Abstract
Myoblast-mediated gene transfer and its repeated applications were tested for achieving a long-term stable systemic production of human factor IX (hFIX) at a therapeutic level in SCID mice. Primary skeletal myoblasts were stably transfected with a hFIX expression plasmid vector, pdLMe4βAhIXm1, which contains a hFIX minigene under the control of a β-actin promoter with muscle creatine kinase enhancers. Myotubes derived from the myoblasts produced 1,750 ng hFIX/106 cells/24 hours in culture. hFIX secretion by the myoblasts and thereof derived myotubes were equally efficient, and myotubes were shown to have a sufficient secretory capacity to handle a substantially elevated production of hFIX. After intramuscular injection of 5, 10, and 20 × 106 myoblasts, SCID mice stably produced hFIX into the systemic circulation proportional to the number of implanted cells, and the expression levels were maintained for at least up to 10 months (end of the experiment). Additional cell injections administered to animals that originally received 10 × 106 cells approximately 2 months later elevated the systemic hFIX levels to an average of 182 ± 21 ng/mL, a therapeutic level, which persisted for at least 8 months (end of the experiment). These results indicate that long-term, stable systemic production of hFIX at therapeutic levels can be achieved by repeated application of myoblast-mediated gene transfer.
HEMOPHILIA B is an X-chromosome–linked, recessive bleeding disorder caused by clotting factor IX (FIX) deficiency in the circulation.1 This disease is currently treated by infusion of FIX protein purified from normal human plasma, which is effective, but exposes patients to potential side effects such as hepatitis or human immunodeficiency virus (HIV) infection. More recently, preparation of safer recombinant human FIX (hFIX) has been achieved, and its clinical testing is under way.2 However, the requirement of frequent infusions still adversely affects the patient's quality of life, and gene therapy may provide a safe and effective alternative treatment for this disease.3-5
Most gene transfer approaches tested to date in animals for hemophilia B have used viral vectors such as retrovirus and adenovirus for delivering FIX gene into various target cells and tissues.3-16 hFIX expression levels in vivo achieved in these experiments, however, were either too low to be therapeutic,3-9 or high, but transient as typically shown for adenoviral gene transfer.10-13 Some studies using genetically modified cell lines, such as NIH 3T3 cells15 and C2C12 cells,16 could give high therapeutic levels of hFIX expression in vivo, but the injected cells formed tumors in animals. In using retroviral vectors, the possible contamination of helper virus remains to be a safety concern.17 Adenovirally transferred FIX gene can survive over months and can continue producing FIX in immune compromised animals; however, expression levels still decline with time.10-13 Multiple adenoviral gene transfers in adults resulted in little improvement in FIX expression levels due to immune rejection of the adenoviral vector.14 Adenovirus-associated virus (AAV)-mediated gene transfer into muscle shows some exciting promise.18 Its successful application to hemophilia B gene therapy, however, has yet to be proved.
Various muscle-targeted gene transfer approaches have been tested, including direct in vivo methods using viral vectors or vector DNAs as well as ex vivo methods.5-8 In particular, ex vivo approaches using myoblast-mediated gene transfer have been intensively tested for their potential as a durable gene delivery method for hemophilia B gene therapy.3-8 Similar ex vivo approaches using skin fibroblasts or myoblasts have also been reported for other therapeutic proteins such as growth hormone, glucocerebrosidase, and erythropoietin.19-22 Recently, we have constructed a series of hFIX expression vectors extensively refined for skeletal muscle. These were designed to be easily used either as retroviral or nonviral gene transfer vectors.23
In the present study, we tested the feasibility of myoblast-mediated gene transfer and its repeat application in SCID mice by using primary skeletal myoblasts stably transfected with a refined hFIX expression vector plasmid. We have demonstrated that it is feasible to achieve long-term stable systemic production of hFIX at therapeutic levels in the animals. Some fundamental issues involved in this approach were also studied in vitro.
MATERIALS AND METHODS
Mouse.Pathogen-free, inbred, male SCID mice 5 to 6 weeks of age were obtained from Taconics (Germantown, NY) and housed in a sterile environment in the Unit of Laboratory Animal Medicine facility of this campus. All the animal studies were carried out in accordance with the institutional guidelines.
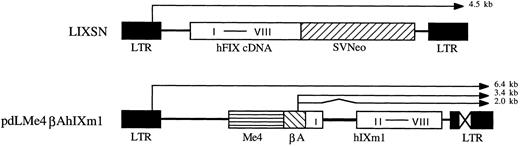
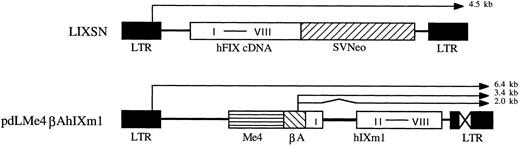
Vectors.Construction of the differentiated muscle cell-specific hFIX expression vector, pdLMe4βAhIXm1, was described elsewhere.23 It contains a hFIX minigene (hIXm1) with a shortened first intron sequence and a transcriptional control unit composed of a β-actin promoter (βA) and four copies of muscle creatine kinase gene enhancer (Me4) (Fig 1). Plasmid vectors pLIXSN (Fig 1) and pBAG contain hFIX cDNA and a bacterial β-galactosidase (β-gal) gene, respectively, in retroviral vector frames under the transcriptional control of the long-terminal repeat (LTR), and a neomycin resistance gene (Neo) under the control of SV40 promoter.24,25 pSV2Neo is a Neo expression vector26 and pCH110 is a β-gal expression vector.27
hFIX expression vectors. pLIXSN contains a hFIX cDNA driven by long terminal repeat (LTR) promoter. pdLMe4βAhIXm1 contains a hFIX minigene (hIXm1) under the transcriptional control of β-actin promoter (βA) and four copies of MCK enhancer (Me4). I to VIII indicates eight exons of hFIX gene. SVNeo indicates a neomycin resistant gene under the control of SV40 immediate early promoter. X in LTR indicates the deleted enhancer region. Thin lines with arrow show the predicted mRNA species; and zigzag line indicates the portion (hFIX intron) to be spliced.
hFIX expression vectors. pLIXSN contains a hFIX cDNA driven by long terminal repeat (LTR) promoter. pdLMe4βAhIXm1 contains a hFIX minigene (hIXm1) under the transcriptional control of β-actin promoter (βA) and four copies of MCK enhancer (Me4). I to VIII indicates eight exons of hFIX gene. SVNeo indicates a neomycin resistant gene under the control of SV40 immediate early promoter. X in LTR indicates the deleted enhancer region. Thin lines with arrow show the predicted mRNA species; and zigzag line indicates the portion (hFIX intron) to be spliced.
Cells and culture conditions.Mouse primary skeletal myoblasts were isolated from muscles of SCID mice and grown in growth medium containing Dulbecco's modified essential medium (DMEM), 20% fetal calf serum (FCS), 0.5% chicken embryo extract (GIBCO/BRL), and antibiotics (streptomycin and penicillin).7,23 hFIX assay medium was composed of the growth medium supplemented with BaSO4-treated FCS (20%) and Vitamin K1 (10 μg/mL).7,23 Differentiation medium was the same as the hFIX assay medium except that FCS was replaced with BaSO4-treated horse serum (2%). In all experiments, culture medium was collected every 24 hours for quantifying hFIX by enzyme-linked immunosorbent assay (ELISA) as previously described,7 23 and replaced with fresh medium. Differentiation of myoblasts to myotubes was induced by changing the medium to the differentiation medium for 3 days. All cells were kept at 37°C in a humidized incubator under 5% CO2 .
Transient expression assay of hFIX expression vectors in muscle cells.Transient expression assays were carried out using LIPOFECTAMINE-mediated cell transfection as previously described.23 Myoblasts at a density of 2 × 105 per well in 6-well plates were transfected with 2 μg of a hFIX expression vector and 0.2 μg of pCH110 DNA. Myoblasts in one well from each group were collected at the end of day 2 and were used for determining cell number, β-gal activity, and total cellular protein content, which were used for normalizing the transfection efficiency as previously described.23 Cell number at the time of switching to the differentiation medium was approximately 2 × 106/well at 80% to 90% confluency.
Stable transfection of myoblasts and hFIX expression assay.Primary myoblasts at a density of approximately 1 × 106 per 10-cm dish were cotransfected with 10 μg pdLMe4βAhIXm1 and 0.8 μg pSV2Neo using LIPOFECTAMINE as described above, and subjected to G418 selection (1 mg/mL). Primary myoblasts transduced with LIXSN and BAG retroviruses were prepared as previously described.28 BAG was used as a background control, and LIXSN was used as a hFIX expression reference control because the cells carrying LIXSN were well characterized in our previous studies for their in vitro and in vivo hFIX expression and served for assessing any improvements in hFIX expression with the new vector. hFIX expression levels were determined by plating myoblasts stably transfected with pdLMe4βAhIXm1, or myoblasts transduced with LIXSN or BAG retrovirus at a density of about 4 × 105 cells per well in 6-well plates. hFIX protein produced into the medium was quantified by ELISA, and its activity was determined by one-stage clotting assay.25
Southern blot analyses.Genomic DNA prepared from cells was digested with Kpn I or BamHI, and subjected to Southern blot analysis using 32P labeled hFIX cDNA as a hybridization probe.25 The transgene band intensity was quantified with PhosphorImager (Molecular Dynamics, Inc, model 400E). Transgene copy numbers in the genomic DNA were determined by using known amounts of vector DNA as the copy number standard.
Analyses of hFIX production and secretion efficiency.Stably transfected or retrovirus-transduced cells were plated at a density of about 6 × 105 cells per dish in 6-cm dishes. The media were collected daily, and cells were collected for preparing protein extracts and total RNA on days 2, 5, and 7. Cellular protein extracts were prepared as previously described23 except that the extraction solution contained Complete protease inhibitor (one Complete protease inhibitor tablet per 25 mL) (Boehringer Mannheim). Intracellular and secreted hFIX was determined by ELISA. Total cellular RNA was prepared using TRIzol total RNA isolation reagent (GIBCO-BRL) and subjected to Northern blot analysis using hFIX cDNA labeled with 32P as the hybridization probe. The filter was exposed to X-ray films, and radioactivity of RNA bands on the filter were quantified by a PhosphorImager (Molecular Dynamics). The filter was then stripped of the hFIX probe and rehybridized with 32P labeled 18S ribosomal RNA cDNA to confirm equal RNA loading to the lanes.
Intramuscular implantation of myoblasts.Myoblasts carrying hFIX or β-gal expression vectors were harvested and washed as described,7,28 and resuspended in serum-free DMEM supplemented with 1 μg/mL basic fibroblast growth factor (bFGF ) (R&D systems) at 50 or 100 × 106 cells/mL. Cells were then injected into the limb muscles of SCID mice through a 30 G needle at five or ten different sites (10 μL per site).7,28 At various time points after cell injection, blood samples (0.1 to 0.3 mL) were collected by orbital sinus bleeding under brief anesthetization with inhalation of methoxyflurane (Pitman-Moore) and plasma samples were prepared for ELISA. At various time points, animals were killed by deep anesthesia with methoxyflurane. Muscles into which cells were implanted were removed for preparing muscle tissue sections and for isolating myoblasts carrying transgenes. The tissue sections were used for H & E staining and immunohistochemical staining. Myoblasts isolated from the muscle tissue which received cell implantation were subjected to G418 selection and used for in vitro hFIX expression assays as previously described.28
Immunostaining analysis.Tissue sections were stained for hFIX using a murine anti-hFIX monoclonal antibody, AHIX-5041 (Haematologic Technologies, Inc), and Histostain-SP Kit (Zymed Laboratories, Inc) according to the manufacturer's instructions. H & E and Masson trichrome stain were carried out using standard histologic methods.29
Western blot analysis.hFIX in aliquots (1 mL) of day 5 culture medium of myotubes was precipitated with barium sulfate (100 mg) and subjected to Western blot analysis as previously described16 25 using a rabbit anti-hFIX polyclonal antibody and the chemiluminescence Western blot kit (Boehringer Mannheim).
RESULTS
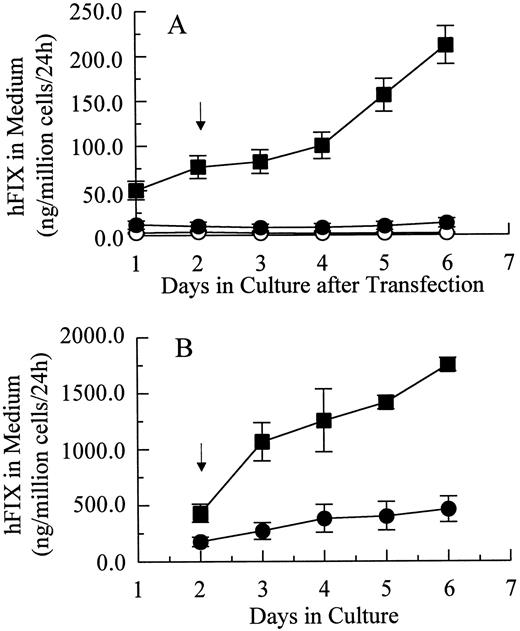
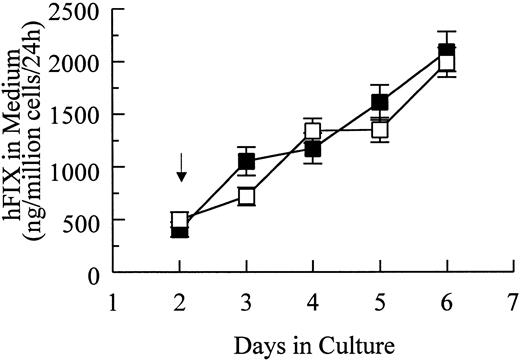
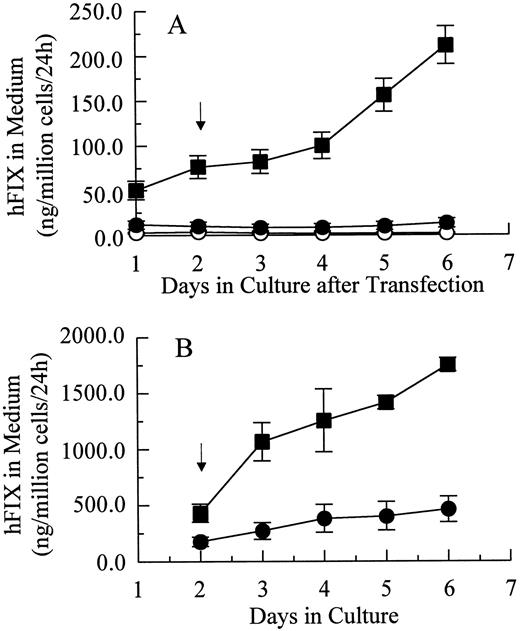
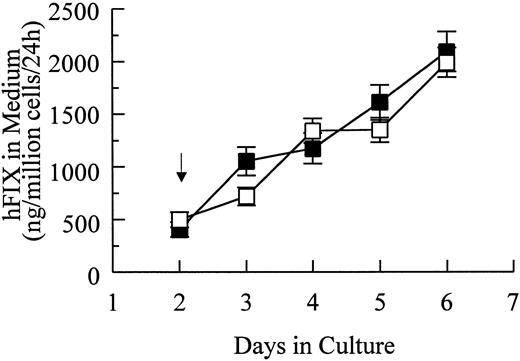
Transient and stable expression of hFIX by muscle cells in vitro.In vitro hFIX production levels of myoblasts transiently transfected with pdLMe4βAhIXm1 were 6- to 10-fold higher than those transfected with pLIXSN (Fig 2A). Myotubes carrying pdLMe4βAhIXm1 secreted hFIX at a rate of 212 ± 21 ng/106 cells/24 hours on day 6, a 15-fold higher level than that of myotubes with pLIXSN (14 ± 5 ng/106 cells/24 h). This was consistent with the presence of the differentiated muscle cell-specific enhancer in pdLMe4βAhIXm1, but not in pLIXSN.
hFIX production in culture by muscle cells carrying pdLMe4βAhIXm1 and LIXSN. (A) Transient expression of hFIX by muscle cells transfected with pdLMe4βAhIXm1 (▪), LIXSN (•), and pSV2Neo (○). Medium was collected every 24 hours for ELISA and replaced with fresh medium. Arrow indicates the differentiation initiation time point. Vertical bars are standard deviations of triplicated assays. (B) Stable expression of hFIX. (▪) indicates pdLMe4βAhIXm1; (•), LIXSN. Results shown are averages of three independent experiments with standard deviations shown in vertical bars. Signals in ELISA from cells transduced with BAG were subtracted as background from the results. The others are the same as described for transient assay (A).
hFIX production in culture by muscle cells carrying pdLMe4βAhIXm1 and LIXSN. (A) Transient expression of hFIX by muscle cells transfected with pdLMe4βAhIXm1 (▪), LIXSN (•), and pSV2Neo (○). Medium was collected every 24 hours for ELISA and replaced with fresh medium. Arrow indicates the differentiation initiation time point. Vertical bars are standard deviations of triplicated assays. (B) Stable expression of hFIX. (▪) indicates pdLMe4βAhIXm1; (•), LIXSN. Results shown are averages of three independent experiments with standard deviations shown in vertical bars. Signals in ELISA from cells transduced with BAG were subtracted as background from the results. The others are the same as described for transient assay (A).
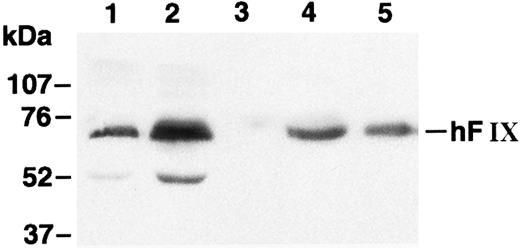
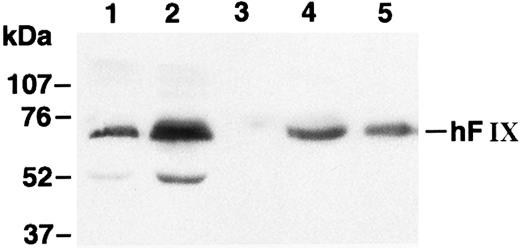
Myoblasts stably transfected with pdLMe4βAhIXm1 were prepared by cotransfection with pSV2Neo followed by G418 selection. Fifty-three myoblast colonies picked were individually expanded and their medium samples were subjected to ELISA screening for hFIX production. Nineteen of them were positive for hFIX expression, and six colonies expressed over 200 ng/106 myoblasts/24 hours. Myotubes derived from one of these myoblast colonies produced hFIX at 1,750 ± 61 ng/106 cells/24 hours on day 6, the highest level among the colonies tested. Myotubes derived from the myoblasts transduced with LIXSN produced 463 ± 114 ng hFIX/106 cells/24 hours (Fig 2B). Southern blot analysis of the genomic DNA indicated that the cells transfected with pdLMe4βAhIXm1 or transduced with LIXSN had approximately four or two copies of the vector DNA per cell, respectively (data not shown). The recombinant hFIX protein produced from myoblasts and myotubes was biologically fully active with a specific activity ranging from 95% to 103% and a molecular size similar to natural hFIX (68 kD), agreeing well with our previous observations (Fig 3).16,25 A minor band with an apparent smaller size (about 52 kD) observed may be a degradation product of hFIX protein generated during the protein preparation procedure as we also observed for canine factor IX.30
Western blot analysis of hFIX. Lane 1, medium of myotubes carrying LIXSN; lane 2, medium of myotubes carrying pdLMe4βAhIXm1; lane 3, medium of myotubes carrying BAG; lane 4, hFIX precipitated from pooled normal human plasma; and lane 5, recombinant hFIX (100 ng). Numbers on the left indicate the molecular size markers. The hFIX band position is shown on the right. Protein bands with an approximate size of 52 kD in lanes 1 and 2 may correspond to degradation products of hFIX during the protein preparation procedure.
Western blot analysis of hFIX. Lane 1, medium of myotubes carrying LIXSN; lane 2, medium of myotubes carrying pdLMe4βAhIXm1; lane 3, medium of myotubes carrying BAG; lane 4, hFIX precipitated from pooled normal human plasma; and lane 5, recombinant hFIX (100 ng). Numbers on the left indicate the molecular size markers. The hFIX band position is shown on the right. Protein bands with an approximate size of 52 kD in lanes 1 and 2 may correspond to degradation products of hFIX during the protein preparation procedure.
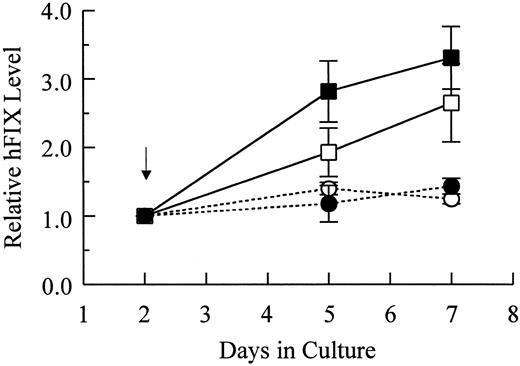
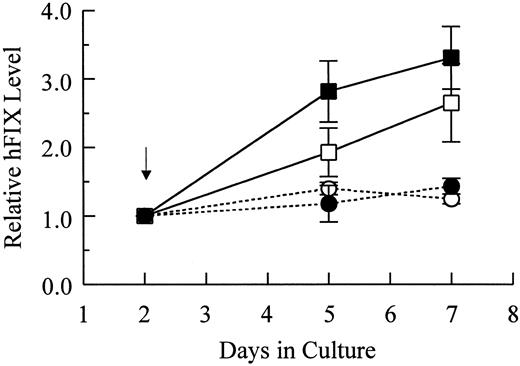
Characterization of production and secretion of hFIX by muscle cells.To test the capacities of primary myoblasts and myotubes in hFIX expression and secretion, hFIX mRNA as well as intracellular and secreted hFIX levels were determined. Following differentiation, intracellular and secreted hFIX levels of cells carrying pdLMe4βAhIXm1 increased by 2.8- and 3.3-fold, respectively, but only marginally increased for cells carrying LIXSN (Fig 4). In agreement with ELISA results, the hFIX mRNA level in myotubes with pdLMe4βAhIXm1 was 2.9-fold higher than that in the parent myoblasts (comparison of day 5 to day 2; mean of three experiments), while it was not significantly different for cells with LIXSN (1.1-fold increase in myotubes over that in myoblasts) (Fig 5). These results indicated that hFIX secretion from myotubes is very efficient even at the level up to 2 μg/106 cells/24 hours.
Relative levels of intracellular and secreted hFIX of myotubes in comparison with those of myoblasts. Arrow indicates the time point of switching to the differentiation medium. Results shown are averages of two independent experiments with observed ranges shown in vertical bars. hFIX expressed by myoblasts at day 2 are 7.7 ± 0.1 ng/106 cells and 593.2 ± 8.9 ng/106 cells/24 hours for intracellular (□) and secreted hFIX (▪) from cells stably transfected with pdLMe4βAhIXm1, 6.2 ± 2.1 ng/106 cells and 581.6 ± 74.9 ng/106 cells/24 hours for intracellular (○) and secreted hFIX (•) from myoblasts transduced with LIXSN, respectively. Intracellular and secreted hFIX levels of myotubes were normalized to those of myoblasts (day 2), which were defined as 1 as shown on the Y-axis.
Relative levels of intracellular and secreted hFIX of myotubes in comparison with those of myoblasts. Arrow indicates the time point of switching to the differentiation medium. Results shown are averages of two independent experiments with observed ranges shown in vertical bars. hFIX expressed by myoblasts at day 2 are 7.7 ± 0.1 ng/106 cells and 593.2 ± 8.9 ng/106 cells/24 hours for intracellular (□) and secreted hFIX (▪) from cells stably transfected with pdLMe4βAhIXm1, 6.2 ± 2.1 ng/106 cells and 581.6 ± 74.9 ng/106 cells/24 hours for intracellular (○) and secreted hFIX (•) from myoblasts transduced with LIXSN, respectively. Intracellular and secreted hFIX levels of myotubes were normalized to those of myoblasts (day 2), which were defined as 1 as shown on the Y-axis.
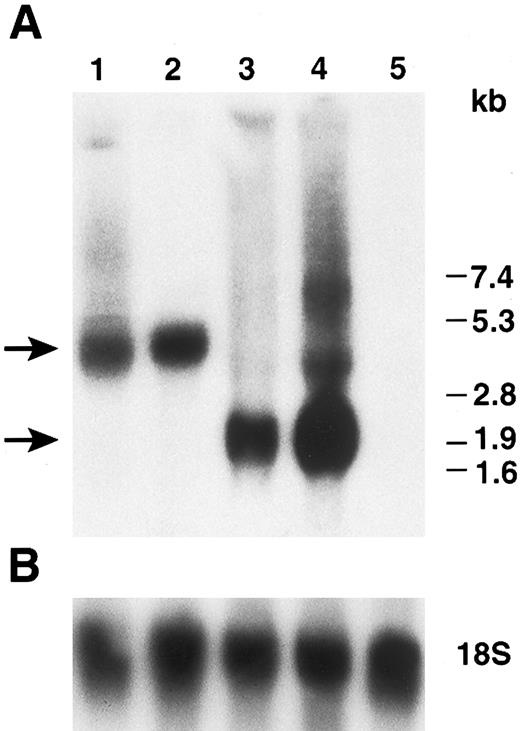
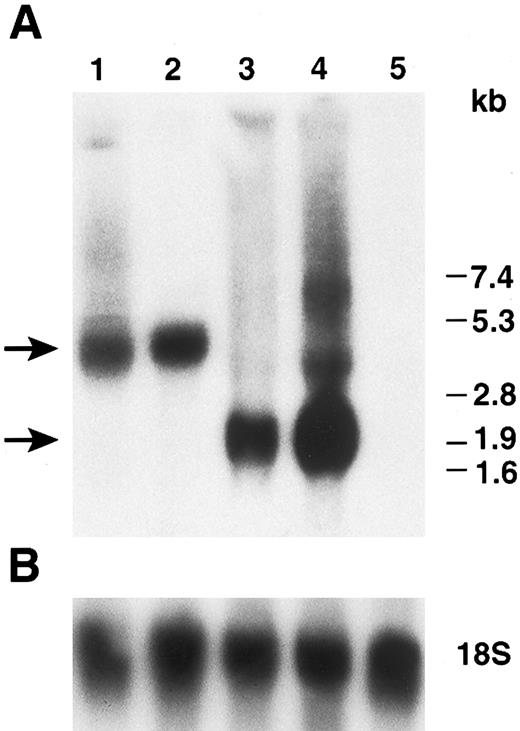
Northern blot analysis of hFIX expressed by myoblasts and myotubes. (A) Total cellular RNAs prepared from cells on day 2 (myoblasts) and day 5 (myotubes) in Fig 4 were subjected to Northern blot analysis. Lanes 1 and 2: myoblasts (day 2) and myotubes (day 5) carrying LIXSN, respectively. Lanes 3 and 4: myoblasts (day 2) and myotubes (day 5) carrying pdLMe4βAhIXm1, respectively. Three major bands are seen in lane 4; they are transcription products from LTR and Me4βA, respectively, corresponding to the predicted mRNA size shown in Fig 1. Lane 5: total RNAs prepared from untransfected myotubes. Numbers on the right indicate the RNA molecular size markers and arrows on the left indicate the major hFIX mRNA bands. (B) Hybridization of the same filter membrane with 18S ribosome RNA probe after the dehybridization of the hFIX probe as RNA loading controls.
Northern blot analysis of hFIX expressed by myoblasts and myotubes. (A) Total cellular RNAs prepared from cells on day 2 (myoblasts) and day 5 (myotubes) in Fig 4 were subjected to Northern blot analysis. Lanes 1 and 2: myoblasts (day 2) and myotubes (day 5) carrying LIXSN, respectively. Lanes 3 and 4: myoblasts (day 2) and myotubes (day 5) carrying pdLMe4βAhIXm1, respectively. Three major bands are seen in lane 4; they are transcription products from LTR and Me4βA, respectively, corresponding to the predicted mRNA size shown in Fig 1. Lane 5: total RNAs prepared from untransfected myotubes. Numbers on the right indicate the RNA molecular size markers and arrows on the left indicate the major hFIX mRNA bands. (B) Hybridization of the same filter membrane with 18S ribosome RNA probe after the dehybridization of the hFIX probe as RNA loading controls.
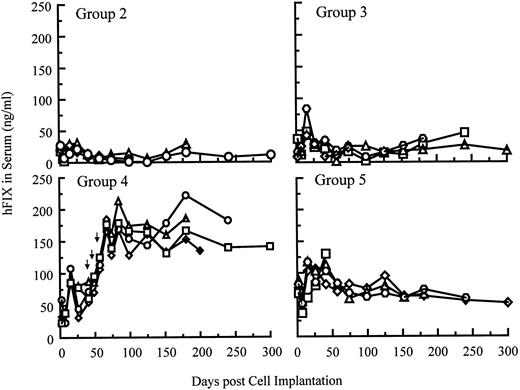
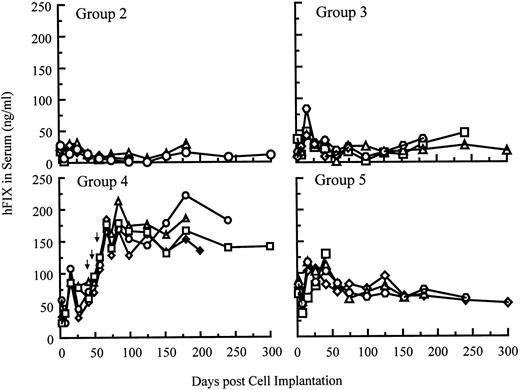
Systemic delivery of hFIX in SCID mice after single and repeated cell implantations.hFIX production into the circulation of SCID mice after myoblast-mediated gene transfer is shown in Fig 6. Five groups of SCID mice (n = 4 per group) were implanted with various numbers of primary myoblasts stably carrying BAG, LIXSN, or pdLMe4βAhIXm1. Animals in groups 1 and 2 were injected with 20 × 106 myoblasts transduced with BAG or LIXSN retroviral vectors. Animals in groups 3, 4, and 5 were injected with 5, 10, and 20 × 106 myoblasts with pdLMe4βAhIXm1, respectively. As assayed 26 days after the first cell injection, animals in groups 2, 3, and 4 produced hFIX at average levels of 23.1, 27.1, and 58.5 ng/mL serum, respectively. Animals in group 5 gave the highest hFIX production (94.1 ng/mL serum on average), 4- to 5-fold higher than those in group 2, which received myoblasts carrying LIXSN.7 These results were remarkably consistent with the in vitro observations (Fig 2B). hFIX expression levels in groups 3, 4, and 5 were approximately proportional to cell numbers injected (Fig 6). Stable hFIX production at levels of approximately 12, 18, and 60 ng/mL serum were observed for groups 2, 3, and 5, respectively, at least up to 10 months (end of observation).
hFIX levels in the systemic circulation of SCID mice after intramuscular implantation of myoblasts carrying pdLMe4βAhIXm1 or LIXSN. The background ELISA signals obtained from group 1 (control group) mice, which were injected with 2 × 107 myoblasts transduced with BAG, were subtracted from the observed levels of other groups. Group 2 mice were injected with 2 × 107 myoblasts transduced with LIXSN. On day 0, group 3, group 4, and group 5 animals were injected with 5, 10, and 20 × 106 myoblasts stably transfected with pdLMe4βAhIXm1, respectively. Mice in group 4 were injected with additional cell doses (1, 1, and 2 × 107 cells, respectively) on days 43, 52, and 59 as shown with arrows. Results from individual animals in each group were shown.
hFIX levels in the systemic circulation of SCID mice after intramuscular implantation of myoblasts carrying pdLMe4βAhIXm1 or LIXSN. The background ELISA signals obtained from group 1 (control group) mice, which were injected with 2 × 107 myoblasts transduced with BAG, were subtracted from the observed levels of other groups. Group 2 mice were injected with 2 × 107 myoblasts transduced with LIXSN. On day 0, group 3, group 4, and group 5 animals were injected with 5, 10, and 20 × 106 myoblasts stably transfected with pdLMe4βAhIXm1, respectively. Mice in group 4 were injected with additional cell doses (1, 1, and 2 × 107 cells, respectively) on days 43, 52, and 59 as shown with arrows. Results from individual animals in each group were shown.
To test the feasibility of repeat cell implantation, animals in group 4 were injected with additional doses of cells on day 43 (10 × 106, left leg muscle), day 52 (10 × 106, right leg muscle), and day 59 (20 × 106, both forearm muscles). Systemic hFIX levels in these animals were elevated to a range of 160 to 210 ng/mL serum, again approximately proportional to the implanted cell numbers, and stayed stable for an additional 8 months (end of the observation) (Fig 6). This range was approximately 2.5-fold higher than that of group 5, and approximately 15-fold higher than that of group 2 (cells with LIXSN).
Two hundred days after the first cell injection, primary myoblasts were obtained in culture from the muscle tissues of a representative animal in group 4. After G418 selection, cells showed a hFIX expression level equivalent to that of the original unimplanted, parent cells (Fig 7). Myoblasts isolated from another animal in group 1 3 months after implantation also gave similar results (data not shown). These results were consistent with our previous observations,28 demonstrating that a fraction of implanted cells can acquire the satellite cell status, and that little inactivation of LTR or Me4βA promoter took place in vivo. Although it is not likely, the possibility of partial inactivation of these promoters in vivo and regaining of their full activities in vitro cannot be denied. Immunohistochemical analysis of muscle tissue sections prepared from mice in groups 4 and 5 after 200 to 300 days of the original cell implantation also confirmed active hFIX production by the implanted cells (Fig 8, A and B).
Production of hFIX in culture by muscle cells stably transfected with pdLMe4βAhIXm1. (▪) indicate uninjected parent cells; (□), cells recovered from the animal muscle tissues 200 days after the original cell implantation. Myoblasts obtained were selected with G418 and pooled for the analysis. Experimental conditions are similar to those in Fig 2B. Averages of triplicate assays are shown with standard deviations (vertical bars).
Production of hFIX in culture by muscle cells stably transfected with pdLMe4βAhIXm1. (▪) indicate uninjected parent cells; (□), cells recovered from the animal muscle tissues 200 days after the original cell implantation. Myoblasts obtained were selected with G418 and pooled for the analysis. Experimental conditions are similar to those in Fig 2B. Averages of triplicate assays are shown with standard deviations (vertical bars).
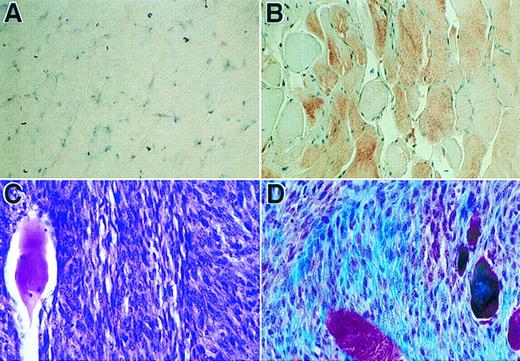
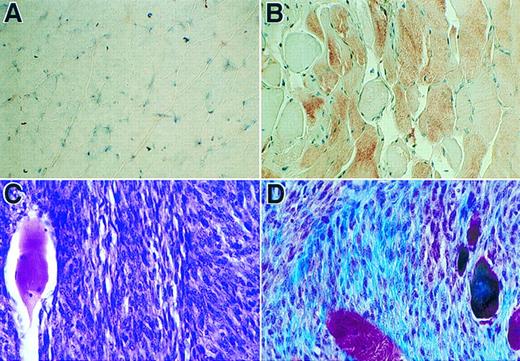
Immunohistochemical analyses of sections of muscle tissue and a spontaneous tumor. (A) Tissue section of muscle with no cell injection. (B) Tissue section of muscle received myoblasts carrying pdLMe4βAhIXm1. Tissue sections in panels A and B were stained with hFIX monoclonal antibody followed by counter-staining with hemotoxylin (×630). Brownish-red color indicates the presence of hFIX. (C) Section of the tumor from a group 4 mouse stained with H & E (×500). The fibrosarcoma cells are small and spindle-shaped; one large muscle fiber is on the left. (D) Tumor section stained with Masson trichrome shows small spindled cells of fibrosarcoma and the collagen produced by the neoplasm in blue color; several muscle fibers are red or purple-red in color and large in size (×500).
Immunohistochemical analyses of sections of muscle tissue and a spontaneous tumor. (A) Tissue section of muscle with no cell injection. (B) Tissue section of muscle received myoblasts carrying pdLMe4βAhIXm1. Tissue sections in panels A and B were stained with hFIX monoclonal antibody followed by counter-staining with hemotoxylin (×630). Brownish-red color indicates the presence of hFIX. (C) Section of the tumor from a group 4 mouse stained with H & E (×500). The fibrosarcoma cells are small and spindle-shaped; one large muscle fiber is on the left. (D) Tumor section stained with Masson trichrome shows small spindled cells of fibrosarcoma and the collagen produced by the neoplasm in blue color; several muscle fibers are red or purple-red in color and large in size (×500).
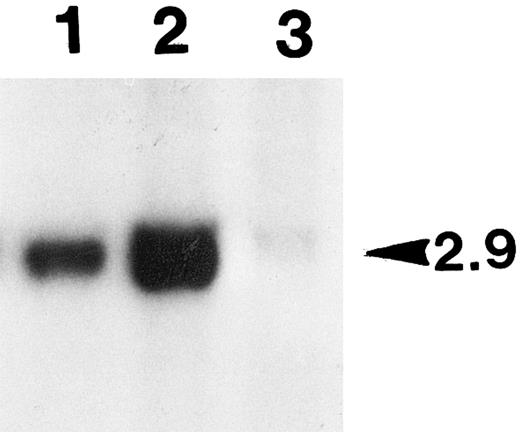
Characterization of tumor.About 6 months post-cell implantation, one of the group 4 animals developed a tumor in the right leg muscle where cells were injected. The left leg muscle injected with the same batch and number of cells was normal and did not grow any tumor. This animal did not show any unusually elevated level of hFIX expression. Such an increase was invariably observed, when the implanted primary cells were transformed and proliferated in vivo. Cells isolated from the tumor biopsy expressed only a background level of hFIX (less than 10 ng/106/24 hours in culture), and they did not survive the G418 selection. Cells isolated from the other leg of the same animal, where the same batch and number of cells were also injected with no subsequent tumor development, survived the G418 selection well. The selected cells expressed hFIX at a comparable level with that of the unimplanted parent cells (Fig 7). Southern blot analysis detected substantially lowered level (23%) of the factor IX transgene in the genomic DNA prepared from the tumor tissue in comparison with that of the other leg where no tumor developed (Fig 9). The lowered transgene level is presumably due to the presence of a massive tumor tissue interspersed with a lesser amount of the normal muscle cells carrying the transgene in the muscle tissue removed for various analyses (Fig 8C). Masson trichrome stain (Fig 8D)29 and electron microscopic studies (data not shown) identified that this tumor is a fibrosarcoma. These results probably determined that the tumor is not derived from the implanted myoblasts.
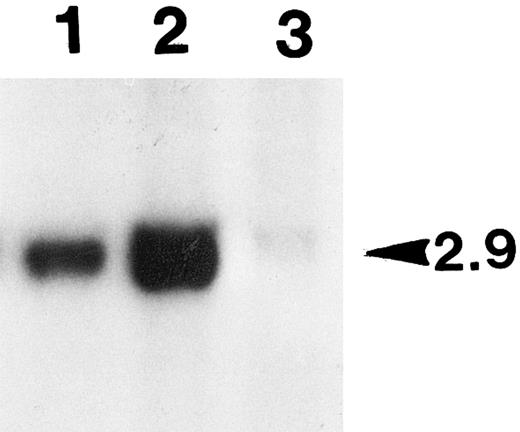
Southern blot analysis of the genomic DNAs prepared from the leg muscle tissues of tumor-bearing animal. Aliquots of the total genomic DNA samples (10 μg) prepared from the leg tissues of a tumor-bearing animal were digested with BamHI, and subjected to Southern blot analysis (see the Materials and Methods). The unique human factor IX transgene bands detected (2.9 kb in size as marked on the right side) were quantified with PhosphorImager. Lane 1, DNA from the right hind leg with tumor; lane 2, DNA from the left leg with no tumor; lane 3, control DNA of primary myoblasts transduced with BAG (β-galactosidase retrovirus).
Southern blot analysis of the genomic DNAs prepared from the leg muscle tissues of tumor-bearing animal. Aliquots of the total genomic DNA samples (10 μg) prepared from the leg tissues of a tumor-bearing animal were digested with BamHI, and subjected to Southern blot analysis (see the Materials and Methods). The unique human factor IX transgene bands detected (2.9 kb in size as marked on the right side) were quantified with PhosphorImager. Lane 1, DNA from the right hind leg with tumor; lane 2, DNA from the left leg with no tumor; lane 3, control DNA of primary myoblasts transduced with BAG (β-galactosidase retrovirus).
DISCUSSION
In recent years, the promising potential of myoblast-mediated gene transfer for systemic production of therapeutic proteins has been reported by multiple groups including ours.3 5-8 In most of these studies, retroviral expression vectors combined with single dose cell implantation were used. In the present study, we have successfully demonstrated that the nonviral myoblast-mediated gene transfer and its repeat application can be used to achieve a long-term stable production of hFIX into the systemic circulation in mice at a therapeutic level. The systemic level achieved in this feasibility study is equivalent to 4% to 5% of the normal hFIX level, sufficient to change a clinically severe hemophilia B to a mild condition.
pdLMe4βAhIXm1, one of our refined hFIX expression vectors,23 was used as a plasmid vector in the present study. In transient assays, this vector, which contains a muscle specific transcription control unit, can express approximately 15-fold higher hFIX than LIXSN (our first generation retroviral vector) in myotubes (Fig 2A). Myotubes derived from 1 × 106 myoblasts stably transfected with this vector produced as high as 1,750 ng of hFIX per 24 hours, approximately 4-fold higher than those of the LIXSN transduced myotubes (Fig 2B). This is a relatively small increase in comparison to that observed in the transient expression assays (∼15-fold) (Fig 2A). This difference cannot be simply explained by the transgene copy number, because cells transfected with pdLMe4βAhIXm1 have approximately four copies of the expression vector per cell, while those transfected with LIXSN have approximately two copies per cell (data not shown). Positional effects may be, in part, responsible for the difference, and/or some changes in expression activities of the promoters used may possibly take place in the stably integrated transgene environment. Alternatively, the difference could be related to possibly poor secretion of hFIX from myotubes. As shown for the LIXSN-transduced cells (Fig 4), however, myotubes can express and secrete hFIX as efficiently as myoblasts. Furthermore, intracellular as well as secreted hFIX levels observed with myotubes derived from primary myoblasts carrying pdLMe4βAhIXm1 are elevated approximately threefold over those of their parent myoblasts in accordance with the increased mRNA level, indicating that the myotubes have a secretory capacity sufficient to handle the elevated hFIX production. These results indicate that hFIX secretion is not the rate limiting step in producing hFIX from myotubes (Figs 4 and 5).
Animals injected with various numbers of myoblasts carrying pdLMe4βAhIXm1 or LIXSN persistently produced hFIX into the circulation for at least 10 months (end of the experiment) (Fig 6). In addition, hFIX levels in the systemic circulation were roughly proportional to the cell numbers injected. Importantly, this repeat cell implantation scheme works well, thus enabling us to achieve much higher, truly therapeutic levels of stable hFIX production into the circulation (Fig 6, group 4). The elevated hFIX levels were again approximately proportional to the total cell number injected. Rando and Blau31 also reported that, after intramuscular injection of myoblasts transduced with the β-gal expression retroviral vector, the expression levels of β-gal are proportional to the injected cell numbers in a given range. The cell numbers as well as cell concentration used by Rando and Blau,31 however, were much smaller than those used in the present study. The hFIX levels in the systemic circulation reported here were 10- to 15-fold higher than the stable levels we previously achieved in mice,7 and much higher than those (approximately 10 ng/mL of canine FIX in mice) reported by Dai et al,8 who also used an approach of primary myoblast-mediated gene transfer with retroviral vectors. Kay et al9 reported a long-term stable canine FIX expression in dogs by in vivo delivery of FIX retrovirus through the portal vein, but only at low levels (<10 ng/mL plasma). Although we must be cautious in direct comparison of our results with those obtained by others because of the different experimental conditions used,30 the stable systemic hFIX level we have achieved and the possibility of its further elevation by repeated therapies are important.
Based on the two-compartment distribution kinetics,16,32 we have estimated that the plasma hFIX concentration level obtained for the group 4 animals is less than 5% of the theoretical steady-state level that could be expected with the total 5 × 107 cells injected. Here we assume that all cells can produce hFIX in vivo as efficiently as in vitro. Although it is a rough estimation, this suggests that most implanted cells are not efficiently contributing to hFIX production in vivo. This may be due to (1) the death of a large percentage of the implanted cells without successful fusion with existing myofiber cells or among themselves to form new myofiber cells, (2) the in vivo environment is substantially different from that in the culture and the transgene cannot be expressed as efficiently as in the cultured cells, (3) a combination of these possibilities, or (4) other yet unknown mechanisms. Generally low survival of nongenetically modified myoblasts implanted in humans (the above second possibility) was also reported by multiple groups6,33 34 suggesting that the myoblast implantation procedure and cell fusion processes are at least two critical steps that need further careful investigation for improving this gene transfer approach.
After 6 months of cell injection, one of the group 4 animals developed a tumor in the right hind leg muscle. The left hind leg muscle, which also received the same batch and number of genetically modified cells, was normal and did not grow any tumor. A series of biochemical and immunohistochemical analyses in addition to the lack of increased production in vivo of the recombinant FIX indicated that this tumor was fibrosarcoma, and not directly derived from the implanted cells. At this stage of the study, however, we should not disregard a possibility that the tumor might have been derived from the implanted cells due to an unknown mechanism. Other possible explanations for its development may include a random tumorigenesis incidence in the immune-deficient SCID mice, possible tumorigenic stimulation in the local tissue due to the damage by multiple needle stabbings or any other unforeseen mechanisms. The implanted cells were mixed with basic FGF just before their implantation to increase the hFIX expression in vivo.7 This effect is presumably due to a brief stimulation of proliferation or possibly augmentation of migration and/or invasion through basal lamina of the injected myoblast.35,36 Although no tumorigenic potential of bFGF has been reported, its angiogenic activity is well established,36,37 suggesting a possibility that once tumorigenesis is triggered due to unknown mechanism(s), it may support subsequent tumor growth. In our experimental conditions, however, bFGF should be cleared from the injection sites within minutes, making it unlikely for bFGF to be directly responsible for such an aberration(s). It has been reported that after subcutaneous implantation in athymic mice, C2 myoblasts (established cells), but not cloned primary myoblasts, develop tumors and also that intramuscularly injected C2 cells can proliferate in vivo.31 In addition, extensive human trials of myoblast implantation therapy for Duchenne muscular dystrophy (DMD) have strongly supported the general safety of myoblast implantation.33,34 Roman et al38 reported that C57BL/J6 mice intramuscularly implanted with either C2C12 cells or primary myoblasts, which are transduced with recombinant retroviruses, invariably developed rhabdomyosarcomas after 60 days of cell implantation. Unfortunately, the investigators provided neither information regarding the potential contamination of replication-competent retrovirus in the viral stock used nor other explanations for the tumor development with the primary myoblasts.
The present study has demonstrated the feasibility to refine the myoblast-mediated gene transfer method in achieving both the long-term systemic production of therapeutic level recombinant FIX and improved safety. The results warrant further intensive studies on its safety aspects and improvement of the overall FIX systemic production, before its clinical application.
ACKNOWLEDGMENT
We thank Dr David L. Allen and Dr Ataç Türkay for their critical reading of the manuscript and the morphology core facility of the Reproductive Science Program of the University of Michigan for preparing tissue sections.
Supported in part by grants from National Institute of Health (HL53713 and HL38644), University of Michigan Multipurpose Arthritis and Musculoskeletal Diseases Center (NIH 5960AR20557), and General Clinical Research Center (NIH MOIRR00042). J.M.W. is a recipient of the fellowship of the Education Commission of P.R. China.
Address reprint requests to Kotoku Kurachi, PhD, Department of Human Genetics, 3712 Medical Science II, University of Michigan Medical School, Ann Arbor, MI 48109-0618.