Abstract
Using a clonal assay of bone marrow (BM) cells from transgenic mice (Tg-mice) expressing the human granulocyte-macrophage colony-stimulating factor receptor (hGM-CSFR), we found in earlier studies that hGM-CSF alone supported the development not only of granulocyte-macrophage colonies, but also of erythrocytes, megakaryocytes, mast cells, blast cells, and mixed hematopoietic colonies. In this report, we evaluated the in vivo effects of hGM-CSF on hematopoietic and lymphopoietic responses in the hGM-CSFR Tg-mice. Administration of this factor to Tg-mice resulted in dose-dependent increases in numbers of reticulocytes and white blood cells (WBCs) in the peripheral blood. Morphological analysis of WBCs showed that the numbers of all types of the cell, including neutrophils, eosinophils, monocytes, and lymphocytes increased; the most remarkable being in lymphocytes that contained a number of large granular lymphocytes (LGLs) in addition to mature T and B cells. However, total cellularity of the BM of the Tg-mice decreased in a dose-dependent manner when hGM-CSF was injected. In sharp contrast to the BM, spleens of the Tg-mice were grossly enlarged. Although all types of blood cells and hematopoietic progenitors increased in the spleen, erythroid cells and their progenitors showed the most significant increase. Increased numbers of megakaryocytes and LGLs were also observed in spleen and liver of the treated Tg-mice. Flow cytometric analysis showed that LGLs expanded in Tg-mice expressed Mac-1+CD3−NK1.1+. The thymus of Tg-mice treated with hGM-CSF exhibited a dose-dependent shrinkage and a remarkable decrease in CD4+CD8+ cells. Thus, hGM-CSF stimulated not only myelopoiesis but also erythropoiesis and megakaryopoiesis of hGM-CSFR Tg-mice in vivo, in accordance with our reported in vitro findings. In addition, hGM-CSF affected the development of lymphoid cells, including natural killer cells of these Tg-mice.
HEMATOPOIESIS is a multistage developmental process in which pluripotent hematopoietic stem cells give rise to cell lineage including granulocytes, monocytes, lymphocytes, megakaryocytes, and erythrocytes. It is widely accepted that growth and differentiation of progenitor cells are stimulated by cytokines. However, it remains unknown whether the differentiation of progenitors can be induced by an exogenous cytokine stimulus (instructive model) or whether a spontaneous random event results in survival of committed cells (stochastic model).1,2 If signal transduction through a lineage-restricted cytokine receptor is a key inductive event in commitment, artificial expression of the receptor at early stages of development would promote differentiation to a particular lineage. To examine the relationship between receptor expression and differentiation of hematopoietic cells, we generated transgenic mice (Tg-mice) expressing the receptor for human granulocyte-macrophage colony-stimulating factor (hGM-CSFR), a lineage-restricted growth factor that acts in almost all lineages during hematopoietic cell development.3 hGM-CSFR was selected for two reasons: (1) mouse (m) GM-CSF has been shown to bind to mGM-CSFR, the expression of which is restricted to GM lineages, and to activate the signal to support proliferation and maturation of only GM progenitor cells into granulocytes and monocytes/macrophages4,5 and (2) because hGM-CSFR expressed on mouse cells interacts with only hGM-CSF in a species-specific manner6 one can specifically monitor the effects of hGM-CSF on cells of various lineages expressing hGM-CSFR in the Tg-mice. In previous work, we characterized proliferation and differentiation of hematopoietic progenitors derived from the hGM-CSFR Tg-mice by an in vitro methylcellulose colony-formation assay. Whereas mGM-CSF yielded only GM colonies, hGM-CSF supported various types of colonies, including GM, eosinophils, megakaryocytes, blast cells, mast cells, and mixed hematopoietic colonies. In addition, hGM-CSF generated erythrocyte colonies in the absence of erythropoietin (EPO), which specifically stimulates the erythroid lineage. These results indicated that the potential of hGM-CSF is not restricted to support of growth and differentiation of cells of GM lineages as long as hGM-CSFR is expressed on the target cells, and it may support a stochastic model rather than an instructive model. This view is also supported by recent findings concerning expression of receptors for more lineage-restricted cytokines, such as the EPOR,7,8 macrophage colony-stimulating factor (M-CSF ) receptor,9 and interleukin-5 (IL-5) receptor.10
In the present study, we extended these observations and further characterized the effects of hGM-CSF on hematopoiesis of hGM-CSFR Tg-mice, in vivo. We found that hGM-CSF stimulated not only myelopoiesis but also erythropoiesis and megakaryopoiesis in accordance with our earlier in vitro findings. In addition, hGM-CSF affected the development of lymphoid cells including the natural killer (NK) cells of the Tg-mice.
MATERIALS AND METHODS
Mice.Tg-mice constitutively expressing high affinity hGM-CSFR were generated, as described.3 Three-month-old male Tg-mice and their normal littermates backgrounds were C3H/HeN, and (C57B1/6J × C3H/HeN) F3 were used for the flow cytometric analysis. The mice were kept in an environmentally controlled clean room with 12-hour light-dark cycles in an authorized animal facility at the laboratory of animal research, Institute of Medical Science, the University of Tokyo, Japan. The mice were maintained in microisolator cages under specific pathogen-free conditions; all the equipment and supplies were sterilized including cages, water bottles, wooden chips for bedding, and food pellets.
Hematopoietic growth factors.Recombinant hGM-CSF produced in Escherichia coli was a gift from Schering-Plough Research Institute (Kenilworth, NJ). The recombinant mIL-3 produced by silkworms (Bombyx mori ) was provided by Dr A. Miyajima,11 Institute of Molecular and Cellular Bioscience, the University of Tokyo, Japan. Recombinant rat stem cell factor (rSCF ) was provided by Amgen Inc (Thousand Oaks, CA), and recombinant hEPO produced by Chinese hamster ovary cells was provided by Kirin Brewery (Tokyo, Japan).
hGM-CSF in vivo administration schedules.The injection schedules of hGM-CSF were according to the modified methods described previously.12 13 In brief, hGM-CSFR Tg-mice were injected subcutaneously with 0.2 mL of phosphate-buffered saline (PBS) containing 1% C3H/HeN serum (serum/PBS) or 20, 100, 500, and 2,500 ng of hGM-CSF twice a day at 12-hour intervals for 7 consecutive days.
Peripheral blood cell counts.The mice were anesthetized with ether, and peripheral blood (PB) was collected from their hearts using a 1-mL syringe with a 24-G needle. After the PB collection, blood was quickly mixed with 2 mg EDTA to avoid aggregation and the samples were divided into two tubes. One tube was used to determine the numbers of red blood cells (RBCs), white blood cells (WBCs), platelets, hemoglobin concentration, and hematocrit using a hemocytometer (Sysmex K1000; Sysmex, Kobe, Japan). Next, smear preparations made on glass slides were stained with May-Grünwald-Giemsa. Reticulocytes on the smear preparations were stained with 0.5% methylene blue staining solution freshly prepared, and the proportion of deep blue-net cells against 1000 RBCs was evaluated.14 The other tube was mixed with 1.5 mL PBS containing 1 U/mL heparin. One and a half milliliters of 2% dextran T500 (Pharmacia LKB, Piscataway, NJ) in PBS was added to the blood sample and the mixture was incubated at 37°C for 30 minutes to allow for sedimentation of the RBC. The upper layer of RBC-depleted fluid was harvested in an ammonium chloride lysis buffer and used for flow cytometric analysis.
Examination of tissues.Portions of the following tissues of the Tg-mice treated with serum/PBS or 500 ng of hGM-CSF were taken for histological examination after fixation in 10% formalin solution: spleen, tibia, thymus, kidney, liver, lung, heart, intestine, and brain. All sections were stained with hematoxylin-eosin. Bone marrow (BM) cells were aseptically collected from both right and left femur shafts, and the spleen was also removed aseptically. The BM and spleen cells were then prepared in α-MEM (Flow Laboratories, Rockville, MD) by repeated pipetting and passed through a 50 μm Nylon cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ). Cytocentrifuge preparations were made from the BM and spleen cell suspensions using a cytocentrifuge (Cytospin II; Shandon Southern Instruments, Sewickley, PA) and stained with May-Grünwald-Giemsa. Hepatic mononuclear cells were isolated by a previously described method.15
Colony assay of hematopoietic progenitor cells.Methylcellulose clonal culture was done using a modification of the technique described previously.16 In most cases, 1 mL of culture mixture containing 1 × 104 BM cells or 1 × 105 spleen cells, α-MEM, 1.2% (1,500 centipoieses) methylcellulose (Shinetsu Chemical, Tokyo, Japan), 30% fetal bovine serum (FBS; HyClone Laboratories, Logan, UT), 1% deionized fraction V bovine serum albumin (BSA; Sigma, St Louis, MO), 1 × 10−4 mol/L mercaptoethanol (Eastman Organic Chemicals, Rochester, NY), and hematopoietic growth factors was plated in 35-mm suspension culture dishes (#171099; Nunc, Naperville, IL). These dishes were incubated at 37°C in a humidified atmosphere flushed with 5% CO2 in air. Unless otherwise specified, concentrations of growth factors used in this study were rSCF 100 ng/mL, mIL-3 10 ng/mL, and hEPO 2 U/mL, which is in the range of optimal concentrations for colony formation, as reported.17 18
Colony types were determined according to described criteria19 on days 5 through 17 of incubation by in situ observations using an inverted microscope. Except for megakaryocyte colonies, cell aggregates consisting of more than 50 cells were scored as colonies. Megakaryocyte colonies were scored as such when they had four or more cells.18 To assess the accuracy of in situ identification of the colonies, individual colonies were lifted with an Eppendorf micropipette under direct microscopic visualization, spread on glass slides using a cytocentrifuge, and then stained with May-Grünwald-Giemsa.
Flow cytometry.After depletion of RBCs, PB, BM, spleen, thymus, and hepatic mononuclear cells were washed twice with staining solution (PBS containing 5% FBS and 0.02% sodium azide). 1 × 106 cells were incubated at 4°C for 30 minutes in 100 μL of staining solution with 500 ng of fluorescein isothiocyanate (FITC)-rat anti-mouse CD8α antibody (Ly-2; clone 53-6.7, Pharmingen, San Diego, CA) in combination with 250 ng of phycoerythrin (PE)-rat anti-mouse CD4 antibody (L3T4; clone RM4-5, Pharmingen), or 500 ng of PE-rat anti-mouse B220 antibody (RA3-6B2, Pharmingen) in combination with 500 ng of FITC-sheep-mouse immunoglobulin M (IgM) antibody (Cappel; Organon Teknica, Belgium). NK cells were detected with (C57B1/6J × C3H/HeN) F3 Tg-mice using 500 ng of PE-rat anti-mouse NK1.1 antibody (clone PK136, Pharmingen) in combination with 500 ng of FITC-rat anti-mouse CD11b (Mac-1) antibody (clone M1/70, gift from Dr K. Ikuta, Kyoto University, Japan) or 1 ng of FITC-hamster anti-mouse CD3ε antibody (clone 145-2C11, Pharmingen). Spleen cells were also stained with 250 ng of PE-rat anti-mouse erythroid cells antibody (clone TER-119, Pharmingen). After staining, cells were washed twice in staining solution and incubated at 4°C for 10 minutes in 300 μL of staining solution containing 2 mg/mL of 7-aminoactinomycin D (Sigma). Debris, erythrocytes, and dead cells were excluded from analysis by forward and side scatter and 7-aminoactinomycin D gatings. Flow cytometry was performed with a Becton Dickinson FACScan (Becton Dickinson, San Jose, CA), using FACScan software, LYSIS II. CD3−NK1.1+ cells in PB were further sorted by FACS Vantage (Becton Dickinson), and their appearance was evaluated on the cytospin preparation stained with May-Grünwald-Giemsa.
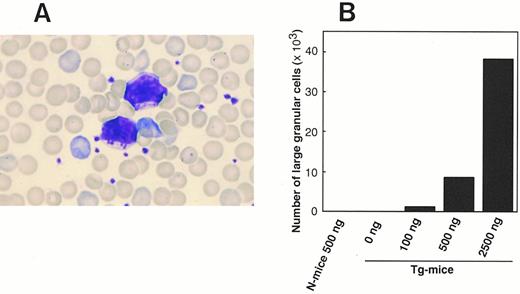
(A) Photomicrograph of LGL in PB of Tg-mice injected with 500 ng of hGM-CSF twice a day for 1 week. This smear preparation was stained with May-Grünwald-Giemsa. (B) Dose-response relationship between hGM-CSF injected and the generation of LGL in PB. Tg-mice and normal littermates (N-mice) were injected with serum/PBS or 100 to 2,500 ng of hGM-CSF twice daily for 1 week, and the proportion to lymphocytes and absolute number of LGL was evaluated on day 8.
(A) Photomicrograph of LGL in PB of Tg-mice injected with 500 ng of hGM-CSF twice a day for 1 week. This smear preparation was stained with May-Grünwald-Giemsa. (B) Dose-response relationship between hGM-CSF injected and the generation of LGL in PB. Tg-mice and normal littermates (N-mice) were injected with serum/PBS or 100 to 2,500 ng of hGM-CSF twice daily for 1 week, and the proportion to lymphocytes and absolute number of LGL was evaluated on day 8.
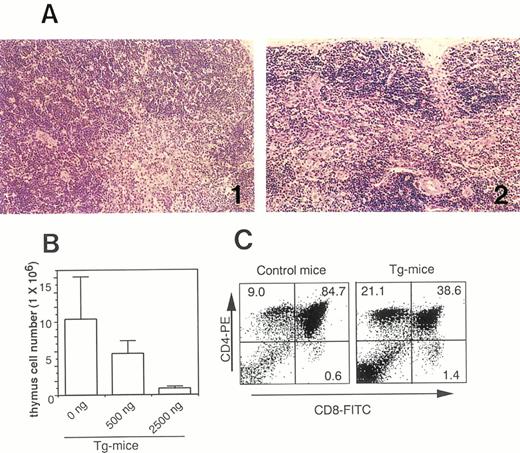
(A) Photomicrographs of thymic tissues of Tg-mice injected with serum/PBS (A-1) and 500 ng of hGM-CSF (A-2) twice daily for 1 week. Note voluminous medulla on Tg-mice treated with serum/PBS as compared with a drastic involution on Tg-mice treated with hGM-CSF. (B) Dose-response effects of hGM-CSF on cell number of thymic cells in Tg-mice. (C) Flow cytometric analysis of thymic cells from normal littermates (left) and Tg-mice (right) treated with 500 ng of hGM-CSF. Thymic cells were stained with FITC-anti-CD8 and PE-anti-CD4.
(A) Photomicrographs of thymic tissues of Tg-mice injected with serum/PBS (A-1) and 500 ng of hGM-CSF (A-2) twice daily for 1 week. Note voluminous medulla on Tg-mice treated with serum/PBS as compared with a drastic involution on Tg-mice treated with hGM-CSF. (B) Dose-response effects of hGM-CSF on cell number of thymic cells in Tg-mice. (C) Flow cytometric analysis of thymic cells from normal littermates (left) and Tg-mice (right) treated with 500 ng of hGM-CSF. Thymic cells were stained with FITC-anti-CD8 and PE-anti-CD4.
RESULTS
Increase of WBCs, large granular lymphocytes, and reticulocytes in PB.hGM-CSFR Tg-mice, whose background was C3H/HeN, and their littermates were injected with 20 to 2,500 ng of hGM-CSF or serum/PBS twice daily for 7 days. After this treatment, both the Tg-mice and littermates looked healthy. In the examination of PB on day 8, however, the number of WBCs in Tg-mice, but not in their littermates, increased in accordance with the dose of hGM-CSF injected (Table 1). The number of WBCs in Tg-mice injected with 500 and 2,500 ng of hGM-CSF increased 7- and 19-fold, respectively. The estimation of absolute numbers, based on morphological analysis, showed that neutrophils, eosinophils, monocytes, and lymphocytes increased, with lymphocytes showing the most significant increase. Interestingly, most increased lymphocytes contained a significant number of large granular lymphocytes (LGLs) (Fig 1A), whereas only a few LGLs were detectable in the PB of littermates injected with 500 ng of hGM-CSF or Tg-mice injected with serum/PBS. The proportion and absolute number of LGLs in the PB of Tg-mice increased in a dose-dependent manner, depending on the hGM-CSF injected (Fig 1B). Flow cytometric analysis of the lymphocytes indicated that absolute numbers of CD4+ and CD8+ single positive (SP) cells and B220+IgM+ cells in PB of Tg-mice increased with administration of hGM-CSF. The ratio and absolute number of reticulocytes in PB of Tg-mice increased approximately three-fold by the administration of over 500 ng of hGM-CSF. The number of RBCs, the hematocrit, and hemoglobin concentration showed no remarkable changes (Table 1), however these numbers increased 10% to 20% after a 2-week injection of hGM-CSF (Nishijima et al, unpublished observation, January 1997). The number of platelets in the PB of Tg-mice decreased as much as 50% following injections of 500 ng hGM-CSF.
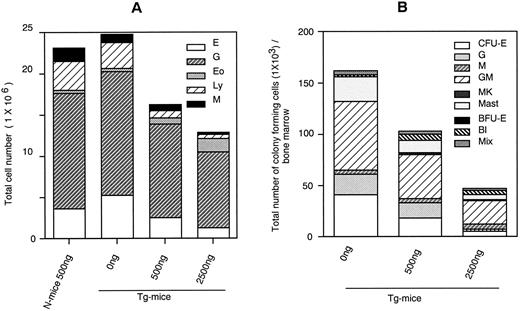
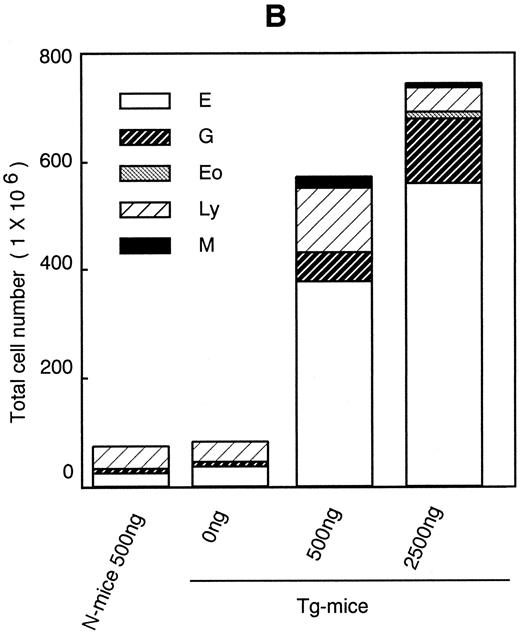
Dose-response effects of hGM-CSF on numbers of constituent cells (A) and hematopoietic progenitors assayed by methylcellulose clonal culture (B) in BM of Tg-mice. Abbreviations: E, erythroblasts or erythrocyte colonies; G, granulocytes or granulocyte colonies; M, monocytes/macrophages or macrophage colonies; Eo, eosinophiles; Ly, lymphocytes; MK, megakaryocytes; BFU-E, erythroid bursts; Bl, blast cell colonies; and Mix, mixed hematopoietic colonies.
Dose-response effects of hGM-CSF on numbers of constituent cells (A) and hematopoietic progenitors assayed by methylcellulose clonal culture (B) in BM of Tg-mice. Abbreviations: E, erythroblasts or erythrocyte colonies; G, granulocytes or granulocyte colonies; M, monocytes/macrophages or macrophage colonies; Eo, eosinophiles; Ly, lymphocytes; MK, megakaryocytes; BFU-E, erythroid bursts; Bl, blast cell colonies; and Mix, mixed hematopoietic colonies.
Decrease in BM cells and hematopoietic progenitors. Histological analysis showed decreased cellularity of the femur of Tg-mice treated with hGM-CSF. The total cell number in BM decreased dose-dependently to half with 2,500 ng of hGM-CSF (Fig 2A). Morphological analysis indicated that although the numbers of neutrophils, eosinophils, monocytes, and megakaryocytes remained relatively constant while numbers of erythroid and lymphoid cells progressively decreased. A substantial number of LGLs was again detected in the BM of Tg-mice treated with hGM-CSF. Next, we analyzed the number of hematopoietic progenitor cells in the BM by methylcellulose colony formation assay. As shown in Fig 2B, the numbers of all types of hematopoietic progenitors were reduced after hGM-CSF injection in a dose-dependent manner (Fig 2B). In Tg-mice treated with 2,500 ng of hGM-CSF, the number of total progenitors decreased by a third, and much less erythroid progenitors were detected compared with findings in Tg-mice injected with serum/PBS.
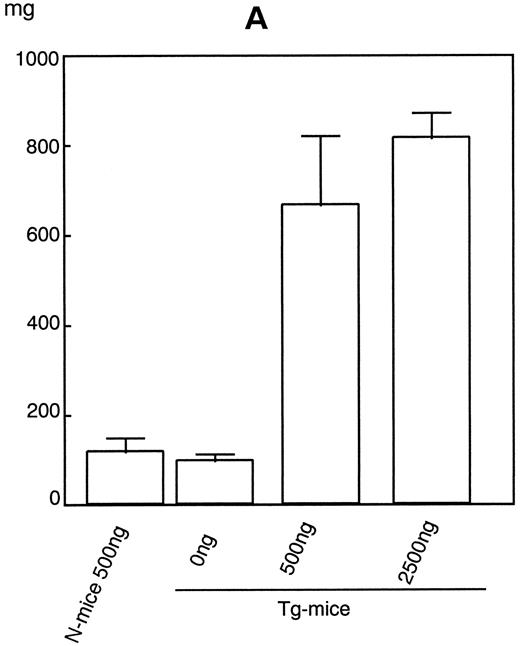
Increase in spleen and liver hematopoietic cells in hGM-CSFR Tg-mice.After 1 week of injections of hGM-CSF, a significant splenomegaly was noted in the Tg-mice. Weight of spleens from Tg-mice injected with 500 ng or 2,500 ng of hGM-CSF rose sixfold to ninefold over that of Tg-mice given serum/PBS or normal littermates treated with 500 ng of hGM-CSF (Fig 3A). The number of cells in the spleen increased in parallel with its weight (Fig 3B). Morphological analysis showed that all types of blood cells increased to various extents, but the most significant increase was that of erythroid cells. Erythroid cells increased approximately 150- to 200-fold by administration of over 500 ng of hGM-CSF in the spleen of Tg-mice, in accordance with the flow cytometric finding that TER-119+ cells significantly increased in the spleen (Nishijima et al, unpublished observation, June 1995). The numbers of all types of hematopoietic progenitors, including GM, erythroid, megakaryocyte, mast cell, and mixed hematopoietic colonies, assayed by methylcellulose clonal culture also increased after the injection of over 500 ng hGM-CSF (Fig 3C). In particular, CFU-E, late erythroid progenitors, expanded approximately 150- to 200-fold. This expansion was also supported by the histological finding that the proportion of white pulp in the spleen was reduced while that of red pulp increased. Histological analysis also showed that infiltrating cells in the spleen contained increased numbers of megakaryocytes.
Dose-response effects of hGM-CSF on weight of spleen (A), numbers of constituent cells (B), and hematopoietic progenitors assayed by methylcellulose clonal culture (C) in spleen of Tg-mice.
Dose-response effects of hGM-CSF on weight of spleen (A), numbers of constituent cells (B), and hematopoietic progenitors assayed by methylcellulose clonal culture (C) in spleen of Tg-mice.
Histological analysis of the liver showed that infiltrating cells were dispersed along the hepatic cell-cords in Tg-mice treated with 500 ng of hGM-CSF. These cells consist of round-nuclear erythroblastic cells, myeloblastic cells (with or without indented nuclei), and medium-sized lymphoid cells. Few, although distinct, megakaryocytes were also identified (Nishijima et al, unpublished observation, June 1995).
Histological analysis of other organs (kidney, lung, heart, intestine, and brain) from Tg-mice revealed no remarkable changes and no infiltration of hematopoietic cells comparable with those observed in the liver.
Decrease of CD4+CD8+DP cells in the thymus.The administration of hGM-CSF induced a dose-related shrinkage of the thymus of Tg-mice. Histological analysis of thymic tissues showed that the thymic cortex as well as the medulla of Tg-mice injected with 500 ng of hGM-CSF had remarkably shrunk compared with those given serum/PBS (Fig 4A). The total number of thymic cells of Tg-mice treated with hGM-CSF also dramatically decreased and, with 2,500 ng of hGM-CSF, fell to approximately 10% of that of Tg-mice treated with serum/PBS (Fig 4B). Flow cytometric analysis indicated that CD4+CD8+ double positive (DP) cells in the thymus had decreased after the injections of 500 ng of hGM-CSF (Fig 4C).
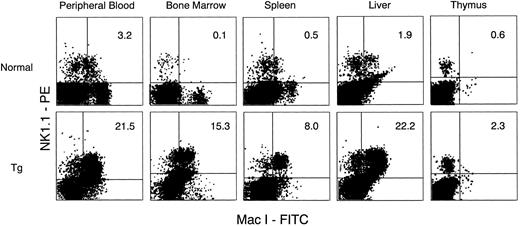
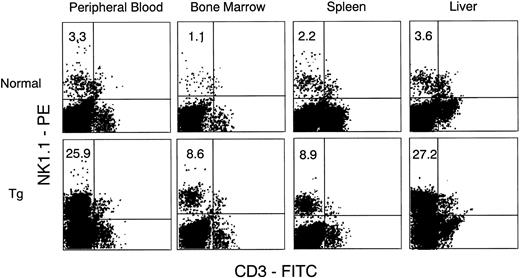
Increase in NK1.1 positive cells.We observed a significant expansion of LGLs, which are morphologically typical features of NK cells, in the PB, BM, spleen, and liver of Tg-mice treated with hGM-CSF. We then asked if these LGLs possessed phenotypic characteristics of NK cells, and for this we used anti-mouse NK1.1 antibody in combination with anti-CD3 antibody or anti–Mac-1 antibody. Because strain C3H/HeN of Tg-mice has no NK1.1 antigen,20 flow cytometric analysis for the detection of NK1.1+ cells was performed with newly generated hGM-CSFR Tg-mice whose background was (C57B1/6J × C3H/HeN) F3 . PB of the Tg-mice showed similar changes to that of C3H/HeN Tg-mice given hGM-CSF treatment. Peripheral WBCs increased and contained a large proportion of LGL. Flow cytometric analysis showed that Mac-1+NK1.1+ cells and CD3− NK1.1+ cells significantly increased in PB, BM, spleen, and liver, but not in the thymus of Tg-mice treated with 500 ng of hGM-CSF, compared with their littermates (Figs 5 and 6). The proportions of Mac-1+NK1.1+ cells and CD3−NK1.1+ cells were close in all samples examined, suggesting that both represented the same NK cell population. Furthermore, most of the FACS-sorted CD3−NK1.1+ cells were morphologically identical with LGLs in the smear preparations of PB, suggesting that hGM-CSF induced in vivo expansion of Mac-1+CD3−NK1.1+ NK cells.
Flow cytometric analysis of cells in PB, BM, spleen, liver, and thymus from normal littermates (upper) and Tg-mice (lower) treated with 500 ng of hGM-CSF. Cells were stained with FITC anti–Mac-1 and PE-anti-NK1.1. Mac-1+NK1.1+ cells in PB, BM, spleen, and liver, but not in thymus, increased in Tg-mice.
Flow cytometric analysis of cells in PB, BM, spleen, liver, and thymus from normal littermates (upper) and Tg-mice (lower) treated with 500 ng of hGM-CSF. Cells were stained with FITC anti–Mac-1 and PE-anti-NK1.1. Mac-1+NK1.1+ cells in PB, BM, spleen, and liver, but not in thymus, increased in Tg-mice.
Flow cytometric analysis of cells in PB, BM, spleen, and liver from normal littermates (upper) and Tg-mice (lower) treated with 500 ng of hGM-CSF. Cells were stained with FITC–anti-CD3 and PE-anti-NK1.1. CD3−NK1.1+ cells in PB, BM, spleen, and liver increased in Tg-mice.
Flow cytometric analysis of cells in PB, BM, spleen, and liver from normal littermates (upper) and Tg-mice (lower) treated with 500 ng of hGM-CSF. Cells were stained with FITC–anti-CD3 and PE-anti-NK1.1. CD3−NK1.1+ cells in PB, BM, spleen, and liver increased in Tg-mice.
DISCUSSION
We reported that although mGM-CSF induces only GM colony formation from the BM of hGM-CSFR Tg-mice in methylcellulose clonal culture, hGM-CSF promotes the formation of various types of colonies, including GM, eosinophils, erythrocytes, megakaryocytes, mast cells, blast cells, and mixed hematopoietic colonies.3 Thus, the potential of hGM-CSF is not restricted to support of the growth and differentiation of cells of GM lineages as long as hGM-CSFR is expressed on the target cells, thereby supporting a stochastic model rather than an instructive model. The present in vivo study confirmed our in vitro observations. In contrast to the previous report that the administration of mGM-CSF to normal mice induced the expansion of only cells of GM lineages,13 hGM-CSF stimulated the increase of all net hematopoiesis in the hGM-CSFR Tg-mice.
The most significant enhancement by hGM-CSF was erythropoiesis. The proportion and absolute number of reticulocytes was remarkably increased in the PB of Tg-mice treated with hGM-CSF. In addition, we observed a 200-fold increase in erythrocytes and their progenitors, CFU-E, in the spleen, the result being a significant enlargement of the spleen. Thus, the in vivo enhancement of hGM-CSF in erythropoiesis of hGM-CSFR Tg-mice was mainly mediated by the proliferation and differentiation of CFU-E, an event similar to the action of EPO in normal mice erythropoiesis.21 The effects of hGM-CSF on neutrophils, monocytes/macrophages, and eosinophils of Tg-mice were less significant than on erythrocytes, although they were comparable with those of mGM-CSF or mIL-3 in normal mice. Mast cell progenitors were expanded in spleens of Tg-mice treated with hGM-CSF. Megakaryocytes and their progenitors were also expanded in the spleen and liver of Tg-mice (Nishijima et al, unpublished observation, June 1995). However, the number of platelets in the PB of Tg-mice was decreased when injections of over 500 ng of hGM-CSF were given. Similar observations were obtained with both preclinical and clinical studies of in vivo treatment of GM-CSF. In dogs there was a dose-dependent decrease in platelet counts during the administration of canine GM-CSF.22 Because the number of platelets in PB did not decrease when we injected hGM-CSF to splenectomized Tg-mice (Nishijima et al, unpublished observation, July 1995), the decreases of circulating platelets caused by the administration of GM-CSF may be explained by promotion of a system that destroys platelets in the spleen.
In the present study, GM progenitors did not increase and erythroid progenitors decreased in BM of Tg-mice treated with hGM-CSF. In marked contrast, all types of hematopoietic progenitors, especially erythroid progenitors, increased in the spleen. Data on normal mice treated with various cytokines such as mGM-CSF, mIL-3, mSCF, and hG-CSF showed a similar redistribution of hematopoietic progenitors through mobilization of hematopoietic progenitors from BM into peripheral hematopoietic organs.12,13,23,24 In addition to the effects on hematopoiesis, hGM-CSF affected lymphopoiesis in the Tg-mice. In Tg-mice injected with hGM-CSF for 1 week, absolute numbers of mature B cells (B220+IgM+ cells) and T cells (CD4+SP or CD8+SP cell) increased in PB. However, immature CD4+CD8+ DP cells in the thymus dramatically decreased. We also injected hGM-CSF for 2 weeks and the same phenomena were observed (Nishijima et al, unpublished observation, January 1997). We found that CD4−CD8−DN cells and CD4+ or CD8+SP cells, but not CD4+CD8+ DP cells, in the thymus of Tg-mice could proliferate in culture medium containing hGM-CSF in vitro. However, the addition of hGM-CSF to the fetal thymus organ culture system resulted in inhibition of the differentiation of CD4−CD8− DN cells into DP cells in the Tg-mice thymus.25 Taken together with these in vitro observations, the present results suggest that although hGM-CSF inhibits the differentiation of CD4−CD8−DN cells into CD4+CD8+DP cells in thymus in vivo, growth of mature CD4+ SP or CD8+ SP cells is stimulated resulting in an increased number of T cells in PB.
The significant expansion of Mac-1+CD3−NK1.1+NK cells morphologically identified as LGLs was also observed in Tg-mice treated with hGM-CSF. It has been proposed that NK cells can differentiate from BM cells in culture medium containing IL-2.26 Peripheral NK cells can proliferate and gain cytotoxicity function in the presence of IL-2,27 and hGM-CSF can substitute to induce the proliferation and cytotoxicity of peripheral NK cells (Nishijima et al, unpublished observation, July 1996). Therefore, it is tempting to speculate that, in our Tg-mice system, hGM-CSF has the potential to substitute for IL-2 and thereby stimulate NK progenitor cells.
In summary, we obtained evidence that hGM-CSF induced in vivo expansion of various types of blood cells and their progenitors conceivable to express hGM-CSFR, thereby supporting our previous in vitro observation. In addition, the development of lymphoid cells including NK cells in Tg-mice was affected by hGM-CSF treatment. In the present study, we have not determined if hGM-CSF can support the self-renewal of multipotent stem cells of Tg-mice. If the self-renewal of multipotent stem cell is regulated by cytokines, hGM-CSF could substitute for cytokines to induce the expansion of multipotent stem cells.
ACKNOWLEDGMENT
We thank Drs T. Yokota, T. Heike, K. Ikuta, K. Ogasawara, and Y. Yasuda for helpful discussions and technical advice and M. Ohara and R. Nishinakamura for comments on the manuscript.
Supported in part by a grant-in-aid for scientific research in priority areas and cancer research from the Ministry of Education, Science, Sports and Culture of Japan.
Address reprint requests to K. Arai, MD, PhD, Department of Molecular and Developmental Biology, Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokane-dai, Minato-ku, Tokyo 108, Japan.