RATIONALE FOR UMBILICAL CORD BLOOD TRANSPLANTATION
HLA-MATCHED RELATED allogeneic stem cell transplantation has been successfully used as the treatment of choice in selected high-risk or recurrent hematologic malignancies, marrow failure syndromes, severe congenital immunodeficiency states, and selected metabolic disorders.1 Recently, the use of matched related allogeneic peripheral blood stem cells as a source of transplantable stem cells has been reported by a variety of groups.2-4 Initial studies have suggested that the risk of graft rejection and the risk of developing severe acute graft-versus-host disease (GVHD) are similar when using matched related allogeneic peripheral blood stem cells as compared with matched related allogeneic bone marrow (BM) stem cells. However, the major limitation of using HLA-matched related sibling donors in BM transplantation (BMT) has been that only 30% to 40% of potential recipients in need of such therapy have an HLA-matched related family donor.1 The recent use of either related or unrelated donor umbilical cord blood stem cells for allogeneic stem cell transplantation has been secondary to a number of factors, most important of which have been (1) the attempt to reduce transplant-related complications and (2) augmentation of the donor pool. Gluckman et al5 first reported the successful use of HLA-matched sibling umbilical cord blood stem cells to reconstitute a child with severe Fanconi anemia. Since 1988, there have been an estimated 500 related and unrelated donor umbilical cord blood transplants.
To increase the pool of potential donors for allogeneic BMT, the National Marrow Donor Program (NMDP) was established in 1987 to develop a registry of available BM donors, promote cooperation with other international BM donor pools, and develop clinical and basic research relative to unrelated donor BMT. As of May 1997, more than 2.7 million donors in the United States have been registered by the NMDP. These potential allogeneic donors have been typed for HLA A and B antigens and approximately one third have additionally been typed for HLA DR. To date, more than 5,700 unrelated donor BMTs have been facilitated by the NMDP (personal communication, May 1997). Despite the recent success in using unrelated donor matched BM, there continue to be limitations and obstacles in using this form of therapy. The average length of time from donor search to transplant is approximately 135 days, and the cost for donor search and marrow procurement ranges from $25,000 to $50,000 dollars.6
UMBILICAL CORD BLOOD STEM/PROGENITOR CELLS
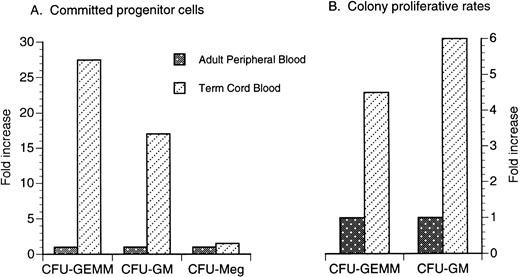
It has been shown in early studies by Broxmeyer et al7 and others that both term and preterm umbilical cord blood contains a significantly higher number of early and committed progenitor cells when compared with adult peripheral blood. The number of colony-forming unit–granulocyte-macrophage (CFU-GM) is greatly increased in umbilical cord blood obtained from term neonates compared with peripheral blood obtained from adults (Fig 1A).8-14 The CFU-GM proliferative rate, as assayed by thymidine suicide studies, is also significantly higher in term umbilical cord blood (Fig 1B).15 The number of circulating colony-forming unit granulocyte, erythroid, monocyte, megakaryocyte (CFU-GEMM) also appears to be significantly increased in term umbilical cord blood (Fig 1A)9 and the CFU-GEMM proliferative rate is also significantly higher in term umbilical cord blood compared with that of adult peripheral blood (Fig 1B).10 Lastly, committed megakaryocytic progenitor cells as identified by circulating colony-forming unit megakaryocyte (CFU-Meg) are also enriched in term umbilical cord blood compared with adult peripheral blood but to a much less degree than CFU-GM and CFU-GEMM (Fig 1A).11
Comparison of the fold increase of circulating committed progenitor cells (A) and proliferative rates (B) in term unrelated cord blood versus adult peripheral blood. Data are a compilation of previous studies.8-15
Comparison of the fold increase of circulating committed progenitor cells (A) and proliferative rates (B) in term unrelated cord blood versus adult peripheral blood. Data are a compilation of previous studies.8-15
Recently, Broxmeyer et al13 showed that umbilical cord blood contains numbers of CFU-GM well within the range of marrow CFU-GM previously shown to allow successful engraftment after autologous BMT. It has recently been demonstrated that umbilical cord blood contains sufficient numbers of hematopoietic progenitor cells to engraft larger size children and adults.14 Others have suggested that ex vivo expansion of umbilical cord blood progenitor cells may be necessary to engraft larger patients.16 In vitro studies have shown that ex vivo expansion is possible; the addition of stem cell factor (SCF ) and granulocyte-macrophage colony-stimulating factor (GM-CSF ) increases umbilical cord blood CFU-GM progenitor cells by 8- to 11-fold in short-term liquid culture.14 Umbilical cord blood progenitor cells also appear to be more sensitive to ex vivo expansion with both lineage-specific and lineage-nonspecific hematopoietic growth factors.7,17,18 In long-term hematopoietic in vitro cultures, umbilical cord progenitor cell production and culture life span are significantly increased compared with progenitor cells from normal adult BM.19
We and others have demonstrated that CD34+ stem cells can be isolated from umbilical cord blood and adult human BM.18,20-22 Fourteen-day expansion of CD34+ cells isolated from umbilical cord blood stimulated with interleukin-11 (IL-11) + granulocyte colony-stimulating factor (G-CSF ) was significantly greater than that from stimulated CD34+ cells isolated from human adult BM (80-fold).21 Additionally, IL-11 + G-CSF also significantly increased the expansion of day-14 CFU-GM and CFU-Meg from umbilical cord blood compared with adult BM.21 Single nonadherent low-density T-lymphocyte–depleted CD34+ cells expanded ex vivo with SCF, GM-CSF, G-CSF, IL-3, and erythropoietin results in an eightfold increase in colony formation from umbilical cord blood CD34+ cells compared with that from adult BM.22 Additionally, single CD34+ cells isolated from umbilical cord blood are capable of at least five serial in vitro replatings, demonstrating a significant level of self-renewal capacity for single-sorted CD34+ umbilical cord blood stem cells.22 Future clinical trials might incorporate ex vivo expansion of umbilical cord blood progenitor cells to accelerate hematopoietic reconstitution.
The CD34+ CD38− immunophenotype reportedly defines a primitive subpopulation of progenitor cells23-28 and continues to be an area of ongoing study. Hao et al23 showed that CD34+ CD38− cells in umbilical cord blood have a higher cloning efficiency than the same immunophenotype in adult BM. CD34+ CD38− cells in umbilical cord blood also proliferate more rapidly in response to cytokine stimulation with IL-3, IL-6, and SCF and generate seven times more progeny than do BM cells.23 Cardoso et al24 found that isolated umbilical cord blood CD34+ CD38− cells yield approximately the same number of CFU-GEMM, twice the CFU-GM, and three times the burst-forming unit-erythroid (BFU-E) as similar cell populations isolated from BM.
UMBILICAL CORD BLOOD IMMUNITY
Hematopoiesis and host defense in the neonate is developmentally immature compared with the adult.29 Dysregulation of neonatal hematopoiesis and the immune response is a significant contributing factor to the increased susceptibility of the neonate to overwhelming infection.30,31 Cairo et al32-38 have recently demonstrated significant dysregulation of a number of hematopoietic cytokines and lymphokines from umbilical cellular sources compared with adult peripheral blood. Lineage-specific hematopoietic cytokines, such as G-CSF, GM-CSF, IL-3, and macrophage colony-stimulating factor (M-CSF ), and negative hematopoietic regulators, including transforming growth factor-β1 (TGF-β1) and macrophage inhibitory protein-1α (MIP-1α), are significantly decreased from activated umbilical cells compared with adult cell sources. There is a significant reduction in mRNA expression from activated umbilical cord blood mononuclear cells (MNC) compared with adult peripheral blood MNC as well as decreased protein production with regards to G-CSF, GM-CSF, IL-3, M-CSF, TGF-β1, and MIP-1α (10% to 40% adult values).33-36 In comparison, expression and protein production of IL-11, SCF, and thrombopoietin (TPO) are significantly increased from umbilical cord blood sources compared with adult fibroblasts and endothelial cells (200% to 400% adult values).37-39 Recent studies examining the complex regulatory mechanisms associated with GM-CSF mRNA expression and protein production have suggested a significant decrease in posttranscriptional stability in umbilical cord blood compared with adult peripheral blood MNC.40 It has additionally been shown that both IL-2 and IL-12, two critically important lymphokines regulating cellular immunity, are significantly decreased in gene expression and protein production from activated umbilical cord versus adult peripheral blood MNC.41 42 Dysregulation of hematopoietic growth factors and cytokines could potentially predispose the recipient of umbilical cord blood to delays in immune or hematopoietic reconstitution.
The immunoreactivity of umbilical cord blood effector cells (monocytes and lymphocytes) appears to be normal to slightly decreased compared with that of adult peripheral blood. Phenotypic comparisons of umbilical cord blood and adult peripheral blood have shown umbilical cord blood total B-cell numbers to be comparable to adult peripheral blood; however, approximately half the population has the immature (CD5+/CD19+) phenotype thought to be involved in anti-self reactions. Lower absolute numbers of CD4+, CD8+, and CD3+ T cells but a higher CD4+/CD8+ ratio have been demonstrated in umbilical cord versus adult peripheral blood. Additionally, umbilical cord blood T cells have an increased number of unprimed (ie, naive) T cells (CD45 RA+) and decreased populations of mature or primed T cells (CD45 RO+) compared with that of adult peripheral blood T cells (Table 1).43 Decreased CD3 expression in umbilical cord blood T cells has also been reported, suggesting the presence of maturational intermediate T cells that may not be fully functional. A unique T-lymphocyte population (CD3−/CD8−) has also been observed in umbilical cord blood but not in adult peripheral blood. These umbilical cord blood T-cell subsets are postulated to be lymphopoietic precursors for the T-cell lineage (Table 1).43 These decreases in mature or primed T cells and subpopulations of T cells also lead to decreased release of certain mediators during activation, including γ interferon and tumor necrosis factor-α (TNF-α) from umbilical cord versus adult peripheral blood T cells (Table 1).41,43,44 Despite the fact that umbilical cord blood T cells have altered subpopulations and decreased mediator release, they appear to have similar natural killer (NK) activity and similar lymphokine-activated killer activity compared with that of adult peripheral blood T cells (Table 1).45-47
The proliferative response after allogeneic stimulation of E-rosetted T cells is similar in umbilical cord blood compared with adult peripheral blood.48 However, in contrast, allogeneic cytolytic activity in mixed leukocyte cultures of umbilical cord blood T cells is significantly decreased compared with that in adult peripheral blood (Table 1).48 Umbilical cord blood has been shown to be 10 to 1,000 times less alloreactive in terms of proliferative T cells and cytotoxic T cells as measured by limiting dilution analyses (Table 1).48 Umbilical cord blood T cells are also significantly decreased in secondary proliferative responses to alloantigens compared with that of adult peripheral blood T cells. The unresponsiveness of umbilical cord blood T cells to allogeneic restimulation appears to be long-lasting and sustained by the induction of anergy and the activity of CD8+ suppressor cells.49 Cytotoxic activity in secondary and tertiary mixed leukocyte cultures of umbilical cord blood T cells has also been shown to be typically less than 20% (Table 1).48 However, the graft-versus-leukemia and GVHD properties of umbilical cord blood remain to be defined.
Properties of the neonatal immune system that might account for umbilical cord blood mediating GVHD reaction are currently being explored. However, some recent reports suggest that the frequencies of helper lymphocyte precursor (HTLp) and cytotoxic T lymphocyte precursor (CTLp) cells in umbilical cord blood are similar to or exceed those of adult peripheral blood50,51 and activation by alloantigen is normal.51 However, other reports suggest that the cytotoxic activity of umbilical cord blood is not mediated by CTLs but primarily by NK cells.52
UMBILICAL CORD BLOOD COLLECTION AND PROCESSING
Successful hematologic reconstitution after myeloablative therapy and umbilical cord blood transplantation has resulted in considerable interest in the techniques of umbilical cord and placental blood collection and storage. Using an open collection procedure,53 a variety of obstetricians have collected umbilical cord blood for purposes of hematopoietic stem cell transplantation and shipped the specimens to Indiana University for storage. The volume of umbilical cord blood collected ranged from 42 to 240 mL, with a median volume of 103 ± 49 mL (n = 38). Needle aspirations of the placental veins produced an additional 8 to 85 mL, with a median volume of 31 ± 16 mL (n = 31). Of specimens (n = 38) sent by overnight courier express mail, the total number of nucleated cells contained in a single collection has ranged from 4.7 × 108 to 4.6 × 109 cells with a median value of 1.4 × 109 ± 0.96 × 109. The nucleated cell concentration varied significantly between umbilical cords (range, 3.1 × 106 to 24.3 × 106 cells/mL). The numbers of day-14 CFU-GM (colonies and clusters) ranged from 5.4 × 105 to 59.2 × 105 (median, 21.5 × 105 ± 3.1 × 105 [SEM]).
A variety of collection methods have subsequently been proposed to optimize the collection volume and reduce the risks of microbial and maternal cell contamination.54-56 Closed collection systems have been principally used by designated umbilical cord blood collection centers with trained staff. Although the open collection system may be technically easier, the most important disadvantage is the greater potential of microbial and maternal cell contamination, yet maternal cell contamination of the umbilical cord blood at the time of collection appears to be of limited clinical importance. Kurtzberg et al (presented by Broxmeyer et al13 ), Wagner et al,57 and Vilmer et al58 failed to demonstrate maternal cells in the umbilical cord blood grafts used for transplantation by cytogenetic or DNA techniques. However, more recent data by Socie et al,59 Hall et al,60 Scaradavou et al,61 and Kugler et al62 suggest that maternal cells are detectable in all umbilical cord blood specimens at a frequency of 1 in 10,000 to 100,000. Although maternal cell contamination might therefore be present in all grafts, the very low incidence of GVHD observed in unrelated sibling donor umbilical cord blood transplant recipients suggests that carefully collected umbilical cord blood has either too little maternal T-cell contamination to be clinically significant or that maternal T cells are not immunologically active in this context.
Different procedures for umbilical cord blood collection, separation, and cryopreservation have been evaluated and reported in anticipation of large-scale banking projects proposed in the United States and Europe. Bertolini et al56 have reported on one of the most extensive evaluations of umbilical cord blood collection procedures thus far, comparing open and closed collection systems, the effect of vaginal versus Caesarian section delivery, and the recoveries of colony-forming cells (CFC) and high proliferative potential-CFC (HPP-CFC) after density gradient centrifugation and gelatin sedimentation of both fresh and cryopreserved cell samples. Bertolini et al56 failed to show any statistical differences in the collection volumes of umbilical cord blood recovered during vaginal delivery (in utero, n = 445) or after Caesarian section deliveries (ex utero, n = 82). The median volume of blood collected was 72 ± 34 mL and 62 ± 19 mL, respectively. Furthermore, no significant difference in collection volume could be discerned between open and closed collection systems. As expected, there appeared to be a lower risk of bacterial contamination for samples collected by venipuncture into a blood collection bag as compared with the open collection method (4% v 14%, respectively).
In the instance in which there are already limited numbers of nucleated cells and hematopoietic progenitors, manipulations that might further reduce the number of these cells in the umbilical cord blood graft must be avoided. Broxmeyer et al13 first reported significant losses in progenitor recovery with umbilical cord blood after density gradient centrifugation (Ficoll-Hypaque, 1.077 g/mL; Sigma, St Louis, MO). They found that CFC were lost by a variety of red blood cell (RBC) separation techniques, suggesting that RBC depletion before clinical transplantation, even if the recipient and donor were ABO-incompatible, should be carefully reconsidered. However, Harris et al,55 Newton et al,63 and Bertolini et al56 failed to observe the same substantial losses of progenitor cells as assessed by in vitro colony-forming assays. Harris et al55 described a double Ficoll-Hypaque procedure in which the final preparation was virtually devoid of RBC and polymorphonuclear leukocytes but contained virtually all CFC. Bertolini et al56 compared the double Ficoll-Hypaque method proposed by Harris et al55 and a 3% gelatin sedimentation method proposed by Nagler et al.64 Umbilical cord blood separation using either Ficoll-hypaque or gelatin sedimentation resulted in only 8% to 14% loss of CFC and HPP-CFC. However, Bertolini et al56 also found that the effectiveness of either separation procedure was markedly reduced when umbilical cord blood was stored for more than 12 hours before the procedure. Although the gelatin procedure took less time relative to the Ficoll-Hypaque method (1.5 v 2.5 hours), in one third of instances, the gelatin procedure failed to result in RBC depletion when performed at room temperature. However, this technical issue was corrected simply by performing the procedure at 4°C. Rubinstein et al65 developed an RBC separation method using hydroxyethylstarch (HES; Hespan; McGaw, Inc, Irvine, CA) sedimentation. This procedure resulted in recovery of 98% of CFC.
These data suggest that RBC depletion by either density-gradient centrifugation, gelatin sedimentation, or HES results in only modest losses of hematopoietic progenitor cells.13 Rubinstein et al65 currently recommend that the frozen umbilical cord blood graft be placed in a sterile bag and then thawed in a 38°C waterbath with gentle agitation. With an equal volume of dextran/albumin solution added after thawing, the cells are centrifuged gently and the supernatant is removed. The cell pellet is then resuspended in dextran/albumin. This procedure removes the bulk of RBC ghosts, free hemoglobin, and dimethyl sulfoxide (DMSO), thus reducing some of the risks associated with the transplant procedure. At the University of Minnesota, this method routinely has provided greater than 90% recovery and greater than 90% viability (n = 35) of nucleated cells (J.E.W., unpublished data).
SIBLING DONOR UMBILICAL CORD BLOOD TRANSPLANTATION
Patient population. Data on 62 patients receiving sibling donor umbilical cord blood transplants for malignant and nonmalignant disorders (Table 2) between October 1988 and November 1995 were reported by 22 transplant teams to the International Cord Blood Transplant Registry. Data on the first 44 were previously reported57 (update by Wagner et al, personal communication, May 1997). Patients were aged 0.5 to 16 years. Eleven patients received HLA 1-3-antigen mismatched grafts; all others were matched. Prophylaxis for acute GVHD (aGVHD) consisted of cyclosporine A alone or in combination with methylprednisolone or an anti–T-cell antibody (n = 40), cyclosporine A with short-course methotrexate (n = 18), or methotrexate alone or in combination with methylprednisolone (n = 2); 2 patients received no prophylaxis. Hematopoietic growth factors were used early after the infusion of umbilical cord blood in 37 patients by study design; 20 received GM-CSF, 14 received G-CSF, and 3 received both simultaneously.
Umbilical cord blood graft characteristics. The method of umbilical cord blood collection varied significantly between institutions; however, the majority of collections were performed by obstetricians or nurse midwives without any prior experience in the large-scale collection of umbilical cord and placental blood. The median volume of umbilical cord blood collected was 98 mL (range, 42.1 to 282 mL). The median number of nucleated cells and CFU-GM in the graft on the basis of the patient's body weight was 4.7 × 107/kg (range, 1.0 to 33.0 × 107/kg) and 1.7 × 104/kg (range, <0.1 to 25.6 × 104/kg), respectively. Despite the diversity of collection systems and inexperience of collectors, the majority of samples were sterile. In 6 of 44 reported cases, the umbilical cord blood was contaminated with bacteria. However, bacterial contamination of the umbilical cord blood graft did not have any demonstrable impact on the posttransplant morbidity in any of these patients.
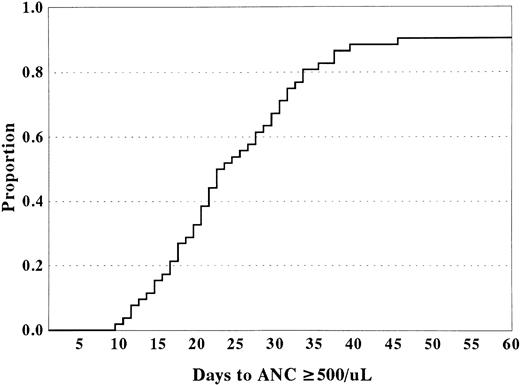
Hematopoietic recovery and engraftment. Patients (n = 56) surviving greater than 30 days after transplantation were considered evaluable for engraftment. For recipients of HLA-matched or HLA-1-antigen mismatched umbilical sibling donor cord blood grafts, the actuarial probability of hematopoietic recovery at 60 days after transplantation was 0.91 ± 0.08 (Fig 2). Median time to neutrophil recovery (defined as time to achieve an absolute neutrophil count [ANC] ≥500/μL) and platelet recovery (defined as platelet count ≥50,000/μL untransfused for 7 days) was 22.0 days (range, 12 to 46 days) and 51 days (range, 15 to 117 days) after transplantation, respectively. Four patients never had signs of hematopoietic recovery and 1 patient had early recovery but cells were entirely host in origin. Of the 5 patients without donor cell engraftment, 4 had undergone umbilical cord blood transplantation for the treatment of a BM failure syndrome and one for the treatment of Hunter syndrome.
Time to neutrophil recovery (ANC ≥500/μL) for recipients of HLA-matched or HLA-1-antigen mismatched sibling donor umbilical cord blood.
Time to neutrophil recovery (ANC ≥500/μL) for recipients of HLA-matched or HLA-1-antigen mismatched sibling donor umbilical cord blood.
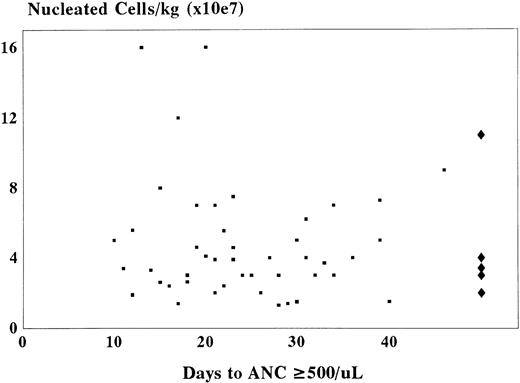
No correlation could be discerned between nucleated cell count or hematopoietic progenitor cell content of the graft and time to neutrophil recovery or probability of engraftment (Fig 3). Although the use of hematopoietic growth factors in 37 of 62 patients did not appreciably shorten the time to neutrophil recovery, the variables of patient and growth factor selection prevent definitive conclusion.
Comparison between the number of nucleated cells per kilogram of recipient body weight and time to neutrophil recovery (ANC ≥500/μL) after sibling related cord blood transplantation. (▪) The nucleated cell count for patients achieving an ANC ≥500/μL (n = 56). (♦) Cell doses in patients failing to engraft.
Comparison between the number of nucleated cells per kilogram of recipient body weight and time to neutrophil recovery (ANC ≥500/μL) after sibling related cord blood transplantation. (▪) The nucleated cell count for patients achieving an ANC ≥500/μL (n = 56). (♦) Cell doses in patients failing to engraft.
GVHD. Patients (n = 51) surviving greater than 30 days after transplantation and demonstrating evidence of donor-derived hematopoiesis were evaluable for aGVHD. The actuarial probability of grade II-IV GVHD at 100 days after transplantation was 0.02 ± 0.02 for recipients with an HLA 0-1-antigen mismatched umbilical cord blood donor. Notably, no patient was reported to have grade III-IV aGVHD. Of the entire cohort of patients, chronic GVHD has been reported in only 3 patients to date, with no patient having extensive disease.
Interestingly, moderate to severe GVHD was infrequently observed in 8 evaluable patients with 1-3 HLA-antigen mismatched haploidentical sibling donors (Table 3). Of these 8 patients, 3 were mismatched at one antigen, 1 was mismatched at two antigens, and 4 were mismatched at three antigens. As shown in Table 3, donor-recipient pairs mismatched at a maternal allele appeared to be less likely to develop grade II-IV GVHD than donor-recipient pairs mismatched at a paternal allele. This observation supports the hypothesis that partial tolerance develops to the noninherited maternal allele during the donor's gestation, but small patient numbers at this time prevent any conclusion.
UNRELATED DONOR UMBILICAL CORD BLOOD TRANSPLANTATION
Patient population. At the present time, more than 450 unrelated donor umbilical cord blood transplants are known to have been performed worldwide. However, thus far few published manuscripts exist summarizing the experiences with unrelated donor umbilical cord blood transplantation.66-68 In these reports, 32 patients received an unrelated donor umbilical cord blood transplant for malignant disorders and 11 patients for nonmalignant disorders (Table 4). The median age and weight of patients were 4.1 years (range, 0.1 to 23.5 years) and 18.7 kg (range, 3.3 to 79.0 kg), respectively (Table 4).66 67 Eight patients received unrelated donor umbilical cord blood transplants that were matched at all three HLA loci (A, B, and DRB1) by serology and molecular typing, and 16 patients received unrelated donor umbilical cord blood transplants that were disparate at 1 HLA locus. Fourteen patients were disparate at 2 HLA loci and 5 patients were disparate at three HLA loci (Table 4).
Umbilical cord blood graft characteristics and hematologic recovery. Unrelated donor umbilical cord blood grafts were provided by the New York Blood Center. In these two reports, the time between search request and donor confirmation ranged between 12 and 291 days.66,67 The median volume of umbilical cord blood collected was 83.0 mL (range, 35 to 214 mL) and the median number of nucleated cells/kg recipient weight was 3.7 × 107 (range, 0.7 to 40 × 107; Table 4).66,67 The median time to ANC ≥500/μL and platelet count ≥50,000/μL was 23 days (range, 14 to 41 days) and 75 days (range, 50 to >120 days), respectively.66 67
GVHD. For the patients in the two reports, the overall incidence of grade II-IV and grade III-IV aGVHD by day 100 was 57% and 9%, respectively (Table 4). In patients fully matched at HLA A, B, DR by serology and DRB1 by high resolution DNA typing, the incidence of grade II-IV and III-IV aGVHD was 33% and 16%, respectively; for a 1-antigen mismatch, 43% and 7%, respectively; and for a 2- or 3-antigen mismatch, 73% and 7%, respectively. The combined groups of fully matched and 1-antigen mismatched (6/5 and 5/6) had an incidence of grade II-IV and III-IV aGVHD of 40% and 5%, respectively, compared with the group with either 2- or 3-antigen mismatch of 73% and 7%, respectively (Table 4).66 67
Survival. Twenty-four patients survived to day 100, 16 patients with malignant disease (16 of 32 [50%]) and 8 patients with nonmalignant disease (8 of 11 [72%]).66 67
Two recent abstracts have reported the preliminary results of larger numbers of recipients of unrelated donor umbilical cord blood transplantation. Gluckman et al69 reported the result of 65 unrelated donor umbilical cord blood transplants reported to the European Cord Blood Transplant Registry. The median nucleated cell dose per kilogram was 3.7 × 107 /kg, and there was a 21% incidence of grade III-IV aGVHD and a 1-year survival rate of 29% in this population undergoing unrelated donor cord blood transplantation. Rubinstein et al70 recently reported the preliminary results of unrelated donor umbilical cord blood transplants that received their unrelated donor umbilical cord blood grafts from the New York Blood Center. Two hundred seventy-two unrelated donor umbilical cord blood transplants (220 from the United States and 52 foreign) were performed with umbilical cord blood from the New York Blood Center, and the incidence of severe grade III-IV aGVHD was 23% and the median time to platelet recovery ≥50,000/μL was 72 days.
These initial results suggest that unrelated donor umbilical cord blood transplantation is feasible in children, is associated with a decreased time of search, and has comparable myeloid engraftment but delayed platelet reconstitution when compared with unrelated donor BMT. It is too early to conclusively determine whether the incidence of severe aGVHD will be decreased compared with unrelated donor BM or whether there is the potential of using more disparate grafts from unrelated donor umbilical cord blood compared with unrelated donor BM. Although there are several reports on the use of umbilical cord blood for adult recipients,71 72 more data are needed. Preliminary results in 19 adult patients undergoing unrelated donor cord blood transplantation at Duke University suggest that the incidence of nonengraftment is similar to that reported in children (J. Kurtzberg, personal communication, May 1997). Carefully designed, future prospective randomized trials will be necessary to answer these important questions.
Comparison to unrelated donor unmanipulated BMT in children. To date, the largest series of unrelated donor BMT in children (n = 88) was reported by Balduzzi et al73 (Table 5). Although the age range of the recipients was similar, the median age was almost twice as high in the unrelated donor BM recipients (9.1 v 4.1 years) and the median number of nucleated cells per kilogram was more than 1 log higher (42 v 3.7 × 107/kg) in the children receiving unrelated donor BM (Table 5). Despite a 1 log higher number of nucleated cells per kilogram, the median time to myeloid engraftment was similar in both groups. However, the median days to platelet recovery was significantly delayed in the children receiving unrelated donor umbilical cord blood versus BM (75 v 23 days; Table 5). The median time of 23 days to recover the platelet count ≥50,000/μL after unmanipulated unrelated donor BMT in children may be somewhat misleading, because 17 patients were not included in the analysis because of discharge before platelet recovery and another 27 patients were eliminated from the analysis because of relapse or death before platelet recovery. This discrepancy between myeloid and platelet engraftment may reflect a 100-fold increase in CFU-GM compared with only a onefold or twofold increase in CFU-Meg in umbilical cord blood versus adult peripheral blood (Fig 1). Additionally, the number of CD34/41+ cells in umbilical cord blood grafts is 1 log less than seen under optimal conditions of platelet reconstitution after mobilized autologous peripheral blood stem cell transplantation.74
The incidence of both grade II-IV and grade III-IV aGVHD appeared to be less after unrelated donor umbilical cord blood versus unrelated donor BMT in children (Table 5). Similarly, matched (6/6) grafts using unrelated donor umbilical cord blood compared with unrelated donor BM in children have a decreased incidence of grade II-IV and III-IV aGVHD (33% v 83% and 16% v 37%, respectively; Table 5). In comparison, the incidence of grade II-IV aGVHD after T-cell–depleted unrelated donor BMT in children was 33%, with similar survival rates.75 Additionally, for HLA 1-antigen mismatched grafts, the incidence of grade II-IV and III-IV aGVHD is less in children after unrelated donor umbilical cord blood versus unmanipulated unrelated donor BM (43% v 98% and 7% v 62%, respectively; Table 5). Although this lower incidence of severe aGVHD after unrelated donor umbilical cord blood could reflect an immaturity in umbilical cord blood effector cells, a decrease in the number of nucleated cells per kilogram in the graft and/or a lower age of the recipient after unrelated donor umbilical cord blood transplant could account for the differences in the incidence of severe aGVHD.
AUTOLOGOUS UMBILICAL CORD BLOOD TRANSPLANTATION AND POTENTIAL FOR GENE THERAPY
BMT has been used successfully to treat a variety of genetic diseases. Parkman76 suggested more than a decade ago that BMT could be used to treat a variety of conditions that were secondary to a single genetic deletion. Hematopoietic stem cells are ideal cells for gene transduction because of their ability to renew themselves and differentiate into progeny cells and generate a self-perpetuating cell population that contains the transduced gene for the lifetime of the patient.77 Specific diseases that could be candidates for gene therapy following gene transduction into hematopoietic stem cells include thalassemia, sickle cell anemia, Fanconi anemia, severe combined immune deficiency secondary to adenosine deaminase deficiency (ADA) or purine nucleoside phosphorylase (PNP) deficiency, chronic granulomatous disease, leukocyte adhesion deficiency, Gaucher's disease, and a variety of other metabolic/storage deficiencies.77 Autologous umbilical cord blood hematopoietic stem cells potentially could be used to correct genetic deficiencies at birth after successful gene transduction and autologous umbilical cord blood stem cell transplantation.
There have been recent reports of treatment using gene therapy after autologous lymphocyte and stem cell transductions.78-84 T lymphocytes transduced ex vivo using a retroviral vector containing the normal human ADA gene and infused have been shown to express the transduced ADA gene and partially improve the immune status of patients with severe combined immune deficiency.78 Additionally, the neomycin-resistance gene (NeoR) has been successfully transduced into tumor-infiltrating lymphocytes, reinfused into 5 patients with advanced melanoma using retroviral gene transduction,79 and transduced into human BM mononuclear cells subsequently transplanted into adult cancer patients, using the retroviral vector, N2.80,85 Recently, several genes, including the human multiple drug resistance gene (MDR) and the murine ADA, were successfully transduced into isolated CD34+ BM stem cells by retroviral-mediated gene transfer.81,82 Human CD34+ stem cells isolated from peripheral blood have also been successfully used as targets for retroviral gene transduction with gene transfer of two genes encoding the components of the superoxide-generating NADPH oxidase (gp91phox and gp22phox).83
Recently, there was noted an increase in the efficiency of gene transfer after retroviral transduction in neonatal (umbilical cord) compared with adult progenitor cells.84 Incubation of hematopoietic stem cells (CD34+) ex vivo with a variety of cytokines, including SCF, IL-3, IL-6, etc, and cotransplantation of marrow stroma expressing human IL-3 has been shown to enhance sustained human hematopoiesis and gene transduction.86 Subsequently, Kohn et al87 transplanted autologous umbilical cord blood CD34+ stem cells that had been transduced with an ADA-containing retrovirus (LASN) into three neonates with severe combined immune deficiency secondary to ADA deficiency. Although all 3 patients are currently being treated with PEG-ADA, reverse transcription polymerase chain reaction (RT-PCR) analysis has shown successful expression of the ADA gene. An analysis of the efficiency of LASN-ADA transduction has demonstrated a transduction frequency of 1/3,000 to 1/100,000 peripheral blood leukocytes and 1/10,000 BM mononuclear cells.84 This pilot study has suggested that autologous umbilical cord blood stem cells can be successfully transduced with long-term expression. Notably, the frequency of transduced T cells has increased to the range of 3% to 10%. with graded reduction in PEG-ADA dose (Parkman and Kohn, personal communication, December 1996). However, the transduction efficiency is still low and may not translate into significant clinical activity. Future studies will be required to determine the clinical success of this form of gene therapy and to develop new and better approaches to obtain a higher degree of gene expression and protein production after gene transduction.
ETHICAL ISSUES ASSOCIATED WITH UMBILICAL CORD BLOOD TRANSPLANTATION
The collection of umbilical cord blood poses a number of ethical issues that remain to be resolved. If umbilical cord blood is considered to be like any other organ or tissue, then consent by the tissue donor must be obtained. In the case of umbilical cord blood, the donor is always a minor, leaving it to the infant's mother to provide consent. Other issues include timing of the consent. For obvious reasons, consent should not be obtained when the infant's mother is in active labor or immediately after delivery. Other questions include whether consent should also be obtained from the infant's father. Presuming the umbilical cord blood remains stored for decades, what rights will the infant donor have at 21 years of age? Will the donor have any rights to umbilical cord blood that was previously given to the unrelated donor umbilical cord blood bank? To make the matter more complex, who gives consent if there is a surrogate mother? Although some of this may seem far-fetched, umbilical cord blood is being collected at an increasing frequency. At some point, many of these issues will likely surface.
Alternatively, umbilical cord blood could be considered as discarded tissue. As such, consent would not be required. Although the collection of umbilical cord blood from the delivered placenta poses no risk to mother or infant, this classification would also raise some difficult questions. What would we do about the issue of human immunodeficiency virus (HIV) testing? How do we protect the rights of individuals whose religious and cultural practices would not allow the collection and transplantation of placental blood? What if a genetic disease (eg, sickle cell disease, thalassemia, G6PD deficiency, hereditary spherocytosis) is detected upon subsequent testing? Although some would argue that a link between donor and umbilical cord blood graft should be maintained, this may be costly and logistically very difficult. Moreover, it should be recalled that such tests were only performed because the umbilical cord blood was collected for future use in an allogeneic recipient; if it were not intended for that use, no tests would have been performed and it would have been discarded. Nonetheless, earlier identification of a disease might benefit some children.
However, ethical issues are not limited to the area of consent and disease screening; other issues include (1) the potential of deliberate conception and embryo cloning for the purpose of producing an HLA-identical tissue donor and (2) commercialization. These are emotionally charged issues that are beyond the scope of these investigators. Clearly, these issues need to be considered by medical ethicists and various members of the medical community so that a consensus opinion might be made available to help guide clinicians and storage services in their practices.
REGULATORY ISSUES ASSOCIATED WITH UMBILICAL CORD BLOOD TRANSPLANTATION
Over the past 5 years, umbilical cord blood has moved from the status of biologic waste to a potentially important source of hematopoietic stem cells. Clinical experience has already shown that placental and umbilical cord blood contains sufficient numbers of hematopoietic stem cells to engraft at least small recipients consistently. As a result, banks of umbilical cord blood have been developed or are being considered throughout the United States and Europe as well as many countries in South America and Asia. Moreover, the potential of storing the child's own umbilical cord blood for future use as a form of biologic insurance has also been considered, resulting in the establishment of commercial banks. Hence, there has been an explosion of banking activity, making the need for standard policies and procedures particularly acute.
Although there will be debate on the optimal procedures for handling umbilical cord blood, there is already extensive experience in handling blood and marrow. Many of the procedures for obtaining consent, collection, testing, cryopreservation, RBC depletion, histocompatibility testing, and sample labeling are likely to be extracted from existing operating manuals for blood. However, a major problem will be in the definition of suitable product. Moreover, the definition of suitable product may vary depending on the type of recipient — self, sibling, or unrelated donor.
In February 1997, the US Food and Drug Administration (FDA) announced a Proposed Approach to Regulation of Cellular and Tissue-Based Products that includes hematopoietic stem and progenitor cells derived from placental/umbilical cord blood and peripheral blood.88 The proposed approach focuses on five major considerations for such products, which include (1) prevention of the transmission of communicable diseases, (2) process controls necessary to preserve the integrity and function of products so that they will work as they are intended, (3) assurance of clinical safety and effectiveness, (4) necessary labeling and permissible promotion for proper use of the product, and (5) a means to monitor and communicate with the cell and tissue industry. The specific regulations to implement the FDA's proposed approach will be phased in during the next several years through a public rule and comment process.
Although it is clear that umbilical cord blood should be considered as a viable source of hematopoietic stem cells for transplantation, we are still in the learning phase. Just as the transplant physician requires assurances that the umbilical cord blood graft product has been processed and stored properly, the director of umbilical cord blood banks may require assurances that the transplant team will use the graft properly. Clinical outcome data may impact upon the bank's methods of processing and graft characterization. Policies for defining the qualifications of transplant teams and the appropriate use of umbilical cord blood should not be overlooked. Similar issues had to be addressed by the NMDP. Perhaps existing societies, such as the International Society of Hematotherapy and Graft Engineering (ISHAGE) and The American Society of Blood and Marrow Transplantation (ASBMT), will aid in addressing these difficult issues.
SUMMARY AND FUTURE PERSPECTIVES
The large-scale collection and storage of umbilical cord blood stem cells is no longer just a concept. Pilot programs for the banking of unrelated donor umbilical cord blood have already begun in the United States and Europe and are being considered in Asia, Canada, and South America.89-91 Not only is there the potential for reducing the time from search initiation to the time of donor stem cell acquisition, but also the potential for reducing the risks associated with unrelated donor BMT and the hope of remedying the shortage of donors from ethnic and racial backgrounds that are currently underrepresented in most unrelated donor programs. Even with the creation of such banks, the collection of umbilical cord blood should certainly still be considered when a child with leukemia, lymphoma, neuroblastoma, marrow failure syndrome, immunodeficiency state, or inborn error of metabolism has a mother who is pregnant.
A multi-institutional cooperative trial is currently being designed by the National Heart, Lung, and Blood Institute (NHLBI) to determine the safety and efficacy of HLA-matched and mismatched unrelated donor umbilical cord blood transplantation in pediatric and adult recipients. Even in instances in which a preliminary search of the unrelated donor marrow registry identifies a potential unrelated BM donor, the use of immediately available, cryopreserved, HLA-compatible umbilical cord blood over BM may be justified in specific patients, such as those with high-risk leukemia, myelodysplastic syndrome, severe aplastic anemia, severe combined immune deficiency, and metabolic disease, because these patients are at high risk of early death due to disease progression or overwhelming infection. Although there have been considerable efforts to shorten the time of marrow acquisition from unrelated donors, the median interval from initial search request to BM harvest remains at 3.5 months. Furthermore, patients with diseases expected to remain stable over a 3- to 5-month period of a donor search without relapse or overwhelming infection (eg, chronic myelogenous leukemia, acute myelogenous leukemia, Wiskott-Aldrich syndrome) might be considered ineligible for umbilical cord blood transplantation unless the marrow donor registry fails to identify a suitable donor in a fixed period of time. As more is learned about the safety of umbilical cord blood transplantation, these restrictive criteria are likely to be modified.
Although the clinical results thus far have been very encouraging, there are potential disadvantages with umbilical cord blood as well: (1) lower risk of GVHD might translate into a higher risk of relapse (ie, absence of graft-versus-leukemia effect); (2) higher risk of genetic disorder transmission due to inability to observe growth and development of stem cell donor; and (3) an insufficient number of stem and progenitor cells in umbilical cord blood for larger recipients, limiting this stem cell source to pediatric patients. Although these risks have yet to be realized, they cannot be excluded.
Despite these limitations, the use of unrelated donor umbilical cord blood has several known advantages, including (1) immediate availability (minimum of 2 weeks for confirmatory HLA testing); (2) absence of donor risk; (3) absence of donor attrition; and (4) a very low risk of transmissible infectious diseases, such as cytomegalovirus (CMV) and Epstein-Barr virus (EBV).92-94 Other advantages potentially include (1) lower risk of aGVHD and (2) the ability to expand available donor pools in targeted ethnic and racial minorities currently underrepresented in all marrow donor registries.
This review of sibling and unrelated donor umbilical cord blood transplants (Table 6) suggests that (1) the rate of neutrophil recovery after umbilical cord blood transplantation is comparable to that observed after allogeneic BMT; (2) there is a potentially higher risk of graft failure in recipients of sibling umbilical cord blood transplants with nonmalignant states; and (3) platelet reconstitution (≥50,000/μL) appears delayed compared with matched related allogeneic BMT.
There are several unresolved issues that will need to be addressed in the future: optimal methods for harvesting, processing, and storing umbilical cord blood units; minimum number of banked umbilical cord blood units necessary to support the needs of recipients who lack matched family member donors; the ethical and regulatory issues of collecting and storing umbilical cord blood units; the minimum number of stem/progenitor cells required for hematopoietic engraftment in larger recipients; the risks of acute and chronic GVHD with mismatched unrelated donor umbilical cord blood transplants; the malignant relapse rate after umbilical cord blood transplantation; the potential for gene therapy with autologous umbilical cord blood transplantation; ex vivo expansion of umbilical cord blood stem/progenitor cells; and immunologic reconstitution after umbilical cord blood transplantation.
Recently, the NHLBI has approved the development of three regional umbilical cord blood banks and a consortium of seven transplant centers to study many of the above-mentioned issues. Additionally, a consortium in Europe and several single-center studies are currently underway to answer many of the questions that still remain regarding this potential form of therapy. Over the next several years, many answers will be forthcoming to provide us with guidance on how to appropriately use this new and exciting form of alternate stem cell therapy.
ACKNOWLEDGMENT
The authors thank Linda Rahl for her assistance in the preparation of this manuscript. We also thank Jeff Buzby, PhD, Vicki Slone, PhD, Yu Suen, PhD, Carmella van de Ven, MA, Robin Ellis, RN, and Leonard Sender, MD, for their critical review of this manuscript.
Supported by grants from the Pediatric Cancer Research Foundation, the CHOC PSF Research and Education Foundation, the Walden W. and Jean Young Shaw Foundation (M.S.C.), The Childrens Cancer Research Fund, Bone Marrow Transplant Research Fund, and the National Institutes of Health Grants No. P01-CA65493 and P01-CA21737 (J.E.W.).
Address reprint requests to Mitchell S. Cairo, MD, Director of Hematology/Oncology Research, Children's Hospital of Orange County, 455 S Main St, Orange, CA 92868.