Abstract
The relationship between differentiation of human myeloid cells and apoptosis remains unclear. Recent studies have shown that terminal differentiation need not necessarily lead to the apoptotic demise of myeloid cells, while other studies have shown that induction of differentiation is associated with increased resistance to apoptosis-inducing agents, such as chemotherapy and γ-irradiation. Such results are pertinent to the treatment of acute myeloid leukemia (AML) and myelodysplastic syndrome, where differentiating agents and hemopoietic growth factors are being combined with chemotherapy to enhance response and limit toxicity. To elucidate the factors governing apoptosis in human AML blasts, we have studied the cytotoxic effect of idarubicin on HL60, U937 and KG1 cells, after incubation with all-trans retinoic acid (ATRA), 1,25(OH)2 D3, and granulocyte-macrophage colony-stimulating factor (GM-CSF ). We show that prior incubation of human myeloid leukemic cells with ATRA or 1,25(OH)2 D3 induced resistance to idarubicin-induced apoptosis, which was modulated by coincubation with GM-CSF. The altered chemosensitivity of cells depended on the degree of G0/G1 cell-cycle arrest induced by incubation with ATRA, 1,25(OH)2 D3, and GM-CSF and was independent of differentiation status or Bcl-2 oncoprotein expression. These findings suggest that cell-cycle arrest in human leukemic cells can be induced by exogenous agents and may promote drug resistance. Determining the mechanisms by which cell-cycle arrest is induced may permit understanding of the processes by which the cells escape cytotoxic drug-mediated apoptosis.
ACUTE MYELOID leukemia (AML) remains incurable in the majority of patients, largely due to resistance to chemotherapy. Ten percent to 20% of patients have de novo refractory disease, while greater than 40% of those who attain remission subsequently relapse.1 The first described and best characterized mechanism of resistance is the mdr1 gene product, P-glycoprotein (Pgp). This molecule spans the cell membrane and acts as an efflux pump for toxins, including chemotherapy drugs such as anthracyclines, vinca alkaloids, and topoisomerase II inhibitors.2 Unfortunately, Pgp expression is often induced by chemotherapy and cross-resistance develops to drugs not previously used,3 which severely limits therapeutic options in refractory and relapsed cases of AML.4 Such “multiple drug resistance” (MDR) has now been described in relation to a large number of other mechanisms, involving Pgp-like membrane transporters, cytoplasmic detoxification enzymes and the cellular machinery for DNA repair and apoptosis (programmed cell death).5,6 Furthermore, redundancy is apparent amongst MDR mechanisms, which limits the benefits of MDR modulation therapy.2 7-9
The clinical limitations of the chemotherapeutic approach in AML have led to the assessment of adjuvant therapies. The underlying biology, with a block in the normal myeloid differentiation program, has led to the use of differentiating agents, either alone, or in combination with chemotherapy.10,11 In vitro studies have shown that all-trans retinoic acid (ATRA) induces differentiation of human AML cell lines, with morphological and functional changes accompanied by a loss of proliferative capacity.12,13 Differentiation responses of blasts from AML patients have been restricted to those with acute promyelocytic leukemia (APL),14 while bone marrow samples from patients with myelodysplasia (MDS) have shown increased myeloid colony formation and the development of increasingly mature granulocytic cells when liquid marrow cultures are incubated with 13-cis retinoic acid.15 ATRA has proven effective in remission induction of APL, however, these remissions are not sustained and chemotherapy is required for durable remission.16,17 Where ATRA is effective, it acts by inducing apoptosis in the leukemic clone.18
Although the active metabolite of vitamin D, 1,25 dihydroxyvitamin D3 (1,25(OH)2 D3) induces monocytic differentiation of HL60 and U937 cells, cell lines such as KG1 generally respond less well to 1,25(OH)2 D3.19 Bone marrow cells from patients with myeloid leukemia have again shown encouraging in vitro responses with monocytoid differentiation and a decrease in myelo/monoblasts induced by various vitamin D analogues.19 Sadly, such responses have not been translated into clinical benefit: in vivo use of 1,25(OH)2 D3 is associated with dose-limiting hypercalcemia,20 and studies combining retinoids and/or vitamin D analogs with low-dose chemotherapy in MDS have failed to produce useful responses in other than occasional cases.21
The relationship between differentiation of human myeloid cells and apoptosis remains unclear. Terminal differentiation of myeloid cells can be associated with apoptosis; Martin et al have shown that HL60 cells cultured with ATRA differentiate and die by apoptosis after 7 to 10 days.22,23 However, two studies have shown that terminal differentiation need not necessarily lead to apoptotic demise of myeloid cells: HL60 cells transfected so as to constitutively overexpress Bcl-2 showed morphological, functional, and phenotypic evidence of differentiation when cultured with ATRA, but remained viable for up to 40 days, with no evidence of apoptosis.24 25
It is not clear what effect differentiation-induction has on the sensitivity of leukemic cells to undergo apoptosis in response to cytotoxic agents, or indeed, whether there is a rationale for using differentiating agents in combination with chemotherapy. If the prevalence of spontaneous apoptosis increases as a result of maturation, then it is possible that sensitivity to cytotoxic drugs would also increase. Previous studies have shown that induction of differentiation is associated with increased resistance to agents such as etoposide, camptothecin, and azacytidine26-30; however, these studies provide conflicting data on the determination of such cytoprotection.
To elucidate the factors governing apoptosis in human AML blasts, we have studied the cytotoxic effect of idarubicin on the AML cell lines HL60, U937 and KG1, after incubation with ATRA, 1,25(OH)2 D3, and GM-CSF. Idarubicin was chosen as it is an anthracycline, which is extensively used in the treatment of AML31 and because it is less affected by Pgp expression than other anthracyclines.32,33 Idarubicin also has the advantage of being available for oral administration, which is often appropriate for the treatment of elderly patients with AML and MDS, as a part of combination treatment regimens.34 35
We show that prior incubation of human myeloid leukemic cells with ATRA or 1,25(OH)2 D3 induced resistance to idarubicin-induced apoptosis, which was modulated by coincubation with GM-CSF. The altered chemosensitivity of cells depended not on differentiation or Bcl-2 oncoprotein expression, but rather on the degree of G0/G1 cell-cycle arrest induced by incubation with ATRA, 1,25(OH)2 D3, and GM-CSF. These findings suggest that differentiating agents may interact in vivo with cytotoxic drugs and hemopoietic growth factors to determine apoptotic cell-death of AML blasts. Such interactions may explain mechanisms by which AML blasts become constitutively resistant to chemotherapy, and thus help to develop treatment strategies to overcome such resistance.
MATERIALS AND METHODS
Cells and reagents. HL60, U937, and KG1 cells were obtained from the European Collection of Animal Cell Cultures (Salisbury, UK). Reagents were purchased from Sigma Chemical Co (Poole, UK). Cells were cultured at 37°C with CO2 enriched to 5% in RPMI, supplemented with 10% fetal calf serum, 2 mmol/L glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. ATRA (Sigma) and 1,25(OH)2 D3 (the kind gift of Dr Kay Colston, St Georges Hospital Medical School, London, UK) were dissolved in ethanol to achieve stock solutions of 1 mmol/L and 20 μmol/L, respectively; these were stored at −20°C until required. The final concentration of ethanol in cultures did not exceed 0.001 vol/vol. GM-CSF (Sandoz, Camberley, UK) was dissolved in sterile water and stored at −20°C until required.
Incubation of cells with ATRA, 1,25(OH)2 D3, and GM-CSF. Cells in exponential growth were seeded at 0.1 × 106/mL in 25-cm2 culture flasks (Costar, High Wycombe, UK) and supplemented with 1 μmol/L ATRA or 1 nmol/L 1,25(OH)2 D3 with or without 1,000 U/mL GM-CSF. Cells were harvested after 72 hours, washed, and used for assay of idarubicin-induced apoptosis (see below); cell proliferation was assessed by counting in a Neubauer hemocytometer (Weber Scientific International, London, UK). Aliquots were analyzed for cell-cycle profile, phenotype and Bcl-2 expression.
Analysis of cell-cycle profile, phenotype and Bcl-2 expression. Cell-cycle profiles were measured by flow cytometry. Washed, pelleted cells were prepared using a Coulter DNA-Prep (Coulter Electronics, Luton, UK), which uses a nonionic detergent permeabilization stage followed by propidium iodide/RNase staining of DNA. Cells were analyzed 1 hour after processing using an EPICS Elite cytometer (Coulter Electronics). Initial gating to exclude doublets was done using peak versus integral red channel signal, such that control HL60 cells gave 2% to 5% apoptosis, which level is observed morphologically on cytospin preparations. G0/G1, S, and G2/M fractions were identified from the resulting cell-cycle profile and apoptosis was measured from the sub-G1 fraction.36
Surface expression of the maturation antigens CD11b, CD13, CD14, CD32, and CD33 was measured using an EPICS Elite cytometer with the following conjugated primary antibodies: CD11b/phycoerythrin and CD14/phycoerythrin (Becton Dickinson, Oxford, UK); CD13/phycoerythrin and CD33/fluorescein isothiocyanate (Coulter); CD32/unconjugated (Immunotec, Marseille, France); rabbit antimouse/fluorescein isothiocyanate (Dako, High Wycombe, UK); appropriate isotype controls were from the same source as primary antibodies.
Bcl-2 protein expression was measured using a modification of the method of Delia et al37; briefly, paired tubes of washed, pelleted cells were fixed on ice with fresh 2% paraformaldehyde (10 minutes) and permeabilized with 0.05% Triton-X (10 minutes; Sigma). After two washes, one tube of each pair was incubated with anti-Bcl-2/fluorescein isothiocyanate conjugated antibody and the other with an isotype-matched control antibody. Percentage expression and mean fluorescence index were measured for the anti-Bcl-2 relative to the control for each cell type/treatment group.
Drug-induced apoptosis. After incubation with ATRA, 1,25(OH)2 D3, and GM-CSF, washed cells were incubated with idarubicin (Pharmacia, Milton Keynes, UK) at concentrations of 2,10, and 50 μg/L in 12-well plates (Costar, High Wycombe, UK); cells were seeded at 0.2 × 106/mL in 3-mL volumes and plates included control wells without idarubicin. Drug concentrations were chosen to reflect in vivo plasma levels as closely as possible: 50 μg/L corresponds to plasma levels achieved with standard doses of intravenous idarubicin, while oral dosing typically achieves levels of 2 to 10 μg/L.38 Cells were harvested after 24 hours and prepared using the DNA-Prep (as above); apoptosis was measured from the sub-G1 fraction of the resulting cell-cycle profile. Cell death was confirmed by loss of the ability to exclude trypan blue; the apoptotic nature of cell death was confirmed morphologically and by DNA fragmentation using agarose gel electrophoresis.39
Statistical analysis. Results were analysed using paired t-test, ANOVA and Spearman Rank correlation by means of Statistica (StatSoft, Tulsa, OK) and STATA (Stata Corp, College Station, TX) software.
RESULTS
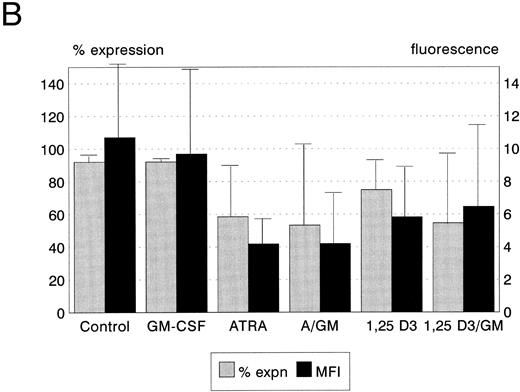
Differentiation status after incubation with ATRA, 1,25(OH)2 D3, and GM-CSF. 1,25(OH)2 D3 induced monocytic differentiation in the U937 cell line as documented by an increase in expression of CD14 (P < .05, Table 1). 1,25(OH)2 D3 reduced the expression of CD33 in HL60 cells (P < .05) and increased the expression of CD11b in the KG1 cell line (P < .001, Table 1). ATRA induced phenotypic evidence of granulocytic differentiation in the HL60 and KG1 cell lines, as indicated by a reduction in CD13 expression (each P < .01), and induced the expression of CD11b in U937 and KG1 cells (P < .05 and P < .01, respectively). GM-CSF had little effect on the differentiation status of HL60 and KG1 cells, either alone or in combination with ATRA or 1,25(OH)2 D3. However, GM-CSF interacted with both ATRA and 1,25(OH)2 D3 to increase monocytic differentiation of U937 in a manner that was at least additive; expression of the CD14 and CD32 antigens increased in cultures coincubated with GM-CSF (CD14, ATRA, and 1,25(OH)2 D3, P < .001; CD32, ATRA, and 1,25(OH)2 D3, P < .05, Table 1).
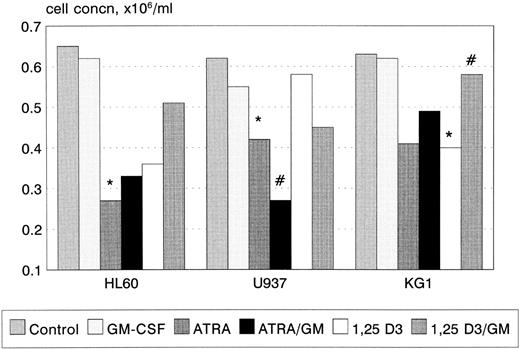
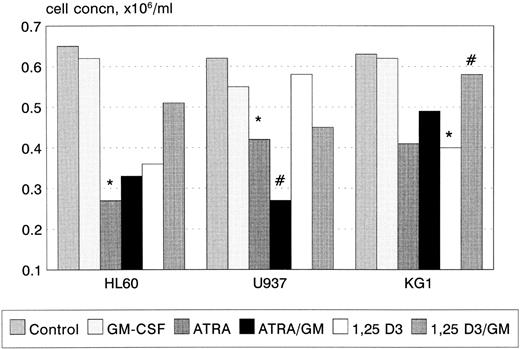
Growth and cell-cycle profile after incubation with ATRA, 1,25(OH)2 D3, and GM-CSF. 1,25(OH)2 D3 inhibited proliferation of KG1 cells (P < .05, Fig 1), with a trend towards a similar effect in U937 and HL60. In contrast, ATRA inhibited the proliferation of U937 and HL60 cell lines (U937, P < .01; HL60 P < .01), with a trend towards an inhibitory response in KG1. GM-CSF had no effect on cellular proliferation when used alone, however, coincubation with ATRA synergistically inhibited the proliferation of U937 cells (P < .05). GM-CSF also reversed the growth inhibitory effect of 1,25(OH)2 D3 on KG1 cells (P < .05). In general, growth inhibition was associated with phenotypic evidence of differentiation; however, U937 cells underwent differentiation in response to 1 nmol/L 1,25(OH)2 D3 without growth inhibition.
Growth inhibition of HL60, U937, and KG1 cells after 72-hours incubation with ATRA, 1,25(OH)2 D3, and GM-CSF. Results shown are mean of n = 3 (HL60 & KG1) or n = 7 (U937) experiments; error bars omitted for clarity. Statistical significance: * result statistically different from control; # result statistically different from ATRA/1,25(OH)2 D3 alone, eg, ATRA versus ATRA+GM-CSF; for P values see text.
Growth inhibition of HL60, U937, and KG1 cells after 72-hours incubation with ATRA, 1,25(OH)2 D3, and GM-CSF. Results shown are mean of n = 3 (HL60 & KG1) or n = 7 (U937) experiments; error bars omitted for clarity. Statistical significance: * result statistically different from control; # result statistically different from ATRA/1,25(OH)2 D3 alone, eg, ATRA versus ATRA+GM-CSF; for P values see text.
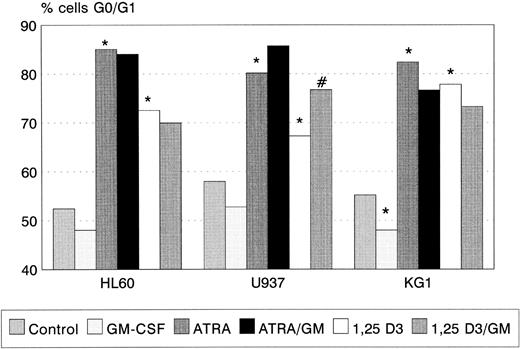
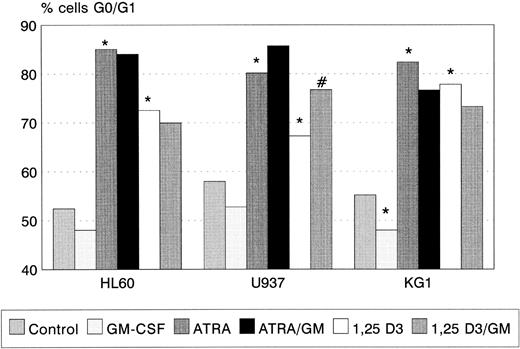
ATRA and 1,25(OH)2 D3 induced significant G0/G1 cell-cycle arrest in all three cell lines (Fig 2), the degree of G0/G1 arrest induced by ATRA being greater than that by 1,25(OH)2 D3 in each case. GM-CSF alone had no effect on cell-cycle status in HL60 and U937, but reduced the proportion of KG1 cells in G0/G1 (P < .05). Coincubation with GM-CSF increased the proportion of U937 cells arrested by 1,25(OH)2 D3 (P < .01). A trend towards a similar interaction between ATRA and GM-CSF (Fig 2) was observed.
G0/G1 arrest of HL60, U937, and KG1 cells after 72-hours incubation with ATRA, 1,25(OH)2 D3, and GM-CSF. Results shown are mean of n = 3 (HL60 & KG1) or n = 7 (U937) experiments; error bars omitted for clarity. Statistical significance: * result statistically different from control; # result statistically different from ATRA/1,25(OH)2 D3 alone, eg, ATRA versus ATRA+GM-CSF; for P values see text.
G0/G1 arrest of HL60, U937, and KG1 cells after 72-hours incubation with ATRA, 1,25(OH)2 D3, and GM-CSF. Results shown are mean of n = 3 (HL60 & KG1) or n = 7 (U937) experiments; error bars omitted for clarity. Statistical significance: * result statistically different from control; # result statistically different from ATRA/1,25(OH)2 D3 alone, eg, ATRA versus ATRA+GM-CSF; for P values see text.
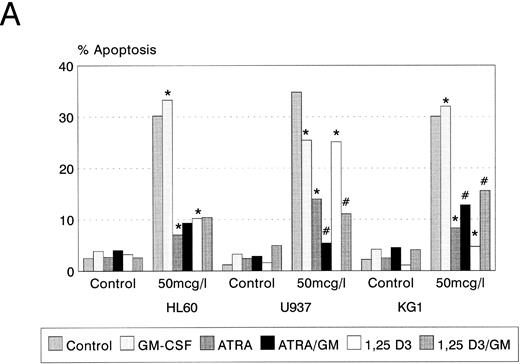
Idarubicin-induced apoptosis. Untreated control cultures in logarithmic growth demonstrated no significant apoptosis (<5% of cells) (Fig 3A). Idarubicin induced dose-dependent apoptotic cell death in all three cell lines after 24-hours exposure at concentrations of 2, 10, and 50 μg/L (data not shown). Cell death was confirmed by loss of the ability to exclude trypan blue; apoptosis was demonstrated by light microscopy and by agarose gel electrophoresis of DNA (data not shown). Apoptosis was quantitated by flow cytometry as described in Materials and Methods.
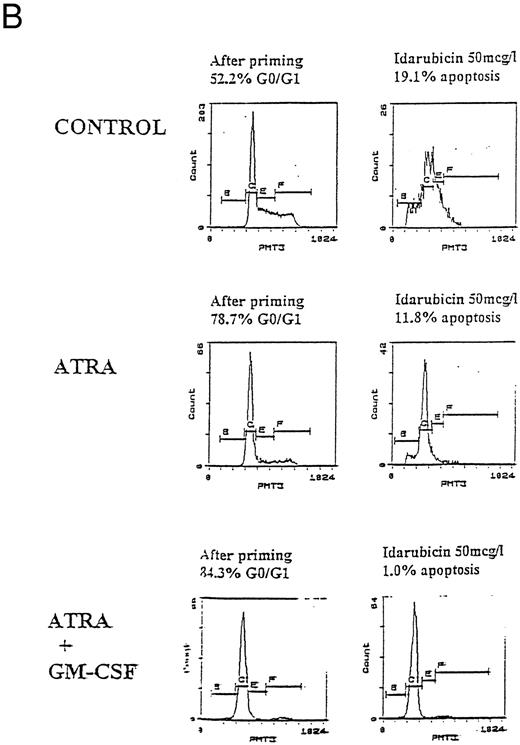
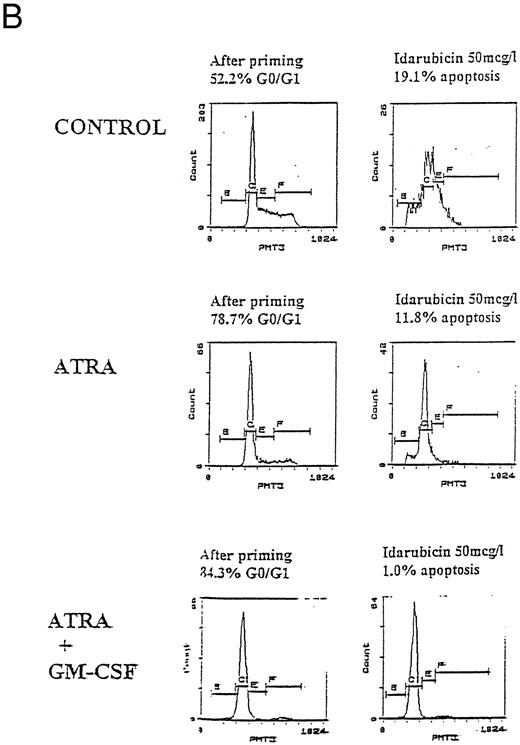
Apoptosis in HL60, U937, and KG1 cells incubated with ATRA, 1,25(OH)2 D3, and GM-CSF for 72 hours and then exposed to idarubicin 50 μg/L for 24 hours. (A) Apoptosis induced by idarubicin 50 μg/L. Results shown are mean of n = 3 (HL60 & KG1) or n = 7 (U937) experiments; error bars omitted for clarity. Statistical significance: * result statistically different from control; # result statistically different from ATRA/1,25(OH)2 D3 alone, eg, ATRA versus ATRA+GM-CSF; for P values see Table 2. (B) Representative cell-cycle profiles for U937 cells incubated with ATRA and ATRA + GM-CSF, and then exposed to idarubicin 50 μg/L; see Materials and Methods for details: X-axis, red fluorescence, Y-axis, cell number; left-hand plots, after initial incubation (ATRA ± GM-CSF, 72 hours), right-hand plots, after exposure to idarubicin (24 hours); gate B denotes apoptotic fraction, C cells in G0/G1, E S phase, and F G2/M.
Apoptosis in HL60, U937, and KG1 cells incubated with ATRA, 1,25(OH)2 D3, and GM-CSF for 72 hours and then exposed to idarubicin 50 μg/L for 24 hours. (A) Apoptosis induced by idarubicin 50 μg/L. Results shown are mean of n = 3 (HL60 & KG1) or n = 7 (U937) experiments; error bars omitted for clarity. Statistical significance: * result statistically different from control; # result statistically different from ATRA/1,25(OH)2 D3 alone, eg, ATRA versus ATRA+GM-CSF; for P values see Table 2. (B) Representative cell-cycle profiles for U937 cells incubated with ATRA and ATRA + GM-CSF, and then exposed to idarubicin 50 μg/L; see Materials and Methods for details: X-axis, red fluorescence, Y-axis, cell number; left-hand plots, after initial incubation (ATRA ± GM-CSF, 72 hours), right-hand plots, after exposure to idarubicin (24 hours); gate B denotes apoptotic fraction, C cells in G0/G1, E S phase, and F G2/M.
Prior incubation of leukemic cells with either ATRA or 1,25(OH)2 D3 significantly inhibited the ability of idarubicin to induce apoptosis (Table 2, Fig 3A). GM-CSF had a protective effect on U937 cells exposed to idarubicin and interacted with ATRA and 1,25(OH)2 D3 to further increase the resistance of U937 to idarubicin-induced apoptosis (Fig 3B). By contrast, GM-CSF sensitized both HL60 and KG1 cells to the effects of idarubicin, and partially reversed the protective effect of ATRA and 1,25(OH)2 D3 incubation on KG1 cells.
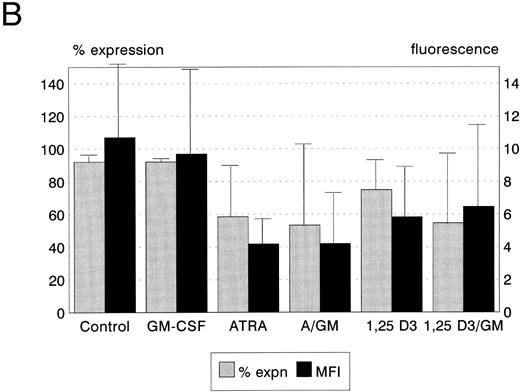
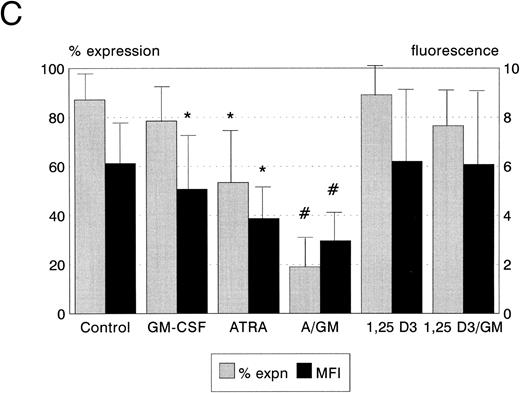
Bcl-2 protein expression. Expression of the Bcl-2 oncoprotein has been shown to be associated with the ability of some hemopoietic cells to resist the induction of apoptosis in certain circumstances. We therefore quantitated the expression of Bcl-2 protein in leukemic cells following incubation with ATRA, 1,25(OH)2 D3, and GM-CSF to establish whether upregulation of Bcl-2 was responsible for the modulation of cytotoxicity which we observed. ATRA, but not 1,25(OH)2 D3 or GM-CSF, reduced Bcl-2 expression by HL60 cells (P < .05, Fig 4A). Neither ATRA, 1,25(OH)2 D3, nor GM-CSF, either alone or in combination, had any significant effect on Bcl-2 protein expression by KG1 cells (Fig 4B). Both ATRA and GM-CSF induced statistically significant reductions in Bcl-2 expression in U937 cells (ATRA P < .01, GM-CSF P < .05, Fig 4c), and the combination of ATRA with GM-CSF reduced Bcl-2 expression still further (P < .01).
Bcl-2 protein expression of HL60, U937, and KG1 cells after 72-hours incubation with ATRA, 1,25(OH)2 D3, and GM-CSF. (A) HL60; (B) KG1; (C) U937. Results shown are mean of n = 3 (HL60 & KG1) or n = 7 (U937) experiments; error bars represent 1 SD above the mean. Statistical significance: * result statistically different from control; # result statistically different from ATRA/1,25(OH)2 D3 alone, eg, ATRA versus ATRA + GM-CSF; for P values see text.
Bcl-2 protein expression of HL60, U937, and KG1 cells after 72-hours incubation with ATRA, 1,25(OH)2 D3, and GM-CSF. (A) HL60; (B) KG1; (C) U937. Results shown are mean of n = 3 (HL60 & KG1) or n = 7 (U937) experiments; error bars represent 1 SD above the mean. Statistical significance: * result statistically different from control; # result statistically different from ATRA/1,25(OH)2 D3 alone, eg, ATRA versus ATRA + GM-CSF; for P values see text.
Correlation of G0/G1 arrest and apoptosis. It is clear from the data presented that susceptibility to idarubicin-induced apoptosis was not related to the differentiation status of cells following incubation with ATRA, 1,25(OH)2 D3, and GM-CSF: 1,25(OH)2 D3 induced greater differentiation of U937 than ATRA, yet ATRA afforded a greater protective effect, while both ATRA and 1,25(OH)2 D3 induced little differentiation of KG1, but both afforded protection. Levels of the Bcl-2 oncoprotein are also unlikely to explain the altered susceptibility of these cells to undergo apoptosis: over the period of incubation, Bcl-2 expression in U937 was downregulated by both ATRA and 1,25(OH)2 D3, while ATRA downregulated expression in HL60, with a trend towards reduced expression in KG1. These changes are consistent with previous observations associating differentiation with downregulation of Bcl-2 expression; however, such downregulation of expression should have increased susceptibility to apoptosis, rather than the reverse, which we observed.
Induction of apoptosis by idarubicin was, however, related to the proportion of cells induced to undergo G0/G1 arrest during incubation. Using Spearman Rank correlation analysis, a negative correlation was observed between the percentage of apoptotic cells after exposure to 50 μg/L idarubicin and the proportion of cells in G0/G1 after incubation with ATRA, 1,25(OH)2 D3, and GM-CSF (rho = −0.9092, P < .001).
DISCUSSION
We have confirmed that prior incubation with ATRA and 1,25(OH)2 D3 protects HL60 cells against drug-induced apoptosis using the anthracycline antibiotic idarubicin, and have demonstrated a similar response in the myeloid leukemic cell lines U937 and KG1, which are less differentiated than HL60.40,41 Several recent studies have shown that HL60 cells have reduced sensitivity to a range of apoptotic stimuli after induction of differentiation26-30; however, there are conflicting data regarding the mechanisms underlying this effect. Zwelling et al30 have shown a 10-fold reduction in etoposide-induced DNA damage after incubation of HL60 cells with PMA; concordant results using isolated nuclei suggested that the protective effect resided in the cell nucleus. By contrast, Solary et al29 have shown an apparent cytoplasmic localization of TPA-induced protection against a range of topoisomerase inhibitors.
We have sought to clarify the relative roles of the nuclear and cytoplasmic consequences of differentiation by comparing the effects of ATRA and 1,25(OH)2 D3 on cell-cycle distribution, Bcl-2 protein expression and immunophenotype of myeloid leukemia cells before their exposure to idarubicin. We show that neither the degree of immunophenotypic differentiation, nor the level of Bcl-2 expression, explain the observed protection of cells against idarubicin-induced apoptosis. By contrast, the degree to which cells were protected was inversely proportional to the extent to which ATRA and 1,25(OH)2 D3 induced G0/G1 cell-cycle arrest.
In this study leukemic cells underwent differentiation and growth arrest in response to 1,25(OH)2 D3 and ATRA; differentiation of U937 cells was further augmented by GM-CSF as previously described.42 43 However, resistance to idarubicin was independent of differentiation per se as there appeared to be discordance between the degree of differentiation observed by phenotypic assessment and idarubicin-associated apoptosis; indeed, ATRA alone conferred greater protection upon U937 cells without inducing detectable phenotypic maturation.
Our observations concerning Bcl-2 expression accord with previous studies that have shown downregulation of Bcl-2 protein in myeloid cells exposed to differentiating agents.26,27,37 However, these studies have shown a paradoxical decrease in drug-induced apoptosis when the reverse would be expected.26,27 It is now clear that protection from a range of apoptotic stimuli is determined not simply by Bcl-2 expression per se but by heterodimers of Bcl-2 and its homologues. Bcl-2 and Bcl-XL act as suppressors of cell death, while Bax and Bcl-XS promote induction of apoptosis.44 Although other members of the Bcl-2 family were not studied here, Sanz et al45 have shown only low-level Bcl-XL expression in parental HL60 cells, with downregulation in response to ATRA-induced differentiation. Nevertheless, it is clear that protection from idarubicin-induced apoptosis conferred by differentiating agents was not mediated by upregulation of Bcl-2 protein expression.
An alternative mechanism of differentiation-induced cytoprotection might involve factors that determine an MDR phenotype, such as Pgp. Upregulation of Pgp in association with differentiation is unlikely to be responsible for the increase in resistance to idarubicin: Xu et al26 and Del Bino et al28 attempted to induce apoptosis with cytarabine, which is not a substrate for Pgp, and with noncytotoxic inducers of apoptosis (calcium ionophore, hyperthermia, and γ-irradiation) and observed a similar protective effect after differentiation. We have confirmed these findings using cytarabine (Ketley et al, unpublished observations, March 1995). Parental HL60 cells have been shown not to express Pgp9 and preliminary data suggest that, although Pgp expression may be differentiation-dependent in K562 cells, differentiation of HL60 and U937 cells does not induce Pgp expression (X.R. Jiang personal communication, July 1995). In addition, idarubicin is not thought to be a substrate for Pgp at physiological levels of expression.32 33
Our data strongly suggest an association between resistance to idarubicin-induced apoptosis and arrest of cell cycling in the G1 phase. This is inferred by three lines of evidence. Firstly, KG1 cells showed limited evidence of differentiation in response to ATRA or 1,25(OH)2 D3, but accumulated in G1 in response to these agents and became resistant to idarubicin. Secondly, there was a strong correlation between G1 arrest in the U937 and HL60 cell lines and protection from idarubicin. Thirdly, GM-CSF was able to alter the sensitivity of both KG1 and U937 cells to idarubicin-induced cell death after incubation with ATRA and 1,25(OH)2 D3, an effect again predicted by the ability of GM-CSF to modulate the induction of G1 arrest.
The relationship between differentiation and G1 cell-cycle arrest is crucial to understanding how the cytoprotection we have observed might be mediated. Bergh et al46 have recently shown that G1 arrest is necessary but not sufficient for differentiation. Using U937 cells transfected with an antisense oligonucleotide (ASO) to the retinoblastoma gene, they showed that ATRA and 1,25(OH)2 D3 inhibit proliferation and induce G1 arrest as in control cells, but without morphological, phenotypic, or functional evidence of differentiation.46 A potential candidate for mediating the G1 arrest seen with differentiation of leukemia cells is p21, and recent data suggest that this cyclin-dependent kinase inhibitor (CKI) may have a role in the modulation of drug-induced apoptosis and DNA repair.47-49
The classical pathway of p21 induction is following DNA damage, which causes upregulation of p53, a transcription factor for the p21 gene.50 p21 inhibits cyclin-dependent kinases (CDKs) and hence CDK-dependent phosphorylation, thereby maintaining the retinoblastoma protein in a hypophosphorylated state, which induces G1 arrest and growth suppression in vitro (to almost the same extent as transfected p53).50-52 p21 can also be induced independently of p53, notably in association with cellular differentiation53-55: HL60 cells show p21 activation after treatment with a range of differentiating agents, including ATRA, with p21 mRNA appearing at 24 hours, before any phenotypic or cell-cycle evidence of differentiation.53
The specificity of p21 mediation of differentiation-induced G1 arrest is supported by the finding of a vitamin D response element in the promoter region of the p21 gene and the demonstration that p21 transfection induces phenotypic differentiation, although not to the extent seen with 1,25(OH)2 D3 itself.56 Furthermore, transfection of p21 ASO is associated with decreased PMA-induced cell-cycle arrest and phenotypic differentiation, as well as an attenuation of PMA-induced inhibition of clonogenic growth.57 The relevance of these in vitro findings to the clinic is provided by Zhang et al,58 who have shown that p21 is an independent risk factor in untreated AML.58 Although the p21 protein expression was heterogeneous amongst the 100 patients studied, high levels were associated with a lower remission rate, which the authors postulated was due to quiescence-associated chemoresistance.58
Such chemoresistance might arise in two main ways: either G1-arrested cells are damaged less by a given dose and duration of chemotherapy, or they are better at repairing that damage before it translates into apoptosis, perhaps simply by having longer in which to do so. Differentiation itself is associated with an increase in DNA strand breaks,59 as well as induction of the repair enzyme poly-ADP-ribose polymerase (PARP).60 PARP seems to act as a “molecular nick sensor,” marking sections of DNA for repair,61 but is itself a target for proteolytic cleavage during apoptosis.62
Again, recent evidence supports a role for p21 in DNA repair following drug-induced damage. Accurate DNA repair requires the suppression of DNA replication, a balance coordinated by proliferating cell nuclear antigen (PCNA).63 Warbrick et al49 have recently demonstrated that p21 has a PCNA binding sequence, and that the formation of p21/PCNA complexes favors DNA repair over replication. The impact of p21 status on cell survival is shown by two studies: Sheikh et al48 have shown increased clonogenic survival and DNA repair of p21-transfected colorectal carcinoma cells exposed to UV irradiation,48 while Fan et al47 have shown that disruption of p21 function is associated with sensitization to cisplatinum-induced cytotoxicity and cell-cycle delay, as well as decreased DNA repair.
Although p21 is a candidate for mediating the differentiation-induced cytoprotection, which we have described, the clinical implications of our findings are complex. The finding by Zhang et al58 of an association between p21 expression and clinical remission in de novo AML suggests that quiescence of tumor cells may be a potent mechanism of chemoresistance. However, they and others have failed to detect mutations or deletions of the p21 gene in leukemia samples, suggesting that this CKI may have a pivotal role in determining tumor therapy responses.58,64 However, despite in vitro evidence for reduced drug-induced cell death after ATRA,27,65 the accumulating clinical evidence from APL trials is of greater remission induction and reduced relapse with ATRA plus chemotherapy as opposed to either modality alone.66
Although there is little clinical data to support the potential for hemopoietic growth factors to recruit cells into cycle and increase their chemosensitivity,67,68 it may be that cell-cycle quiescence is associated with chemoresistance. Constitutive changes or external stimuli that induce cell-cycle arrest in leukemic cells may substantially increase the resistance of these cells to chemotherapy-induced apoptosis. Our data suggest that further elucidation of the mechanisms underlying differentiation-induced cytoprotection will enable novel therapeutic strategies to be developed. Furthermore, if tumor cell quiescence can be accurately assessed at presentation, stratification of chemotherapy based on predicted response may become more sophisticated. The studies of Banker et al69 where multiparametric analyses of cell-cycle characteristics and apoptosis were able to sensitively and reproducibly detect the effects of apoptosis modulators on individual patient samples may be important in this respect.
Supported by the Leukaemia Research Fund (London, UK) as a Clinical Training Fellow (to N.J.K.).
Address reprint requests to Nicolas J. Ketley, MRCP, Department of Haematology, Great Ormond Street Hospital for Children, Great Ormond St, London, WC1N 3JH, United Kingdom.