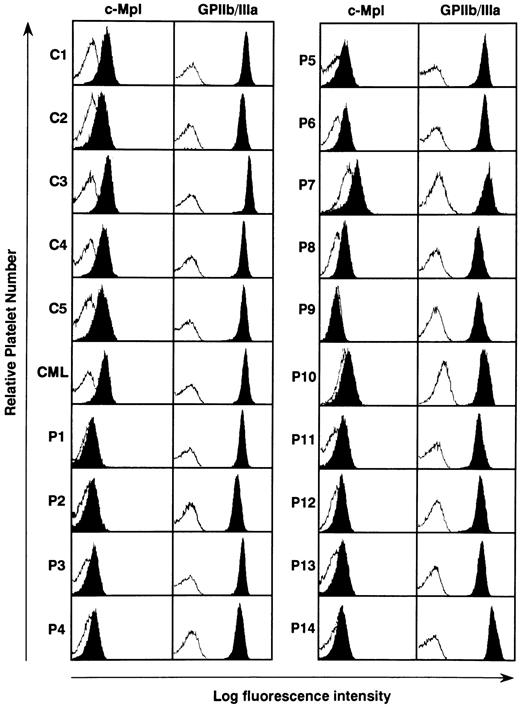
Expression of c-mpl protein in the platelets from ET patients. The serum TPO level has been reported to be regulated by masses of platelets and megakaryocytes, both of which express c-Mpl on their surface.36-40 We therefore examined the c-Mpl expression on the platelets prepared from 14 ET patients, five normal controls, and one CML patient. Flow cytometric analysis showed that when compared with normal controls and a CML patient surface expression levels of c-Mpl on platelets were markedly reduced in all of ET patients, although a patient-to-patient variation in the fluorescence intensity was noted (Fig 2). Especially in three patients with ET, furthermore, c-Mpl expression was scarcely detectable on platelets (P1, P9, and P10). In contrast to the decreased expression of c-Mpl, there was no significant difference in the expression level of GPIIb/IIIa antigen on platelets between ET patients, normal subjects, and the CML patient (Fig 2).
Flow cytometric analysis of c-Mpl and GPIIb/IIIa expression on the platelets from 14 ET patients (P1 to P14), one CML patient (CML), and four normal controls (C1 to C4). The expression levels of c-Mpl and GPIIb/IIIa was examined by staining with rabbit anti–c-Mpl antiserum and AP2 MoAb, respectively (solid). The log fluorescence intensity was examined by reference to nonspecific isotype control MoAb (open).
Flow cytometric analysis of c-Mpl and GPIIb/IIIa expression on the platelets from 14 ET patients (P1 to P14), one CML patient (CML), and four normal controls (C1 to C4). The expression levels of c-Mpl and GPIIb/IIIa was examined by staining with rabbit anti–c-Mpl antiserum and AP2 MoAb, respectively (solid). The log fluorescence intensity was examined by reference to nonspecific isotype control MoAb (open).
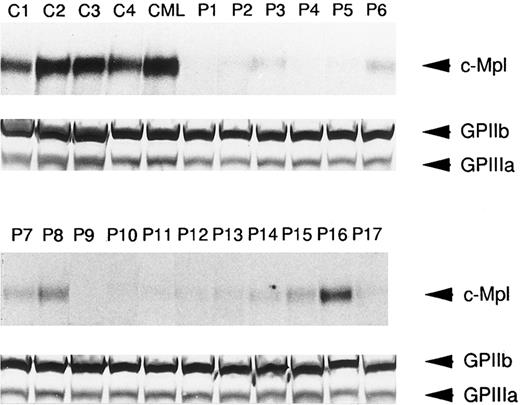
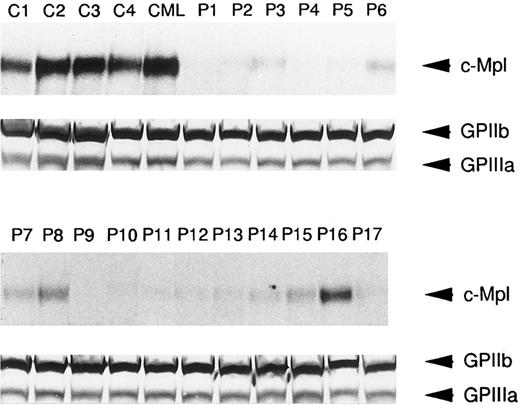
In order to further determine if platelet c-Mpl expression was reduced in ET patients, immunoblots with anti–c-Mpl or anti-GPIIb/IIIa antisera were performed in 17 ET cases as well as four normal subjects and one CML case. As shown in Fig 3, the expression of c-Mpl with an approximate molecular mass of 82 kD was easily detectable in lysates of normal and CML platelets. In contrast, significantly reduced expression of c-Mpl was observed in the lysates of platelets from the 17 patients with ET, whereas GPIIb and GPIIIa were expressed in the lysates of normal, CML, and ET platelets at a similar level (Fig 3). This observation was consistent with the findings on flow cytometric analysis, and in most cases the intensity of c-Mpl band in the immunoblot roughly corresponded with the fluorescence intensity in the flow cytometry.
Western blot analysis of c-Mpl and GPIIb/IIIa expression in the platelets from ET patients (P1 to P17), a CML patient (CML), and normal controls (C1 to C4). The platelet lysates (15 μg each lane) were separated by SDS-PAGE and probed with either anti-c-Mpl or anti-GPIIb/IIIa antiserum.
Western blot analysis of c-Mpl and GPIIb/IIIa expression in the platelets from ET patients (P1 to P17), a CML patient (CML), and normal controls (C1 to C4). The platelet lysates (15 μg each lane) were separated by SDS-PAGE and probed with either anti-c-Mpl or anti-GPIIb/IIIa antiserum.
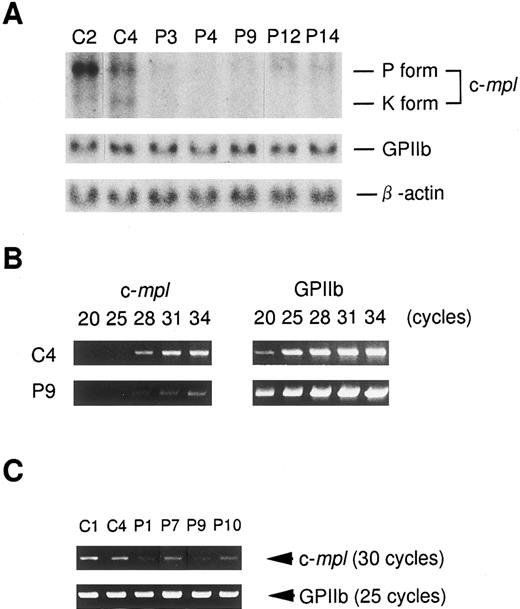
Expression level of c-mpl mRNA in the platelets from ET patients. In an effort to clarify the mechanism of low c-Mpl expression in the platelets from ET patients, we investigated the expression of c-mpl, GPIIb, and β-actin mRNA by means of Northern blot and semi-quantitative RT-PCR analyses. Consistent with the previous findings on nucleated cells such as megakaryocytic and erythroid leukemia cell lines,35 Northern blot analysis revealed two types of c-mpl mRNA transcripts, a major 3.7-kb mRNA (P form) and a minor 2.7-kb (K form), in normal platelets (Fig 4A). In platelets from five patients with ET, the expression of c-mpl mRNA was significantly decreased in three patients (P3, P4, and P9), and modestly in two patients (P12 and P14), while similar levels of GPIIb or β-actin mRNA expression were observed in platelets from normal controls and ET patients (Fig 4A). In the case of other ET patients from which sufficient amounts of mRNA could not be obtained, the expression levels of c-mpl mRNA was examined by a semi-quantitative RT-PCR analysis. As shown in upper panels of Fig 4B, both c-mpl and GPIIb cDNAs from normal platelets (C4) were amplified exponentially during 20 to 34 cycles of PCR; at each cycle of PCR, significantly less c-mpl cDNA was detected in the platelets of an ET patient (P9), while no significant difference was observed in the intensity of GPIIb bands between normal (C4) and ET (P9) samples. Furthermore, when the expression levels of c-mpl and GPIIb were assessed at 30 and 25 cycles of PCR, respectively, the platelets of ET patients (P1, P7, P9, and P10) were found to express a lower dose of c-mpl transcripts than those of normal controls (C1 and C4) (Fig 4C).
Northern blot (A) and semi-quantitative RT-PCR analysis (B, C) of c-mpl and GPIIb mRNA expression in the platelets from ET patients (P1, P3, P4, P7, P9, P10, P12, and P14) and normal controls (C1, C2, and C4). (A) Four micrograms of total cellular RNA obtained from the platelets was electrophoresed in 1% agarose gels. The blot was hybridized with 32P-labeled probe for c-mpl and then rehybridized with probes for GPIIb and β-actin. (B) Two-and-a-half micrograms of total cellular RNA was reverse-transcribed with oligo dT primers. Each of 1.5 μL of the cDNA product was amplified at various (20 to 34) cycles of PCR. (C) Expression levels of c-mpl and GPIIb mRNA was evaluated at 30 and 25 cycles of PCR, respectively.
Northern blot (A) and semi-quantitative RT-PCR analysis (B, C) of c-mpl and GPIIb mRNA expression in the platelets from ET patients (P1, P3, P4, P7, P9, P10, P12, and P14) and normal controls (C1, C2, and C4). (A) Four micrograms of total cellular RNA obtained from the platelets was electrophoresed in 1% agarose gels. The blot was hybridized with 32P-labeled probe for c-mpl and then rehybridized with probes for GPIIb and β-actin. (B) Two-and-a-half micrograms of total cellular RNA was reverse-transcribed with oligo dT primers. Each of 1.5 μL of the cDNA product was amplified at various (20 to 34) cycles of PCR. (C) Expression levels of c-mpl and GPIIb mRNA was evaluated at 30 and 25 cycles of PCR, respectively.
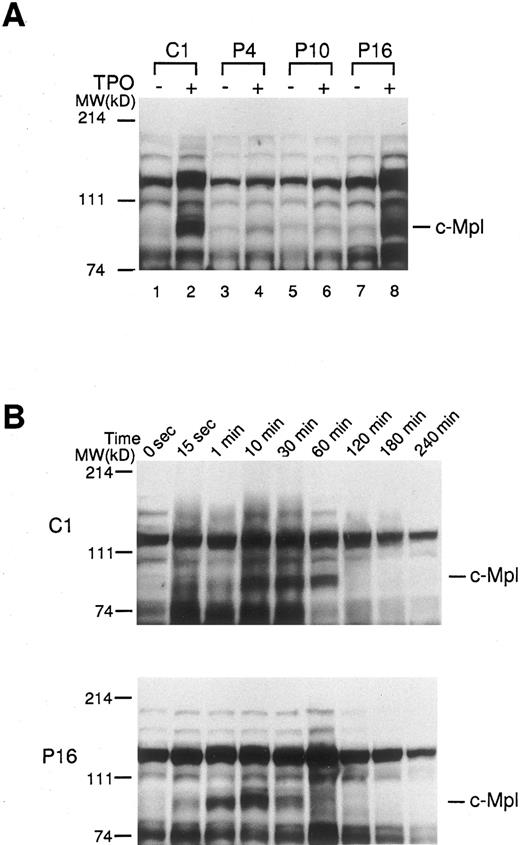
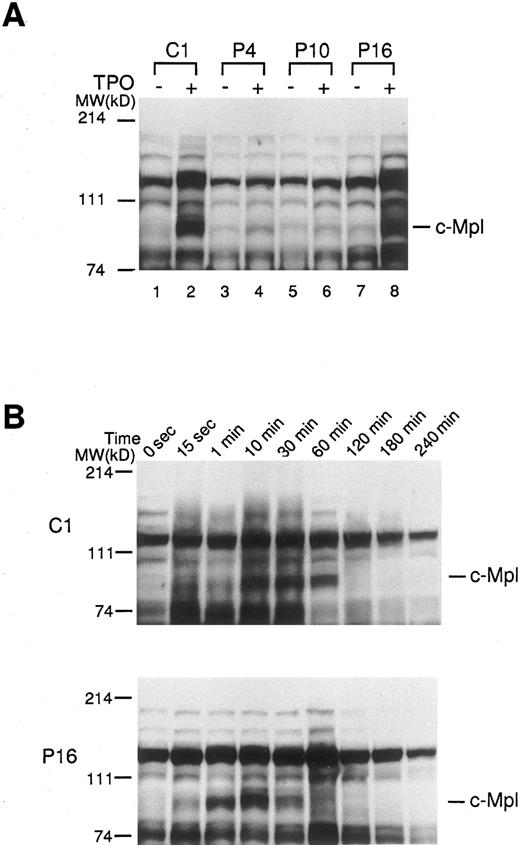
Effect of TPO on protein tyrosine phosphorylation in platelets of ET patients. In order to further determine the activation state of c-Mpl–mediated signaling molecules, platelets from ET patients and normal controls were treated with rhTPO (200 ng/mL) for 10 minutes, and changes in protein tyrosine phosphorylation were detected by Western blotting with anti-phosphotyrosine MoAb. Before treatment with rhTPO, the patterns of protein-tyrosine phosphorylation detected in normal and ET platelets were similar; and aberrant types of protein-tyrosine phosphorylation were not detected in any of ET samples tested (Fig 5A). When normal platelets (C1) were stimulated with rhTPO, increased phosphotyrosine was observed in proteins, particularly at molecular weights 82 kD corresponding to c-Mpl, after exposure to rhTPO (Fig 5A). By contrast, the rhTPO treatment led to only a minimal change in tyrosine phosphorylation pattern in two ET cases (P4 and P10), reflecting a marked decrease in c-Mpl expression. In an ET case (P16) showing a considerable level of c-Mpl expression, tyrosine phosphorylation pattern was almost identical to that observed in normal platelets (Fig 5A). Furthermore, we investigated the time course and dose response of rhTPO-induced protein-tyrosine phosphorylation in platelets from a normal subject (C1) and an ET patient (P16). The kinetics of protein-tyrosine phosphorylation induced by rhTPO were similar in the normal and ET samples (Fig 5B); and the protein-tyrosine phosphorylation was dose dependent, with readily detectable activity at 10 ng/mL rhTPO and maximum activity at 100 ng/mL in both samples (data not shown). These results suggested that c-Mpl–mediated signaling cascade was not constitutively activated in platelets from patients with ET.
Tyrosine phosphorylations of platelet proteins before and after the stimulation with rhTPO. (A) The platelets from three ET patients (P4, P10, and P16) and one normal control (C1) were stimulated with rhTPO (200 ng/mL) for 10 minutes. The lysates were separated by SDS-PAGE and probed with anti-phosphotyrosine MoAb. (B) The platelets from an ET patient (P16) and a normal control (C1) were stimulated with rhTPO (200 ng/mL) for the time indicated. The lysates were subjected to SDS-PAGE and the changes in tyrosine phosphorylation were analyzed by probing with anti-phosphotyrosine MoAb.
Tyrosine phosphorylations of platelet proteins before and after the stimulation with rhTPO. (A) The platelets from three ET patients (P4, P10, and P16) and one normal control (C1) were stimulated with rhTPO (200 ng/mL) for 10 minutes. The lysates were separated by SDS-PAGE and probed with anti-phosphotyrosine MoAb. (B) The platelets from an ET patient (P16) and a normal control (C1) were stimulated with rhTPO (200 ng/mL) for the time indicated. The lysates were subjected to SDS-PAGE and the changes in tyrosine phosphorylation were analyzed by probing with anti-phosphotyrosine MoAb.
DISCUSSION
Hematopoiesis is tightly regulated by a number of hematopoietic growth factors that positively or negatively modulate the survival, proliferation, and differentiation of hematopoietic stem/progenitor cells. It has been reported that the dysregulated expression of the factors and/or their cognate receptors could result in excessive proliferation and aberrant differentiation of hematopoietic cells.41 In the case of TPO-c-Mpl system, c-mpl mRNA was found to be expressed in about half of samples from patients with acute myeloblastic leukemia (AML) regardless of its French-American-British (FAB) classification.34 In addition, we have shown that rhTPO-stimulated proliferation of AML cells in approximately 70% of c-mpl–positive cases, and it could induce megakaryocytic differentiation of AML cells, albeit in a limited number of cases.27 34 These findings suggested that TPO-c-Mpl system might contribute, at least in part, to abnormal growth and differentiation of AML cells. However, it is not known yet how TPO-c-Mpl system is involved in pathophysiology of ET.
It has been hypothesized that serum TPO levels are mainly controlled by platelet and/or megakaryocyte mass through c-Mpl–mediated uptake and metabolism.36-39 This hypothesis was supported by the findings that TPO gene expression was constant in the liver in which TPO is primarily produced, and that serum levels of TPO were inversely related to the numbers of circulating platelets and of bone marrow megakaryocytes.38,39 However, the present study demonstrated that serum TPO levels in most patients with ET were similar to or slightly higher than those in normal controls. In addition, there was no significant correlation between serum TPO levels and platelet counts in ET patients. These results were largely comparable with the previous report,32 and suggested that almost normal or slightly elevated levels of TPO in ET patients might be due to overproduction of TPO or reduced expression of c-Mpl, or both. In view of clonal origin of thrombocythemia, however, overproduction of TPO was unlikely to be involved in pathophysiology of ET. We therefore focused on the expression of c-Mpl on platelets in a series of ET patients. Both flow cytometric and Western blot analyses revealed that c-Mpl expression were markedly reduced in platelets from all of ET patients, although the reduction levels varied from case to case. In addition to c-Mpl protein, the expression of c-mpl mRNA transcripts was found to be severely or moderately decreased in platelets prepared from ET patients. By contrast, there was no significant difference in the expression levels of GPIIb/IIIa protein and mRNA between platelets of ET patients and normal controls. These findings suggested that quantitative reductions in the levels of c-Mpl expression in ET patients may result, at least partially, from a defect in transcription and/or stability of c-mpl mRNA, but not from internalization and degradation of c-Mpl by TPO. Furthermore, it was suggested that almost normal or slightly elevated levels of serum TPO in ET patients may be attributable to the low c-Mpl expression through impaired uptake and catabolism of TPO.
The c-mpl proto-oncogene was originally identified as the cellular homologue of oncogene v-mpl transduced in the myeloproliferative leukemia retrovirus.35 Furthermore, various types of growth factor receptors have been reported to function as oncogenic proteins, especially by constitutively activating mutations, and to play causal roles in cell transformation.42,43 In addition, autonomous CFU-Meg formation from peripheral blood cells from ET patients but not from normal subjects have been observed in the previous studies.6-8 These lines of evidence raised the possibility that constitutively activating mutations of c-mpl might lead to excessive growth of megakaryocytes and platelets in ET. To test this possibility, changes in protein-tyrosine phosphorylation was investigated in platelets before and after treatment with TPO, because platelets and nucleated cells were reported to bear almost identical sets of downstream targets of c-Mpl, including Janus family of protein tyrosine kinases (JAKs), signal transducers and activators of transcription (STATs), phosphatidylinositol 3 kinase (PI3 kinase) and Shc.22-26 44-47 Immunoblotting analysis with anti-phosphotyrosine MoAb showed that no aberrant pattern of protein-tyrosine phosphorylation was observed in platelets of ET patients, and that tyrosine phosphorylation of c-Mpl and its downstream targets was induced in a ligand-dependent manner, although its level was decreased in ET cases, reflecting the reduced levels of c-Mpl expression. These results suggested that c-Mpl–mediated signaling was not constitutively activated in platelets of ET patients, and that constitutively activating mutations of c-mpl may rarely contribute to the autonomous CFU-Meg formation from peripheral blood cells from ET patients and to development of ET.
Although regulatory mechanisms of c-mpl expression are not clearly elucidated, the promoter regions of both human and murine c-mpl genes are known to have putative binding motifs for GATA and Ets family proteins.48 Deveaux et al have demonstrated that, despite constitutive binding of both GATA-1 and Ets proteins, Ets-binding site is a crucial cis-regulatory element in transactivation of the c-mpl promoter in a human c-mpl–positive erythroleukemia cell line, HEL, whereas GATA-binding site has only a partial effect on c-mpl gene expression.48 Lemarchandel et al have shown that GATA- and Ets-binding motifs are also found in the promoter of human GPIIb gene, and that ectopic overexpression of either GATA-1, Ets-1, or Ets-2 can actually transactivate the GPIIb promoter, suggesting functional equivalence and redundancy of GATA-1 and Ets proteins in transactivation of GPIIb gene.49 Since c-mpl mRNA, but not GPIIb mRNA, is downregulated in platelets of ET patients, it is possible that genetic abnormality associated with development of ET may impair the expression or function of Ets protein, and thereby lead to downregulation of c-mpl expression. In addition to c-mpl, furthermore, a variety of molecular defects such as reduced α-adrenergic receptors, 12-lipoxygenase deficiency, reduced prostaglandin D2 receptors and increased Fc receptors50-53 have been described in platelets from ET patients. Therefore, it is also possible that gene(s) mutated in ET might be important for regulating the expression of multiple gene products in platelets.
In summary, the results presented here show that the expression of c-mpl protein and mRNA is dramatically reduced in platelets from patients with ET, and suggest that the low c-Mpl expression may account for normal or slightly elevated levels of serum TPO in patients with ET. By using a cDNA library prepared from ET platelets, current and future efforts are directed to identify gene(s) that downregulate c-mpl expression. It is hoped that a better understanding of the molecular processes that regulate c-mpl expression will lead to greater insights into the pathogenesis of ET.
Supported in part by grants from the Ministry of Education, Science and Culture, the Inamori Foundation, the Senri Life Science Foundation, and the Mochida Memorial Foundation.
Address reprint requests to Yuzuru Kanakura, MD, PhD, The Department of Hematology and Oncology, Osaka University Medical School, 2-2, Yamada-oka, Suita, Osaka 565, Japan.