Abstract
Tumor necrosis factor (TNF ) and Fas ligand (FasL) have been implicated in the pathogenesis of graft-versus-host disease (GVHD), which is a major complication after allogeneic bone marrow transplantation. We examined here the ameliorating effect of a metalloproteinase inhibitor (KB-R7785) that inhibits TNF-α and FasL release in a lethal acute GVHD model in mice. Administration of KB-R7785 into (BALB/c × C57BL/6) F1 that received C57BL/6 spleen cells markedly reduced the mortality and weight loss in association with minimal signs of GVHD pathology in the liver, intestine, and hematopoietic tissues. The ameliorating effect of KB-R7785 was superior to that of anti–TNF-α antibody. Our results suggest that KB-R7785 could be a potent therapeutic agent for GVHD.
ALLOGENEIC bone marrow transplantation (BMT) has been a clinical treatment modality for hematopoietic disorders and hematologic malignancies.1 Graft-versus-host disease (GVHD) remains a principal complication after allogeneic BMT, occurring in up to 75% of unmanipulated HLA-matched marrow recipients.2 An acute lethal form of GVHD is caused by activation of the host-reactive donor T cells as represented by a murine model that is caused by transfusion of C57BL/6 spleen cells into (DBA/2 × C57BL/6)F1 or (BALB/c × C57BL/6)F1 mice.3 Acute GVHD affects the skin, liver, gastrointestinal tract, and lymphoid tissues, where inflammatory reactions characterized by mononuclear cell infiltration and histopathologic damages take place, which lead to erythroderma, diarrhea, wasting, and, finally, death. The effector mechanisms leading to the GVHD-associated tissue damage have not been well clarified.
Tumor necrosis factor-α (TNF-α) has been implicated in the pathogenesis of GVHD. TNF-α has been identified as a principal mediator of cachexia in rodents4 and is a potent mediator of various inflammatory diseases.5 It has been shown that serum levels of TNF-α were increased in patients undergoing GVHD after allogeneic BMT6 and that administration of anti–TNF-α antibody markedly reduced the weight loss and mortality in a mouse model of GVHD.7 Some beneficial effects of an anti–TNF-α monoclonal antibody (MoAb) for the treatment of refractory acute GVHD have been obtained in the phase I-II clinical trials.8 These observations substantiate that TNF-α is an important target for the clinical treatment of GVHD.
Recently, the ligand for Fas (FasL) has been also implicated in the pathogenesis of GVHD. Fas (APO-1, CD95) is a member of the TNF receptor family and transmits an apoptotic cell death signal upon ligation by FasL.9
Fas is expressed in various tissues, including the skin, liver, and intestine, that are target tissues of GVHD.10 A critical involvement of FasL in the development of hepatic and cutaneous GVHD pathology has been shown in a murine model of acute GVHD.11 A partial contribution of FasL to the mortality was also noted in a different murine model of acute GVHD.12
TNF-α and FasL are type II integral membrane proteins belonging to the TNF family and predominantly expressed on activated macrophages and activated T cells, respectively.13 It has been known that TNF-α is efficiently shed from the macrophage surface as a soluble cytokine. Several recent studies have shown that some metalloproteinase mediates the TNF-α processing and that some metalloproteinase inhibitors protected mice against a lethal endotoxin shock by reducing the serum TNF-α levels.14-16 We recently found that FasL is also efficiently released from the activated T-cell surface and that some metalloproteinase inhibitors could inhibit the shedding.17 Moreover, elevated serum levels of soluble FasL have been reported in some patients with large granular lymphocytic leukemias, which may be responsible for systemic tissue damage, including hepatic failure and neutropenia.18 In the context of critical contribution of both TNF-α and FasL to the systemic GVHD pathology, we here examined the therapeutic effect of a metalloproteinase inhibitor (KB-R7785) that blocks both TNF-α and FasL release in a lethal acute GVHD model in mice. KB-R7785 exhibited a potent ameliorating effect superior to anti–TNF-α MoAb. Pathologic and clinical implications are discussed.
MATERIALS AND METHODS
Mice.Six-week-old female BALB/c (H-2d), C57BL/6 (B6;H-2b), and (BALB/c × C57BL/6)F1 (CBF1;H-2b/d) mice were purchased from SLC (Shizuoka, Japan) and maintained in our animal facilities.
Reagents.The following hydroxamic acid-based metalloproteinase inhibitors were synthesized by Kanebo Ltd (Osaka, Japan): [4-(N-hydroxyamino)-2R-isobutyl-3S-methylsuccinyl]-L-phenylglycineN-methylamide (KB-R7785)19 and [4-(N-hydroxyamino)-2R-isobutyl-3S-methylsuccinyl]-L-3-(5,6,7,8-tetrahydro-1-naphthy1) alanine-N-methylamide (KB-R8301).17 A nonhydroxamate derivative of KB-R8301, (4-hydroxy-2R-isobutyl-3S-methylsuccinyl)-L-3-(5,6,7,8tetrahydro-1-naphthyl) alanine-N-methylamide (KB-R8845), was also synthesized as a negative control.17 For in vitro studies, these compounds were dissolved in dimethyl sulfoxide at 10 mmol/L as stock solutions. For in vivo administration, these compounds were suspended in 0.5% carboxymethyl cellulose (CMC) at 10 mg/mL. D-galactosamine and Escherichia coli lipopolysaccharide (LPS) were purchased from Sigma (St Louis, MO). A neutralizing antimouse TNF MoAb (MP-6 XT22) was obtained from PharMingen (San Diego, CA).
Cell culture.A human monocytic leukemia cell line THP-1 (3 × 105 cells) was stimulated with 1 μg/mL LPS for 72 hours in the presence or absence of the inhibitors in RPMI1640 medium containing 10% fetal calf serum, 100 μg/mL each streptomycin and penicillin, and 2 mmol/L glutamine (culture medium) for estimating the TNF-α release. A human FasL cDNA-transfected mouse T-lymphoma cell line (hFasL/L5178Y) was generated as described previously17 and 1 × 106 cells were cultured with or without the inhibitors for 24 hours in the culture medium for estimating the FasL release.
Enzyme-linked immunosorbent assay (ELISA).Human TNF-α in the culture supernatants and mouse TNF-α in the sera were evaluated with commercial ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer's instruction. FasL in the culture supernatants was quantitated by sandwich ELISA using two anti-FasL MoAbs, as described previously.17
Induction of lethal endotoxin shock.BALB/c mice (8 mice in each group) received 30 or 100 mg/kg of the inhibitors or 0.5% CMC vehicle alone intraperitoneally (IP). After 1 hour, these mice were intravenously (IV) injected with 30 mg of D-galactosamine and 3 μg of LPS. Sera were obtained at 1 hour after the LPS injection and subjected to the TNF-α ELISA.
Induction of lethal acute GVHD.CBF1 mice (10 mice in each group) were IV injected with 1 × 108 spleen cells from B6 mice on day 0 and day 7. Some mice received 0.5 mg of anti–TNF-α MoAb IP on day 0 and day 7. Some mice received 2 mg of KB-R7785 in 200 μL of 0.5% CMC or 200 μL of 0.5%CMC alone IP every day from day 0 to 20. Survival was monitored until day 28. The body weight of the survivors was measured at days 0, 7, 14, and 21. Sera were obtained weekly and subjected to the TNF-α ELISA. On day 10 for the GVHD group or day 14 for the other groups, some mice in each group were killed and their liver, intestine, spleen, and bone marrow were subjected to histopathologic examination.
Histopathology.Tissues were fixed in 10% buffered formalin and paraffin embedded. Sections were stained with hematoxylin and eosin and examined under microscopy.
Flow cytometric analysis.Splenocytes were prepared from normal CBF1, GVHD, or KB-R7785-treated mice on day 14 and stained with fluorescein isothiocyanate-conjugated anti–H-2Kd (SF1-1.1; PharMingen), biotin-conjugated anti–H-2Kb (AF6-88.5; PharMingen), and phycoerythrin-conjugated anti-CD4 (RMG4-5; PharMingen), anti-CD8 (53-6.7; PharMingen), or anti-B220 (RA3-6B2; PhaMingen) MoAbs followed by allophycocyanin (APC)-conjugated avidin (PharMingen). Cells (1 × 104) were analyzed on FACStar Plus equipped with C32.Lysis2 program (Becton Dickinson, San Jose, CA). Recipient and donor lymphocytes were identified as H-2Kd+Kb+ and H-2Kd-Kb+ cells, respectively. Cell numbers of CD4+ T, CD8+ T, and B220+ B cells of recipient or donor origin were calculated from the total numbers of splenocytes recovered and the percentages of each subpopulation were determined by the three-color analysis.
RESULTS
Characterization of KB-R7785.KB-R7785 (Fig 1A)19 and KB-R830117 are hydroxamic acid-based metalloproteinase inhibitors, which exhibit potent inhibitory effects against matrix metalloproteinases (MMP). IC50s of KB-R7785 and KB-R8301 for MMP-1 (collagenase), MMP-3 (stromelysin), and MMP-9 (gelatinase) are 3.0, 1.9, and 3.9 nmol/L and 0.3, 0.6, and 0.3 nmol/L, respectively. We previously showed that KB-R8301 efficiently inhibited the release of both TNF-α and FasL from activated T cells.17 KB-R7785 inhibited the TNF-α release from LPS-stimulated THP-1 cells to a similar extent to KB-R8301 (Fig 1B). KB-R7785 also inhibited the FasL release from FasL/L5178Y cells in a dose-dependent manner but to a lesser extent than KB-R8301 (Fig 1C). In contrast, a nonhydroxamic acid control compound (KB-R8845) inhibited the release of neither TNF-α nor FasL. We verified the in vivo effects of KB-R7785 and KB-R8301 in a murine lethal endotoxin shock model, which is predominantly mediated by soluble TNF-α and has been prevented by a similar metalloproteinase inhibitor.16 As represented in Fig 1D, all control mice died within 24 hours after the injection of D-glactosamine and LPS, whereas all the mice receiving 100 mg/kg of KB-R7785 were protected. A lower dose of KB-R7785 (30 mg/kg) exerted a partial protection (4 of 8 mice survived). The serum TNF-α level in the control mice peaked (1.70 ± 0.37 ng/mL) at 1 hour after the LPS injection, but was barely detectable (22 ± 18 pg/mL) in the mice receiving a previous dose of 100 mg/kg KB-R7785 (not shown). Despite rather stronger or comparable inhibitory effects in vitro, KB-R8301 exerted a less protective effect than KB-R7785 and only a partial protection was obtained even at 100 mg/kg. This limited effect of KB-R8301 in vivo might result from the inferior pharmacokinetics of this compound. Based on its superior effect in vivo, we then applied KB-R7785 to the following acute GVHD study.
Characterization of KB-R7785. (A) Structure of KB-R7785: [4-(N-hydroxyamino)-2R-isobutyl-3S-methylsucciny1]-L-phenylglycine-N-methylamide. (B) Inhibition of TNF-α release from LPS-simulated THP-1. THP-1 cells were stimulated with 1 μg/mL LPS for 72 hours in the presence of the indicated dose of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). TNF-α in the supernatants was measured by ELISA. Data are indicated as a percentage of control TNF-α release in the absence of inhibitors (112 ± 39 pg/mL) and are the mean ± SD of triplicate samples. (C) Inhibition of FasL release from hFasL/L5178Y. hFasL/L5178Y cells were cultured for 24 hours in the presence of the indicated doses of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). FasL in the supernatants was measured by ELISA. Data are indicated as a percentage of control FasL release in the absence of inhibitors (13.9 ± 3.3 ng/mL) and are the mean ± SD of triplicate samples. (D) Prevention of lethal endotoxin shock. BALB/c mice received IP 30 mg/kg (▴) or 100 mg/kg (▪) of KB-R7785, 100 mg/kg of KB-R8301 (♦), or an equal volume of 0.5% CMC (•). After 1 hour, these mice were injected IV with 30 mg of D-galactosamine and 3 μg of LPS. The results are shown as a survival rate of 8 mice in each group at the indicated time points.
Characterization of KB-R7785. (A) Structure of KB-R7785: [4-(N-hydroxyamino)-2R-isobutyl-3S-methylsucciny1]-L-phenylglycine-N-methylamide. (B) Inhibition of TNF-α release from LPS-simulated THP-1. THP-1 cells were stimulated with 1 μg/mL LPS for 72 hours in the presence of the indicated dose of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). TNF-α in the supernatants was measured by ELISA. Data are indicated as a percentage of control TNF-α release in the absence of inhibitors (112 ± 39 pg/mL) and are the mean ± SD of triplicate samples. (C) Inhibition of FasL release from hFasL/L5178Y. hFasL/L5178Y cells were cultured for 24 hours in the presence of the indicated doses of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). FasL in the supernatants was measured by ELISA. Data are indicated as a percentage of control FasL release in the absence of inhibitors (13.9 ± 3.3 ng/mL) and are the mean ± SD of triplicate samples. (D) Prevention of lethal endotoxin shock. BALB/c mice received IP 30 mg/kg (▴) or 100 mg/kg (▪) of KB-R7785, 100 mg/kg of KB-R8301 (♦), or an equal volume of 0.5% CMC (•). After 1 hour, these mice were injected IV with 30 mg of D-galactosamine and 3 μg of LPS. The results are shown as a survival rate of 8 mice in each group at the indicated time points.
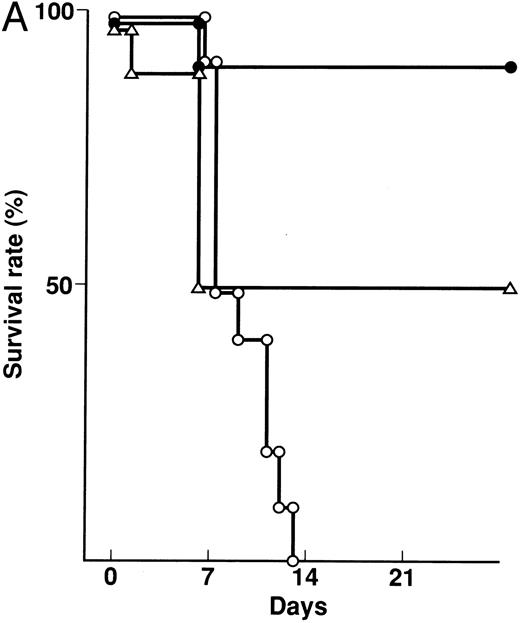
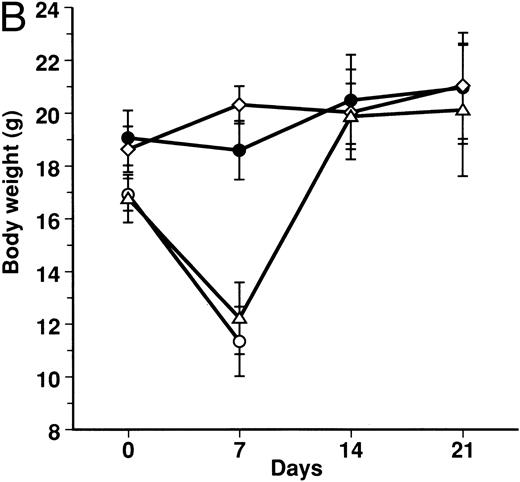
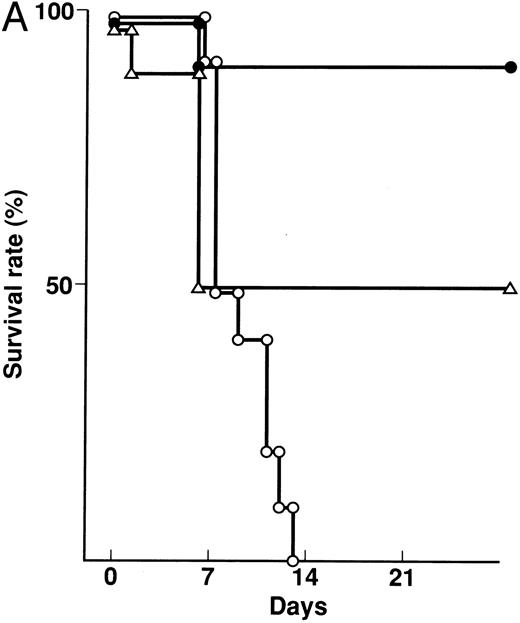
Effect of KB-R7785 on GVHD-induced mortality and weight loss.A lethal acute type of GVHD was induced by IP injection of B6 splenocytes into CBF1 mice. These mice receiving 2 mg of KB-R7785 in 0.5% CMC or 0.5% CMC vehicle alone daily IP from day 0 to 20. For comparison, another group of mice received neutralizing anti-TNF MoAb. As represented in Fig 2A, all of the 0.5% CMC control mice undergoing GVHD died within 14 days. In these mice, clinical symptoms of acute GVHD, such as hair ruffling, lesser mobility, and weight loss (Fig 2B), became apparent within 1 week. However, skin lesions, such as alopecia and desquamative rash, apparently were not developed.
Prevention of lethal acute GVHD by KB-R7785. Lethal acute GVHD was induced by IV injection of B6 splenocytes into CBF1 mice on days 0 and 7. Ten mice in each group received IP 2 mg of KB-R7785 in 0.5% CMC (•) or 0.5% CMC (○) every day from day 0 to 20 or 0.5 mg anti-TNF MoAb (▵) on days 0 and 7. Survival (A) was monitored every day until day 28. Body weight (B) was measured at the indicated days and is indicated as the mean ± SD of 5 to 10 mice. In (B), the body weight of age-matched normal CBF1 (⋄) is also plotted.
Prevention of lethal acute GVHD by KB-R7785. Lethal acute GVHD was induced by IV injection of B6 splenocytes into CBF1 mice on days 0 and 7. Ten mice in each group received IP 2 mg of KB-R7785 in 0.5% CMC (•) or 0.5% CMC (○) every day from day 0 to 20 or 0.5 mg anti-TNF MoAb (▵) on days 0 and 7. Survival (A) was monitored every day until day 28. Body weight (B) was measured at the indicated days and is indicated as the mean ± SD of 5 to 10 mice. In (B), the body weight of age-matched normal CBF1 (⋄) is also plotted.
Administration of KB-R7785 markedly reduced the mortality, and 9 of 10 mice survived indefinitely (Fig 2A). No significant weight loss was observed as compared with the age-matched normal mice until day 21 (Fig 2B) and even after discontinuation of the treatment at day 20 (not shown). No apparent clinical symptoms of acute GVHD was observed in these mice.
In contrast, anti-TNF treatment exerted lesser protective effect. Half (5 of 10) of mice died within 7 days (Fig 2A), and the weight loss was not significantly ameliorated during this period (Fig 2B). However, body weight of the survivors was improved thereafter and recovered to normal level during days 7 to 14 (Fig 2B).
An elevated serum level of TNF-α (147.3 ± 64.4 pg/mL) was observed in the GVHD control mice on day 7. In contrast, serum TNF-α was not detectable (<10 pg/mL) in the KB-R7785– or anti-TNF–treated mice (data not shown).
Effect of KB-R7785 on GVHD-associated histopathologies.The development of skin lesions was not apparent in the present model of GVHD as also estimated by histologic examination (not shown).
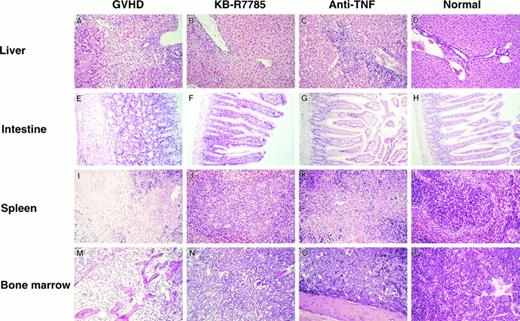
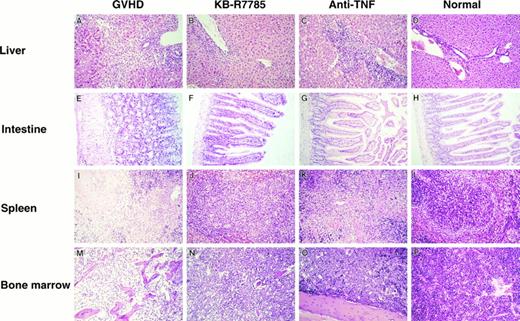
In the liver from the control mice undergoing GVHD, a massive infiltration of mononuclear cells and fibrosis were observed mainly in the periportal areas (Fig 3A). A similar hepatic pathology was observed in the liver from the anti-TNF–treated mice (Fig 3C). In contrast, such inflammatory changes were minimal in the liver from the KB-R7785-treated mice (Fig 3B).
Histopathological examination. Induction of lethal acute GVHD and administration of KB-R7785 or anti-TNF MoAb were performed as described in Fig 2. On day 10 for the GVHD group or day 14 for the other groups, 3 mice in each group were killed. Paraffin section of the liver, intestine, spleen, and bone marrow were stained by hematoxylin and eosin. Sections from age-matched normal CBF1 are also represented. (Original magnification × 100.)
Histopathological examination. Induction of lethal acute GVHD and administration of KB-R7785 or anti-TNF MoAb were performed as described in Fig 2. On day 10 for the GVHD group or day 14 for the other groups, 3 mice in each group were killed. Paraffin section of the liver, intestine, spleen, and bone marrow were stained by hematoxylin and eosin. Sections from age-matched normal CBF1 are also represented. (Original magnification × 100.)
The gut from the control mice undergoing GVHD exhibited a dilatation, flattening of the villi, and elevation of the crypts, which are characteristics of intestinal GVHD (Fig 3E). There was no marked infiltration of mononuclear cells in this model. All of these lesions were almost absent in the KB-R7785–treated mice (Fig 3F ) as well as in the anti-TNF–treated mice (Fig 3G).
The spleen from the control GVHD mice showed a marked lymphoid atrophy and structural disorganization (Fig 3I). A similar change was observed in the anti-–TNF–treated mice (Fig 3K). In contrast, such a change was minimal in the KB-R7785–treated mice (Fig 3J).
The bone marrow from the control GVHD mice showed a severe atrophy of most hematopoietic cells (Fig 3M). In contrast, such a change was not observed in the KB-R7785–treated mice (Fig 3N) as well as in the anti-TNF–treated mice (Fig 3O).
Effect of KB-R7785 on GVHD-associated lymphoid hypoplasia.Cell numbers of CD4+ T, CD8+ T, and B220+ B cells of recipient (H-2Kd+Kb+) or donor (H-2Kd−Kb+) origin in the spleen of normal CBF1, GVHD, or KB-R7785–treated mice on day14 were calculated from the total numbers of splenocytes recovered and the percentages of each subpopulation were determined by three-color flow cytometric analysis (Table 1). In the splenocytes from GVHD mice, CD4+ T cells and especially B220+ B cells of host origin were substantially decreased as compared with normal CBF1 mice. In contrast, no decrease of host B cells was observed and both CD4+ and CD8+ host T cells were rather increased in the KB-R7785–treated mice. It was also noted that donor-derived CD4+ T, CD8+ T, and B220+ B cells were increased in the KB-R7785–treated mice as compared with the GVHD mice, representing a chimeric state of the KB-R7785–treated recipients. This chimeric state appeared to be stable, because no further change in the numbers of host and donor lymphocytes in the KB-R7785–treated mice was observed on day 21 (not shown).
DISCUSSION
In this study, we found a profound ameliorating effect of an MMP inhibitor, KB-R7785, in a murine model of lethal acute GVHD. Administration of KB-R7785 almost completely abolished the mortality and weight loss as well as the GVHD-associated pathologies in the liver, intestine, and hematopoietic tissues. The ameliorating effect of KB-R7785 was obviously superior to that of anti–TNF-α MoAb and some differential modes of action were noted between these two regimens.
The ameliorating effect of anti-TNF treatment observed in this study was essentially consistent with that reported by Piguet et al.7 They reported that the administration of an anti-TNF polyclonal antibody reduced the mortality at day 40 by 50% and abolished the weight loss on day 18. In our present study, the administration of an anti-TNF MoAb similarly reduced the mortality by 50%. However, the weight loss was not abolished during the first 7 days but recovered thereafter. This might result from a difference in the experimental model systems used. They also showed that the GVHD-associated pathologies in the skin and gut, but not those in the liver or spleen, were prevented by the anti-TNF treatment. These observations are also consistent with our findings. It was also reported that the gut lesions responded best, followed by skin and liver lesions to the phase I-II trial of anti-TNF treatment in GVHD patients.8 These observation substantiate a critical contribution of TNF-α to intestinal and cutaneous lesions, but in a lesser degree to hepatic lesions. We also noted that the lymphoid atrophy in the spleen was not TNF-mediated, but TNF-α was critical in the atrophy of hematopoietic cells in the bone marrow. This appears to result from a potent suppressive effect of TNF-α on hematopoiesis.20-22 In contrast to the limited effect of anti-TNF treatment on the hepatic and splenic pathologies, all these GVHD-associated pathologies were prevented by the KB-R7785 treatment. It may be possible that the inferior effect of anti-TNF to KB-R7785 resulted from an insufficient dose of anti-TNF MoAb we used. However, this seems unlikely, because serum TNF-α was not detectable in the anti-TNF–treated mice and the intestinal and marrow complications were completely inhibited by the anti-TNF treatment. Therefore, it is more likely that the potent ameliorating effect of KB-R7785 is not solely mediated by the blockade of systemic TNF-α.
Recently, it has been shown that FasL plays a critical role in the development of hepatic and cutaneous GVHD and lymphoid atrophy. Baker et al11 showed that, when the FasL-defective gld mice were used as the T-cell donor, only minimal signs of hepatic and cutaneous GVHD pathology were observed and the lymphoid atrophy in the spleen was improved. In contrast, intestinal GVHD was not abrogated and neither weight loss nor mortality was improved. A major role for FasL in the GVHD-associated elimination of host lymphocytes was also recently shown by other groups.23,24 In the present study, we showed that the KB-R7785 treatment abrogated the host lymphocytes elimination. This suggests a predominant role for soluble FasL in the elimination of host lymphocytes, especially B cells. Although it may be also possible that the KB-R7785 treatment inhibited the donor T-cell activation and thus prevented GVHD, this seems unlikely because the donor lymphocytes rather expanded in the KB-R778–-treated recipients as compared with the GVHD mice. This expansion of host T cells seems to result from inhibition of activation-induced apoptosis, in which autocrine or paracrine FasL plays a critical role.25 Consistent with such an in vivo observation, KB-R7785 does not inhibit the proliferative response of B6 T cells to alloantigens or the cytotoxic activity of CTL against Fas-bearing target cells in vitro (unpublished observation). The preservation of both host and donor lymphocytes by the KB-R7785 treatment resulted in a chimeric state of the recipients. It remains to be determined whether the chimeric state is stable after cessation of the treatment and a tolerance to the host alloantigen has been established in the donor T cells.
Considering together the previous and present results, TNF-α and FasL appear to differentially contribute to the GVHD pathologies as follows: (1) hepatic GVHD and lymphoid atrophy in the spleen are predominantly mediated by FasL; (2) intestinal GVHD, weight loss, and mortality are predominantly mediated by TNF-α; and (3) cutaneous GVHD is mediated by both TNF-α and FasL. Therefore, the superior effect of KB-R7785 might result from the blockade of FasL release in addition to that of TNF-α. It should be noted that KB-R7785 blocks the release, but not the functional activity, of TNF-α and FasL. It has been known that membrane TNF-α on activated macrophages or T cells and membrane FasL on activated T cells are functionally active in a cell contact-dependent manner.26-29 This finding suggests that TNF-α and FasL may not be pathogenic when locally confined onto the producer cells but may cause extensive tissue damage when released.
It should also be noted that KB-R7785 is a potent inhibitor of MMPs, including collagenases, stromelysins, and gelatinases, which are directly involved in tissue degeneration and tissue infiltration of inflammatory cells. A major role for MMPs in T-cell–mediated injury in the gut has been suggested recently.30 Therefore, the blockade of these processes in the GVHD-associated inflammation may also contribute to the beneficial effect of KB-R7785.
The hydroxamic acid-based metalloproteinase inhibitors have been developed against degenerative MMPs as the main targets. One of such compounds (Marimastat) has already been subjected to phase I/II studies for the treatment of pancreatic, prostatic, and ovarian cancers, with no serious adverse effect and minimal toxicity.31-33 Therefore, the MMP inhibitors are expected to be immediately applicable for the clinical treatment of refractory severe acute GVHD patients.
ACKNOWLEDGMENT
The authors thank K. Saito for technical assistance and helpful suggestions and N. Kimura and Y. Ikeda for preparing the manuscript.
Supported by grants from the Ministry of Education, Science and Culture and the Ministry of Health, Japan.
Address reprint requests to Ko Okumura, MD, Department of Immunology, Juntendo University School of Medicine, 2-1-1 Hongo, Bunkyo-ku, Tokyo 113, Japan.

![Fig. 1. Characterization of KB-R7785. (A) Structure of KB-R7785: [4-(N-hydroxyamino)-2R-isobutyl-3S-methylsucciny1]-L-phenylglycine-N-methylamide. (B) Inhibition of TNF-α release from LPS-simulated THP-1. THP-1 cells were stimulated with 1 μg/mL LPS for 72 hours in the presence of the indicated dose of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). TNF-α in the supernatants was measured by ELISA. Data are indicated as a percentage of control TNF-α release in the absence of inhibitors (112 ± 39 pg/mL) and are the mean ± SD of triplicate samples. (C) Inhibition of FasL release from hFasL/L5178Y. hFasL/L5178Y cells were cultured for 24 hours in the presence of the indicated doses of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). FasL in the supernatants was measured by ELISA. Data are indicated as a percentage of control FasL release in the absence of inhibitors (13.9 ± 3.3 ng/mL) and are the mean ± SD of triplicate samples. (D) Prevention of lethal endotoxin shock. BALB/c mice received IP 30 mg/kg (▴) or 100 mg/kg (▪) of KB-R7785, 100 mg/kg of KB-R8301 (♦), or an equal volume of 0.5% CMC (•). After 1 hour, these mice were injected IV with 30 mg of D-galactosamine and 3 μg of LPS. The results are shown as a survival rate of 8 mice in each group at the indicated time points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.542/4/m_bl_0056f1a.jpeg?Expires=1769195370&Signature=F4~QUP91Em1LMmUqE9btfonZi9QV6q3krQ~DMCtUPoegZxlmtQDPSPxTzW4Z5E2VvMGsjfw6h74U0f6qCoP2l1IUSblcjFvZp3PWVbItfcgWrN2i59bR83suf3J5~hwHAsxRlw8~BnjsszeiBpUH5f-NjlAkn0Pvv4WFdXKjdETbSvVbtjEiLXLysAw8zNuS3d2RFr8Cxl8AfeFkk5l9UTxdVG0hPu42wKIhtfQAwsITSI8GRZutT7NBU7i9JPblSqQYNHtLCIWoaroW4KG3NpEfcroDvDEwxWrnxsK~5vwwUoSQxtlxMnij~n8w1UhL4~VuTSqbr0zTqWYitcyohA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Characterization of KB-R7785. (A) Structure of KB-R7785: [4-(N-hydroxyamino)-2R-isobutyl-3S-methylsucciny1]-L-phenylglycine-N-methylamide. (B) Inhibition of TNF-α release from LPS-simulated THP-1. THP-1 cells were stimulated with 1 μg/mL LPS for 72 hours in the presence of the indicated dose of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). TNF-α in the supernatants was measured by ELISA. Data are indicated as a percentage of control TNF-α release in the absence of inhibitors (112 ± 39 pg/mL) and are the mean ± SD of triplicate samples. (C) Inhibition of FasL release from hFasL/L5178Y. hFasL/L5178Y cells were cultured for 24 hours in the presence of the indicated doses of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). FasL in the supernatants was measured by ELISA. Data are indicated as a percentage of control FasL release in the absence of inhibitors (13.9 ± 3.3 ng/mL) and are the mean ± SD of triplicate samples. (D) Prevention of lethal endotoxin shock. BALB/c mice received IP 30 mg/kg (▴) or 100 mg/kg (▪) of KB-R7785, 100 mg/kg of KB-R8301 (♦), or an equal volume of 0.5% CMC (•). After 1 hour, these mice were injected IV with 30 mg of D-galactosamine and 3 μg of LPS. The results are shown as a survival rate of 8 mice in each group at the indicated time points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.542/4/m_bl_0056f1b.jpeg?Expires=1769195370&Signature=cBFuGU5NzcyLMnCIJ0j6MwTg49hiiRI5Y7OPRX08YFjZKg51E1kB9mDGSkhTppTNWPJ-~M3R2MyVeGlqC4WBv0MqQD3HQoLIeQ7iV3L2kQK7pbGQyk4fSQ9hsWxL66ubM-H-GtIji-48uYWuss7pFXsiVUPeCt4p1h7FPWAvS1wtmgqnPBNeXavpqjmA4kZy-iDqUTtBG4ABd7LS0HZo4st44YiDEAocGUkHmliwZL2EtlkQBaIim3Cp7yQ6T3hc2hcXl-HdK65GJO8v9EDwlvHyWsTPN7WwVi1vAZdOqj9SgiVAqbOOXRbXJnr6~xIJBKb024FTY4MMZZusHWzBMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Characterization of KB-R7785. (A) Structure of KB-R7785: [4-(N-hydroxyamino)-2R-isobutyl-3S-methylsucciny1]-L-phenylglycine-N-methylamide. (B) Inhibition of TNF-α release from LPS-simulated THP-1. THP-1 cells were stimulated with 1 μg/mL LPS for 72 hours in the presence of the indicated dose of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). TNF-α in the supernatants was measured by ELISA. Data are indicated as a percentage of control TNF-α release in the absence of inhibitors (112 ± 39 pg/mL) and are the mean ± SD of triplicate samples. (C) Inhibition of FasL release from hFasL/L5178Y. hFasL/L5178Y cells were cultured for 24 hours in the presence of the indicated doses of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). FasL in the supernatants was measured by ELISA. Data are indicated as a percentage of control FasL release in the absence of inhibitors (13.9 ± 3.3 ng/mL) and are the mean ± SD of triplicate samples. (D) Prevention of lethal endotoxin shock. BALB/c mice received IP 30 mg/kg (▴) or 100 mg/kg (▪) of KB-R7785, 100 mg/kg of KB-R8301 (♦), or an equal volume of 0.5% CMC (•). After 1 hour, these mice were injected IV with 30 mg of D-galactosamine and 3 μg of LPS. The results are shown as a survival rate of 8 mice in each group at the indicated time points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.542/4/m_bl_0056f1c.jpeg?Expires=1769195370&Signature=WWVerPbACFhj3PdCKSiC4YBIFHOzWPUuMX~2tMgEMY1MbI4ABxb6xsUvLfq7jBAD3w27URAouOE3G3qlnHMTahxNu7LrXHUqXJunxa~B2kWSa707~iD3OLrjiOr0Pf2HFI6pS2-xLRvqQB6Y3TTvmuc-kEFn4-cECSYDz8UqezrfdL578RTOGMsBousso9WORcsu2dTwPN1BMDoFnhlw4zJURQ-DbWUTdbqDq3RK1f3yLbfQ7sPde5CTtsBeOf8Txq4Qvv4c0NWwC4LDeJ9TLmP-KLv81d6qLV~vBZlQaZUPCqv2ljR9VbPo04bkJIdqBEGsHx3L4ZYQOjylLXD-Bw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Characterization of KB-R7785. (A) Structure of KB-R7785: [4-(N-hydroxyamino)-2R-isobutyl-3S-methylsucciny1]-L-phenylglycine-N-methylamide. (B) Inhibition of TNF-α release from LPS-simulated THP-1. THP-1 cells were stimulated with 1 μg/mL LPS for 72 hours in the presence of the indicated dose of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). TNF-α in the supernatants was measured by ELISA. Data are indicated as a percentage of control TNF-α release in the absence of inhibitors (112 ± 39 pg/mL) and are the mean ± SD of triplicate samples. (C) Inhibition of FasL release from hFasL/L5178Y. hFasL/L5178Y cells were cultured for 24 hours in the presence of the indicated doses of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). FasL in the supernatants was measured by ELISA. Data are indicated as a percentage of control FasL release in the absence of inhibitors (13.9 ± 3.3 ng/mL) and are the mean ± SD of triplicate samples. (D) Prevention of lethal endotoxin shock. BALB/c mice received IP 30 mg/kg (▴) or 100 mg/kg (▪) of KB-R7785, 100 mg/kg of KB-R8301 (♦), or an equal volume of 0.5% CMC (•). After 1 hour, these mice were injected IV with 30 mg of D-galactosamine and 3 μg of LPS. The results are shown as a survival rate of 8 mice in each group at the indicated time points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.542/4/m_bl_0056f1d.jpeg?Expires=1769195370&Signature=d4xt~ng4hgvQ0InYPleigWjHnpFXJvJuBuI2WuJjiMd4lQrEZADacmwIzKbvUiNqTYqsT~D-yRIhltBlCvoDT5FjS5wCnTmrA6lwMbe2wvmNbM7AOb2U040ObzzSvD-bng43ydu24fFHg1jp0CSoxlyk9Ad9J6jC~9fpXVUfOHnpPGW-hXmxQEkuL~Cm6MKGA1NPN75gijJZdvO0oMZO~OEEmv~Eq49XCd9aCnA6h94FS0ywygKQZp2c-yfJh5gttyuQjMXv7UQVit0qqK9IY2~gnPht4d2Yatmq0A4qGovWOH25MRB09rZTUHFQGZCLj~tqRiVD74hXVArNold86A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 1. Characterization of KB-R7785. (A) Structure of KB-R7785: [4-(N-hydroxyamino)-2R-isobutyl-3S-methylsucciny1]-L-phenylglycine-N-methylamide. (B) Inhibition of TNF-α release from LPS-simulated THP-1. THP-1 cells were stimulated with 1 μg/mL LPS for 72 hours in the presence of the indicated dose of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). TNF-α in the supernatants was measured by ELISA. Data are indicated as a percentage of control TNF-α release in the absence of inhibitors (112 ± 39 pg/mL) and are the mean ± SD of triplicate samples. (C) Inhibition of FasL release from hFasL/L5178Y. hFasL/L5178Y cells were cultured for 24 hours in the presence of the indicated doses of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). FasL in the supernatants was measured by ELISA. Data are indicated as a percentage of control FasL release in the absence of inhibitors (13.9 ± 3.3 ng/mL) and are the mean ± SD of triplicate samples. (D) Prevention of lethal endotoxin shock. BALB/c mice received IP 30 mg/kg (▴) or 100 mg/kg (▪) of KB-R7785, 100 mg/kg of KB-R8301 (♦), or an equal volume of 0.5% CMC (•). After 1 hour, these mice were injected IV with 30 mg of D-galactosamine and 3 μg of LPS. The results are shown as a survival rate of 8 mice in each group at the indicated time points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.542/4/m_bl_0056f1a.jpeg?Expires=1769466320&Signature=Bu1In7V5EErTK8bcY~pWXF0NbhVg-PUMXwfeLznhPE-p5FzeGZS3uMteNNfBEH1BlewAkuGCa5FOBI3J7Og8KRrTy1zHnjsGNV8tD1b41~auoQhpREcwyZ8HnAqxNlg~BSOK~efi0dqMJsq94ISU4F7ott3~mWxQycRJ9quB1tpX51bIMjkuw2uAklwmRL1NAuk4krHeJlMbmAxTWV80PUT46oJ5JUd9qw8oOEGVHW-C7faOMx3mm2b4aF8yfL-LTrOAQHHwNN14XLyS1UmBJ8nQRpiI-gG6ufey18huIafWtbYBqEBeDReEmb3Z7gkBcG6Dz4bYQrRzDslVnF3aXA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Characterization of KB-R7785. (A) Structure of KB-R7785: [4-(N-hydroxyamino)-2R-isobutyl-3S-methylsucciny1]-L-phenylglycine-N-methylamide. (B) Inhibition of TNF-α release from LPS-simulated THP-1. THP-1 cells were stimulated with 1 μg/mL LPS for 72 hours in the presence of the indicated dose of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). TNF-α in the supernatants was measured by ELISA. Data are indicated as a percentage of control TNF-α release in the absence of inhibitors (112 ± 39 pg/mL) and are the mean ± SD of triplicate samples. (C) Inhibition of FasL release from hFasL/L5178Y. hFasL/L5178Y cells were cultured for 24 hours in the presence of the indicated doses of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). FasL in the supernatants was measured by ELISA. Data are indicated as a percentage of control FasL release in the absence of inhibitors (13.9 ± 3.3 ng/mL) and are the mean ± SD of triplicate samples. (D) Prevention of lethal endotoxin shock. BALB/c mice received IP 30 mg/kg (▴) or 100 mg/kg (▪) of KB-R7785, 100 mg/kg of KB-R8301 (♦), or an equal volume of 0.5% CMC (•). After 1 hour, these mice were injected IV with 30 mg of D-galactosamine and 3 μg of LPS. The results are shown as a survival rate of 8 mice in each group at the indicated time points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.542/4/m_bl_0056f1b.jpeg?Expires=1769466320&Signature=x9QEn2WLFG0jzDVq1-oVQTu7sViduzwwRPpBalmpAbnQzAybIZtqfurDx3wg2nOW0gfYKF1kqXZIWNXW4lrjJOjVFmPsigELLC9wJzwxKuSQXMLcizmYZBLtf6NuhYhKN1lynLulLdoRFo~fljciyF7taN3yNm2j9JdTAbpMYtqoWhA~JkVbtQwTsiDSJ718~0vDenYgPgk54xDC4RhkA0GBIO~o3VQ~4X~Wd0e9WktKm11LwG9d450xp4xGMnWDDmOSNPlqfEPT1L0eNvqHaNF~FRqh2BmEkqvjIGFS~L1ulJBnt-l~NH0ELqLb4EJ6ZqPWr77fBRG8XUGDTEIYMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Characterization of KB-R7785. (A) Structure of KB-R7785: [4-(N-hydroxyamino)-2R-isobutyl-3S-methylsucciny1]-L-phenylglycine-N-methylamide. (B) Inhibition of TNF-α release from LPS-simulated THP-1. THP-1 cells were stimulated with 1 μg/mL LPS for 72 hours in the presence of the indicated dose of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). TNF-α in the supernatants was measured by ELISA. Data are indicated as a percentage of control TNF-α release in the absence of inhibitors (112 ± 39 pg/mL) and are the mean ± SD of triplicate samples. (C) Inhibition of FasL release from hFasL/L5178Y. hFasL/L5178Y cells were cultured for 24 hours in the presence of the indicated doses of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). FasL in the supernatants was measured by ELISA. Data are indicated as a percentage of control FasL release in the absence of inhibitors (13.9 ± 3.3 ng/mL) and are the mean ± SD of triplicate samples. (D) Prevention of lethal endotoxin shock. BALB/c mice received IP 30 mg/kg (▴) or 100 mg/kg (▪) of KB-R7785, 100 mg/kg of KB-R8301 (♦), or an equal volume of 0.5% CMC (•). After 1 hour, these mice were injected IV with 30 mg of D-galactosamine and 3 μg of LPS. The results are shown as a survival rate of 8 mice in each group at the indicated time points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.542/4/m_bl_0056f1c.jpeg?Expires=1769466320&Signature=Pc5QCeAXIf6p3xMTUOw4hvRjnodcCCoGYTJPUQQDPC5T3p-vTRGsUcROEOizwgM05-CzsNSvmymtrYAZvXG2RmpFUJmzToet4T-vMr36FFIhpA2X9umBOJDu44edXtLvQm6Um0AF5e0WV7XJGflvaAoFh0hB5NpcxmtNNN~dsNNoo3tLtoCAlD~EUtL-XZTJxOiykhsGrpLDaA3jKVvElOiZUFnH4PF7RpF5-uJUJgvggcFskGUENs5CB-weYWWGrLK0O6uQax2YgRRd-M0vazB919JcspMu9y8pI-zKm-DBBv~Q~~Uvnn308nQERRdiFskEGYlKeOGFRP5pUyryfQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. Characterization of KB-R7785. (A) Structure of KB-R7785: [4-(N-hydroxyamino)-2R-isobutyl-3S-methylsucciny1]-L-phenylglycine-N-methylamide. (B) Inhibition of TNF-α release from LPS-simulated THP-1. THP-1 cells were stimulated with 1 μg/mL LPS for 72 hours in the presence of the indicated dose of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). TNF-α in the supernatants was measured by ELISA. Data are indicated as a percentage of control TNF-α release in the absence of inhibitors (112 ± 39 pg/mL) and are the mean ± SD of triplicate samples. (C) Inhibition of FasL release from hFasL/L5178Y. hFasL/L5178Y cells were cultured for 24 hours in the presence of the indicated doses of KB-R7785 (▴), KB-R8301 (▪), or KB-R8845 (•). FasL in the supernatants was measured by ELISA. Data are indicated as a percentage of control FasL release in the absence of inhibitors (13.9 ± 3.3 ng/mL) and are the mean ± SD of triplicate samples. (D) Prevention of lethal endotoxin shock. BALB/c mice received IP 30 mg/kg (▴) or 100 mg/kg (▪) of KB-R7785, 100 mg/kg of KB-R8301 (♦), or an equal volume of 0.5% CMC (•). After 1 hour, these mice were injected IV with 30 mg of D-galactosamine and 3 μg of LPS. The results are shown as a survival rate of 8 mice in each group at the indicated time points.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.542/4/m_bl_0056f1d.jpeg?Expires=1769466320&Signature=Fkm8O2LvGNi5xaWkWcHD1LkvIKQnFQ07ulHt97JCM0okDFSTrBA7cyQX4Nskh8iyzMXQvmo4~tfcfp-rpUbC~tC8P-uGSdLE-WSbJuN5Ddasrsa7tq5lD2RG1yFu~MpOffRiifiqTV3IRysMcweqHHOzvzxX0GS3zA7vG~Ma~K-1ItEnMdot3WFEBFAGRKanFYPAQCAohF284RjyLPtx1s~E3eLF~Hh3jNwbCRE9rV1eb1PKkST5h7ndLCiCMoM81WSX50BZH1Atls9DeEBCh~WnSEed-wxZXvufRw-yEYAIMF4tTj1iYFUJZzEi2v-72uHzL7qnazH9wq4sZLM~uQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)