Abstract
Infection by human immunodeficiency virus type 1 (HIV-1) results in a progressive depletion of CD4+ T lymphocytes, leading to fatal immunodeficiency. The mechanisms causing the marked loss of CD4+ T lymphocytes are incompletely understood. However, several lines of evidence indicate that direct cytopathology mediated by HIV-1 is a key element in such CD4+ T-cell depletion. In this study, we investigated whether the previously reported incorporation of host-derived major histocompatibility class II glycoproteins (MHC-II) on HIV-1 can alter its replicative capacity. To achieve this goal, virus stocks were produced in parental MHC-II–expressing RAJI cells and in MHC-II–negative RAJI mutants (RM3), both of which have been stably transfected with human CD4 cDNA to allow productive infection with HIV-1. An enhancement of the rate/efficiency of virus entry was seen after infection with normalized amounts of virions carrying host-derived MHC-II on their surface as compared with inoculation with virions devoid of cellular MHC-II. Data from time-course and infectivity experiments showed that the kinetics of infection were more rapid for virions bearing host-derived MHC-II glycoproteins than for MHC-II–free HIV-1 particles. These results suggest that virally embedded cellular MHC-II glycoproteins are functional and can have a positive effect on early events in the virus replicative cycle. Therefore, we show that the acquisition of cellular MHC-II glycoproteins by HIV-1 can modify its biologic properties and might, consequently, influence the pathogenesis of this retroviral disease.
INDIVIDUALS INFECTED with the human immunodeficiency virus type 1 (HIV-1) experience a dramatic loss of CD4+ T lymphocytes, which is responsible for the immunodeficiency seen in virally infected patients. A variety of hypotheses have been postulated in an attempt to elucidate the CD4+ T lymphocyte loss in HIV-1 infection. Direct lysis of the cells after the virus infection has been suggested as a putative mechanism that could account for the depletion of CD4+ T cells in infected individuals.1 2 Therefore, any viral or cellular factors that may modulate the viral life cycle may also influence the pathogenesis of the disease.
Most enveloped viruses are released from infected cells by budding through cell membranes. During the budding process, it is thought that the incorporation of host molecules within the virus occurs because the cellular membrane plays an active role during virion assembly.3 The nascent viral particle is constituted by a lipid bilayer similar to the plasma membrane of the target cell leading to the insertion of host cell membrane molecules within the nascent viral particle. The first demonstration that cellular proteins were efficiently incorporated in HIV came from a study that has determined that false-positive reactions in enzymatic assays were due to virally incorporated cellular major histocompatibility complex (MHC) glycoproteins.4 Several investigators have also analyzed virally embedded host molecules and it was shown that numerous host proteins were acquired by HIV, including β2-microglobulin, intercellular adhesion molecule-1, lymphocyte function-associated antigen 1 (LFA-1), CD43, CD44, CD55, CD59, CD63, CD71, and the HLA-DR determinant of MHC class II (MHC-II).5-16
The physical presence of MHC-II glycoproteins on the virion surface is of interest considering that surface CD4 is acting both as the primary cellular receptor for HIV17,18 and the physiological ligand of MHC-II.19 The interaction between CD4 and MHC-II has been shown to be required to achieve optimal activation of CD4-expressing T lymphocytes during antigenic presentation.20-25 MHC-II antigens are heterodimeric and highly polymorphic glycoproteins constituted of noncovalently bound α (34 kD) and β (28 kD) chains belonging to at least three series of multiallelic products: HLA-DR, HLA-DP, and HLA-DQ.26 MHC-II glycoproteins are expressed on limited types of cells such as B lymphocytes, dendritic cells, Langerhans cells, monocytes and macrophages, microglial cells, and activated T lymphocytes.27 Expression of MHC-II molecules has been reported to be induced in human T cells after their in vivo28 or in vitro29 30 activation.
It is logical to postulate that binding of virally embedded host MHC-II to cell surface CD4 may result in stronger and/or longer interactions between HIV-1 and susceptible cells. This putative interaction would help the initial process of virus entry since a certain threshold number of interactions between viral and cellular surface molecules must occur to achieve an efficient viral infection because the surfaces of both CD4+ T cells and HIV-1 are highly negatively charged.31 Such interactions between virally acquired host MHC-II and its physiological counterligand might be even more important after taking into consideration the reported spontaneous shedding of the external envelope glycoprotein gp120 from mature HIV.32 33 To define whether the acquisition of host MHC-II glycoproteins by HIV-1 particles may affect the course of the HIV-1 life cycle, virus stocks were generated in two CD4+ cell lines that differed only in the absence or the presence of surface MHC-II. Using virus internalization and infectivity assays, we determined that the acquisition of host-derived MHC-II glycoproteins by HIV-1 is associated with a faster kinetics in the process of viral infection. Our data indicate that the physical presence of virally acquired host MHC-II glycoproteins on HIV-1 positively modulates the biologic properties of such virions.
MATERIALS AND METHODS
Cells and transfection.HeLa-CD4-LTR-β-gal cells have been previously used to quantify HIV-1 infectivity34; Molt-4 clone 8 is a T-cell line that is highly susceptible to HIV-1 infection35; Sup-T1 cells express high levels of surface CD436; and We17/10 is a human interleukin-2–dependent T-lymphoblastoid cell line.37 These cell lines were provided by the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (Rockville, MD). RAJI is an Epstein-Barr virus-carrying B-cell line that has been reported to express very high levels of cell surface MHC-II.38 RM3 is a RAJI derivative cell line produced by mutagenesis and is negative for MHC-II expression due to a defect at the level of transcription initiation.39 RAJI and RM3 have been kindly provided by Dr R.-P. Sékaly (Institut de Recherches Cliniques de Montréal, Montréal, Québec, Canada). RAJI and RM3 cells were rendered susceptible to HIV-1 infection by stably transfecting them with cDNA encoding for human CD4 using an amphotropic retrovirus as described previously.40 In brief, the cDNA encoding for human CD4 was subcloned in the eukaryotic expression retroviral vector pMNC, which contains the neomycin (G418) resistance gene and a cytomegalovirus promoter for eukaryotic gene expression.41 The amphotropic helper packaging cell line DAMP42 was transfected with the pMNC molecular construct by the calcium phosphate technique. The resulting recombinant amphotropic retrovirus particles, which contains the cDNA encoding for CD4 driven by the cytomegalovirus promoter region and the neo gene driven by the long terminal repeat of the Moloney murine leukemia virus, was next used to infect RAJI and RM3 cells. Cell lines were maintained in complete culture medium made of RPMI 1640 supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT), glutamine (2 mmol/L), penicillin G (100 U/mL), and streptomycin (100 μg/mL). RAJI and RM3 cells stably expressing CD4 (RAJI-CD4 and RM3-CD4) were cultured in complete culture medium supplemented with 1 mg/mL of the selective agent G418 (GIBCO-BRL, Gaithersburg, MD).
Antibodies.Anti-CD4 monoclonal antibody (MoAb) Leu-3A was purchased from Becton Dickinson Immunocytometry System (San Jose, CA). Hybridomas secreting monoclonal anti–HLA-DR L243 (recognizes the αβ dimer),43 anti–HLA-DR 2.06,44 anti-CD3 OKT3,45 and anti–MHC-I W6/3246 antibodies were obtained from the American Type Culture Collection (Rockville, MD). Anti-HLA-DP B7.2147 and anti–HLA-DQ BT3.448 antibodies were kindly provided by Dr R.-P. Sékaly. All antibodies were isolated from hybridoma culture supernatants and purified by protein G-Sepharose (GIBCO -BRL) affinity chromatography according to the manufacturer's instructions.
Flow cytometry analysis.Levels of surface molecules were detected by indirect immunofluorescence using a cytofluorimeter. Briefly, 1 × 105 cells were first incubated with an experimentally determined saturating concentration of an appropriate murine antibody for 30 minutes on ice. Samples were washed twice in phosphate-buffered saline (PBS) and incubated for 30 minutes on ice with a fluorescein isothiocyanate-conjugated goat antimouse IgG. After two washes with PBS, samples were fixed with 1% (vol/vol) of paraformaldehyde and analyzed by a cytofluorimeter (EPICS XL; Coulter Corp, Miami, FL). Controls consisted of commercial isotype-matched murine monoclonal antibodies (Sigma Chemicals, St Louis, MO). Ten thousand cells were analyzed for each sample.
Production of virus stocks bearing or not bearing host-derived MHC-II.RAJI-CD4 (MHC-II–positive) and RM3-CD4 (MHC-II–negative) cell lines were infected with HIV-1IIIB at a multiplicity of infection of 0.01 (infectious particle/target cell). At the peak of viral production and before extensive cytopathic effects were seen, cells were centrifuged at 300g for 5 minutes. Thereafter, the virus-containing supernatant was clarified at 2,000g for 30 minutes and was filtered through a 0.45-μm cellulose acetate membrane to remove cellular debris. The virus-containing supernatant was stored at −80°C in aliquots until used. Infectivity of our virus stocks was monitored by terminal dilution microassay using the highly susceptible MT-4 cell line.49 End-point titration was performed in flat-bottomed microtiter wells using four parallel series of 10-fold dilutions. After 5 to 7 days of incubation, cells from each well were stained for immunofluorescence as described previously.50 Cells were scored as positive or negative and the 50% tissue culture infectious dose (TCID50) was calculated by the method of Reed and Muench.51 The IIIB strain of HIV-1 was kindly provided by Dr R.C. Gallo through the AIDS Research and Reference Reagent Program as cell-free supernatant from infected H9 cells. The percentage of pelletable virus, as measured by the amount of precipitable p24 over the total p24 value in each virus preparation, was evaluated by subjecting virus stocks to ultracentrifugation conditions sufficient to sediment whole viruses (Heraeus type HTA 13.8 rotor, 12,000 rpm for 90 minutes at 4°C).
Antibody virus capture assay.The wells of an enzyme-linked immunosorbent assay plate (Immulon 2; Dynatech, Chantilly, VA) were coated for 2 hours at 37°C with different murine antibodies (50 μg/mL, 100 μL/well) or with human anti–HIV-1 polyclonal antibodies (HIV-1 neutralizing serum 1; diluted 1:100) before blocking with 3% bovine serum albumin in Tris-buffered saline (TBS). Wells were washed several times with TBS and HIV-1 particles (1,000 TCID50 ) originating from acutely HIV-1IIIB–infected RAJI-CD4 and RM3-CD4 cells were added. After an incubation of 1 hour at 37°C, wells were washed several times to remove unabsorbed viruses. The captured viruses were solubilized by adding TBS supplemented with Triton X-100 (1% vol/vol final concentration). The supernatant was transferred to another plate into which the p24 concentration was monitored using a commercially available quantitative p24 capture assay (Organon Teknika, Durham, NC). HIV-1 neutralizing serum 1 was obtained through the AIDS Research and Reference Reagent Program (catalogue no. 1984).
Capture of HIV-1 by immunomagnetic beads.This assay was performed as described previously.52 Briefly, magnetic particles (BioMag goat antimouse IgG particles Fc specific; PerSeptive Diagnostics, Inc, Cambridge, MA) were washed four times using a magnetic separation unit with binding buffer made of PBS, pH 7.2, supplemented with 0.1% bovine serum albumin and 0.02% thimerosal. Thereafter, washed magnetic particles (2.5 × 108) were incubated overnight at 4°C using gentle mixing with 40 μg of each appropriate MoAb. Antibody-coated magnetic particles were washed four times with binding medium and were stored at 4°C until used. Similar amounts of each virus stock, standardized by their p24 content (2,500 pg), were incubated with antibody-coated magnetic particles (12.5 × 106) for 60 minutes at 4°C under gentle mixing. Complexes consisting of immunomagnetic particles and viruses were washed four times with binding medium and were resuspended in 100 μL of binding medium. Samples were immediately tested for their p24 content by a commercial enzymatic assay.
Virus internalization assay and kinetics of HIV-1 entry.Cells (5 × 105) were incubated with 1,000 TCID50 of progeny virus originating from HIV-1IIIB–infected RAJI-CD4 or RM3-CD4 cells. Incubation was first performed at 4°C for 30 minutes to allow virus binding. Cells were washed and incubated at 37°C for 90 minutes to allow virus entry. Thereafter, cells were washed and treated with trypsin (Sigma Chemicals) to remove viruses that had not entered cells. Cells were washed and lysed using Triton X-100, and virus p24 protein was quantitated using a commercial enzymatic assay. Incubation period at 37°C was omitted for controls and allowed us to evaluate amounts of uninternalized virions that cannot be removed from the cell surface despite trypsin treatment. Levels of virus particles that had entered studied cell lines were determined by subtracting the amount of viruses that could not be removed by trypsin treatment from the total amount of viruses (viruses that could not be removed by treatment with trypsin and viruses that had entered cells). The kinetics of virus entry into susceptible cells were determined as follows. Sup-T1 cells were inoculated with 1,000 TCID50 of HIV-1IIIB stocks bearing (RAJI-CD4) or not bearing host-derived MHC-II (RM3-CD4). At specific time points after incubation with HIV-1 at 37°C, cells were washed and treated with trypsin before evaluation of the levels of internalized viral p24.
Multinuclear activation of galactosidase indicator cells (MAGI) assay.The MAGI assay was performed as described previously.34 In brief, a 30% confluent monolayer of HeLa-CD4-LTR-β-gal cells (24-well plate) was incubated for 15 minutes at 37°C with 100 TCID50 of progeny virus harvested from HIV-1IIIB–infected RAJI-CD4 or RM3-CD4 cells. Cells were washed several times to remove unabsorbed viruses and incubated for 48 hours in a 37°C, 5% CO2 incubator. The culture medium was removed and cells were fixed for 5 minutes at room temperature. Cells were washed before the addition of the staining solution made of 0.4 mg/mL X-gal, 4 mmol/L potassium ferrocyanide, 4 mmol/L potassium ferricyanide, and 2 mmol/L MgCl2. After an overnight incubation period at 4°C, wells were washed twice and the number of blue-stained cells was counted using a light microscope.
Reverse transcriptase and p24 enzymatic assays.The reverse transcriptase activity was monitored as described previously.53 In brief, enzymatic activity was measured in 50 μL of clarified supernatant fluid to which 10 μL of a solution A (5 mmol/L dithiothreitol, 50 mmol/L KCl, 0.05% Triton X-100) and 40 μL of a solution B [5 mmol/L MgCl2 , 0.5 mol/L EGTA, 0.04 mg/mL poly (rA)-oligo (dT)12-18 , 3 mCi [3H]-TTP (40 to 70 Ci/mmol)] had been added. After incubation for 1 hour at 37°C, samples were precipitated before filtration on glass fiber filters using a cell harvester system (TOMTEC, Orange, CT). The filters were dried and radioactivity was counted in a liquid scintillation counter (1205/1204 BS BETAPLATE; Wallac Oy, Turku, Finland). Quantitative detection of the main viral core p24 protein was achieved with the use of a commercial enzyme-linked immunosorbent assay (Organon Teknika).
Statistical analysis.Results shown are expressed as means ± standard deviations (SDs) of triplicate samples. Statistical analysis of the differences between groups was first performed by analysis of variance. If P values were less than .05, group comparisons were performed using the Fisher least-significance difference post-hoc test. A P value less than .05 was considered significant. Calculations were made using the Statview Software (Abacus Concepts, Inc, Berkeley, CA).
RESULTS
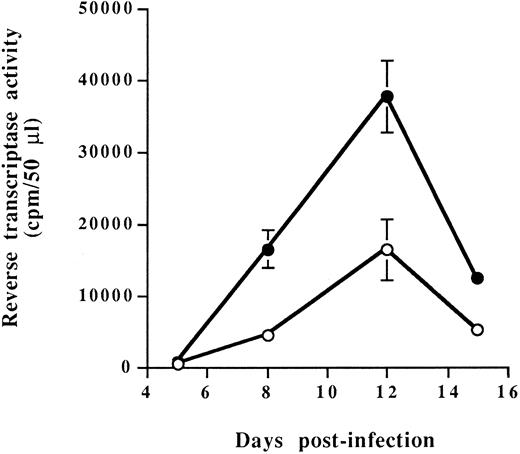
Characterization of RAJI-CD4 and RM3-CD4 cells and susceptibility to HIV-1 infection.Our studies were performed using RAJI, a human B-cell line expressing very high levels of all MHC-II isotypes (DR, DP and DQ), and RM3, a RAJI derivative cell line known to express no mRNA encoding HLA-DR, -DP, and -DQ MHC-II glycoproteins.39 Both cell lines were transfected with the cDNA encoding for human CD4 glycoprotein to allow infection with HIV-1. Two independent stable CD4-expressing transfectants termed RAJI-CD4 and RM3-CD4 were used for our subsequent studies. Flow cytometry analysis showed that the level of cell surface CD4, as reflected by the percentage of positive cells and the mean fluorescence intensity (indicative of the number of molecules per single cell shown on a logarithmic scale), was higher on RAJI-CD4 as compared with RM3-CD4 cells (Table 1). We also determined that, as expected, RAJI-CD4 cells expressed very high levels of each MHC-II isotypes, whereas RM3-CD4 cells were negative for expression of HLA-DR, -DP, and -DQ. Next, these cell lines were infected with HIV-1IIIB and were found to support efficiently virus replication (Fig 1). Peaks of viral replication were reached at the same time for both cell lines, namely at 12 days after infection. However, it should be noted that the peak of viral replication was higher for RM3-CD4 than for RAJI-CD4 cells. These results could be explained by a more rapid and extensive virus-induced cell death in RAJI-CD4 than in RM3-CD4 cells (data not shown).
Virus replication in RAJI-CD4 and RM3-CD4 cells. RAJI-CD4 (○) and RM3-CD4 (•) cells were infected with HIV-1IIIB (multiplicity of infection, 0.1) and virus production was monitored by measuring reverse transcriptase activity in cell-free culture supernatants. Results shown are the mean ± SD of triplicate samples. Reverse transcriptase activity in mock-infected cells was always less than 1,000 cpm/50 μL.
Virus replication in RAJI-CD4 and RM3-CD4 cells. RAJI-CD4 (○) and RM3-CD4 (•) cells were infected with HIV-1IIIB (multiplicity of infection, 0.1) and virus production was monitored by measuring reverse transcriptase activity in cell-free culture supernatants. Results shown are the mean ± SD of triplicate samples. Reverse transcriptase activity in mock-infected cells was always less than 1,000 cpm/50 μL.
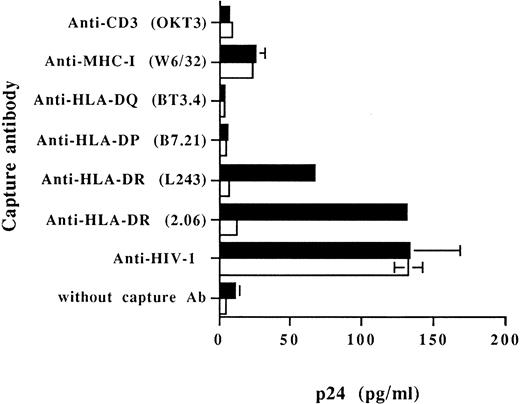
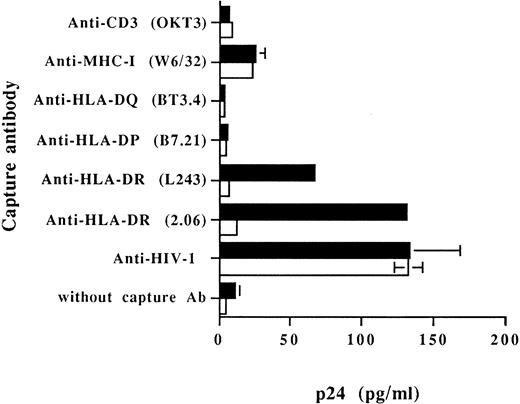
Cellular MHC-II glycoproteins are found embedded on virions produced by RAJI-CD4 and are absent on progeny viruses harvested from RM3-CD4 cells.To examine the acquisition of specific host membrane proteins by HIV-1 particles budding out from RAJI-CD4 and RM3-CD4 cells, we first performed a virus capture assay based on the use of immobilized monoclonal and polyclonal antibodies. Results from Fig 2 indicate that HLA-DR was found to be physically present on viruses harvested from HIV-1–infected RAJI-CD4 cells and absent on virus particles generated by RM3-CD4 cells. These data were obtained using two different MoAbs recognizing distinct nonpolymorphic epitopes of HLA-DR (L243 and 2.06). In this assay, nonspecific adsorption of viruses to the bottom of the wells (background levels of p24) was evaluated by using wells without a capture antibody or wells coated with an antibody specific for a cell surface molecule absent on the surface of both RAJI-CD4 and RM3-CD4 cells (eg, anti-CD3). Data from this set of experiments confirmed also previous studies by us and others showing the acquisition of host MHC-I by HIV-1.7-9 52
Capture of progeny virus harvested from RAJI-CD4 and RM3-CD4 cells acutely infected with HIV-1IIIB . Purified virus stocks (1,000 TCID50 ) from acutely infected RAJI-CD4 (▪) and RM3-CD4 (□) cells were added to wells precoated with different MoAbs or an HIV-1 neutralizing sera. The presence of captured viral particles was next investigated with the use of a commercial p24 enzymatic assay. Results shown represent the average ± SD of triplicate samples.
Capture of progeny virus harvested from RAJI-CD4 and RM3-CD4 cells acutely infected with HIV-1IIIB . Purified virus stocks (1,000 TCID50 ) from acutely infected RAJI-CD4 (▪) and RM3-CD4 (□) cells were added to wells precoated with different MoAbs or an HIV-1 neutralizing sera. The presence of captured viral particles was next investigated with the use of a commercial p24 enzymatic assay. Results shown represent the average ± SD of triplicate samples.
No virus capture was observed in wells coated with MoAbs against HLA-DP and HLA-DQ. This is in contrast with our previous report showing that host HLA-DP and HLA-DQ glycoproteins can also be found embedded on HIV-1.52 It should be noted that these observations were made using a system based on the capture of progeny virus with immunomagnetic beads. Therefore, we have also analyzed the presence of host-derived molecules acquired by budding HIV-1 particles using this test. Magnetic beads coated with anti–HLA-DR antibodies efficiently captured progeny virus harvested from HIV-1–infected RAJI-CD4 cells (Table 2). Immunomagnetic beads coated with anti–HLA-DP and HLA-DQ antibodies also captured RAJI-CD4-produced viruses but to a lesser extent. In the case of progeny virus harvested from HIV-1–infected RM3-CD4 cells, MoAbs against HLA-DR, -DP, and -DQ could not capture these viruses (data not shown). In this assay, background amounts of captured viruses were estimated using magnetic coated with anti-CD3 antibodies. These results suggest that host-derived HLA-DR molecules are relatively more abundant on HIV-1IIIB particles produced by RAJI-CD4 cells than host HLA-DP and HLA-DQ glycoproteins. Moreover, because both assays were run using the same anti–HLA-DP and -DQ antibodies, it also indicates that immunomagnetic beads are more sensitive than antibodies immobilized on a plastic surface to investigate the presence of low levels of virally acquired host membrane components.
The process of virus entry is modulated by the acquisition of host-derived MHC-II proteins by HIV-1.The next step was to investigate whether the physical presence of host cell membrane MHC-II glycoproteins on HIV-1 can positively affect specific steps of the HIV-1 replicative cycle, such as the process of virus entry within susceptible cells. The standardization of virus preparations represents a critical step in these experiments because similar amounts of viruses have to be used to quantitatively assess the process of virus entry. In most experiments, viral stocks are normalized according to their p24 content. However, it has been shown that capsid proteins, such as p24, may be shed as free soluble antigen.54-57 Therefore, we investigated the possibility that virion-associated p24 proteins may differ for virus preparations harvested from HIV-1IIIB–infected RAJI-CD4 and RM3-CD4 cells. This possibility was assessed by monitoring the proportion of pelletable virus-associated p24 for each virus stock. Table 3 shows that the percentage of pelletable HIV-1 particles was markedly different between the viral preparation harvested from HIV-1IIIB–infected RAJI-CD4 (41%) and RM3-CD4 (68%) cells. The presence of higher amounts of soluble nonpelletable free p24 proteins in the culture supernatant from HIV-1IIIB–infected RAJI-CD4 (59%), as compared with the cell-free supernatant from HIV-1IIIB–infected RM3-CD4 cells (32%), is most likely attributed to the more rapid and dramatic virus-induced cytopathic effect (data not shown). These observations imply that each viral stock could not be normalized for our experiments in terms of amounts of p24.
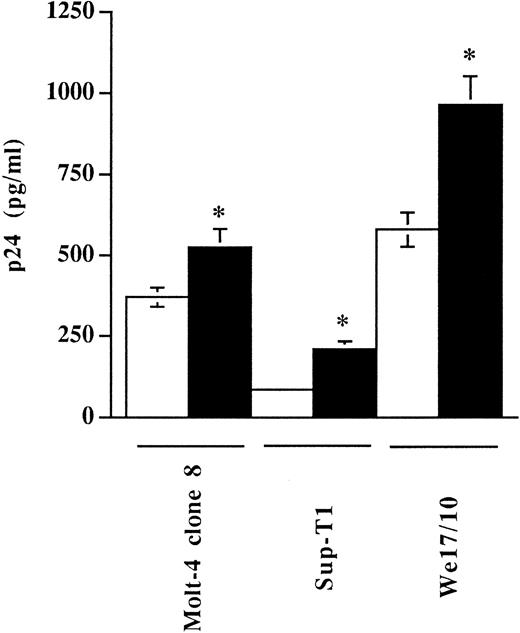
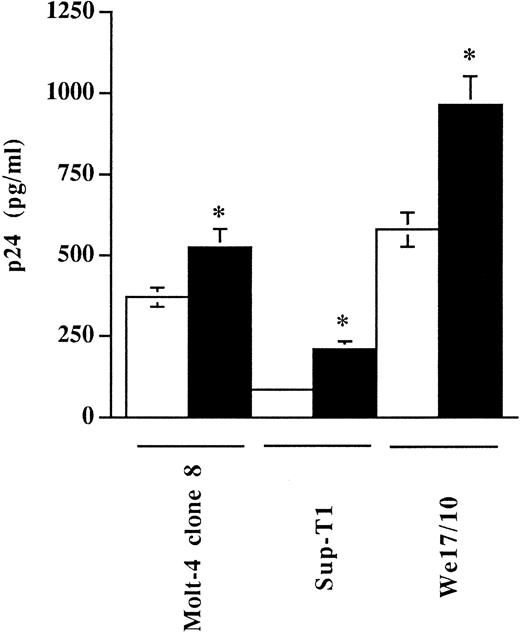
Studies to analyze if virally embedded host MHC-II could influence virus entry were thus performed with our virus preparations standardized in terms of infectivity as we determined that virus stocks produced in RAJI-CD4 and RM3-CD4 cells had similar noninfectious to infectious particle ratios (TCID50 per picogram of p24; data not shown). The extent of virus entry was first monitored by incubating three different CD4-expressing T-lymphoid cell lines with equivalent amounts of progeny virus (1,000 TCID50 ) harvested from either HIV-1IIIB–infected RAJI-CD4 (MHC-II–positive) or RM3-CD4 (MHC-II–negative) cells. The efficiency of virus entry was monitored by measuring the p24 antigen uptake. In this assay, cells were incubated with normalized amounts of virions either at 4°C, a temperature allowing virus binding but not entry, or at 37°C, a temperature permissive for both processes. After 90 minutes of incubation, cells were washed several times and uninternalized viruses were removed with trypsin. The data from Fig 3 indicate that a 1.4-, 2.5-, and 1.7-fold increase in the levels of internalized viruses was seen in Molt-4 clone 8, Sup-T1, and We17/10 cells, respectively, when using a virus preparation produced by RAJI-CD4 (MHC-II–positive) as compared with a virus stock harvested from RM3-CD4 cells (MHC-II–negative). Results from these experiments show that, depending of the cell line tested, a 1.4- to 2.5-fold increase in the amount of viruses entering such cells was noticed when cells were inoculated with virions carrying host MHC-II proteins (originating from HIV-1IIIB–infected RAJI-CD4 cells) as compared with cells infected with progeny virus harvested from HIV-1IIIB–infected RM3-CD4 (MHC-II–negative cells).
The process of HIV-1 entry in different T-lymphoid cells is enhanced by virus-bound cellular MHC-II glycoproteins. Molt-4 clone 8, Sup-T1, and We17/10 were incubated with similar amounts of virus preparations, standardized in terms of infectivity (TCID50 ), produced by either RAJI-CD4 (▪) or RM3-CD4 (□) cells. Next, cells were washed several times with PBS and treated with trypsin to remove uninternalized viruses. Cells were washed and lysed using Triton X-100, and the amounts of internalized viral p24 were determined using a p24 enzymatic assay. Results shown represent the mean ± SD of triplicate samples. Experiments were repeated three times and gave reproducible results. Asterisks indicate significant differences from infection with virions produced by RM3-CD4 cells at P < .05. Levels of viral p24 in mock-infected cells were less than 20 pg/mL.
The process of HIV-1 entry in different T-lymphoid cells is enhanced by virus-bound cellular MHC-II glycoproteins. Molt-4 clone 8, Sup-T1, and We17/10 were incubated with similar amounts of virus preparations, standardized in terms of infectivity (TCID50 ), produced by either RAJI-CD4 (▪) or RM3-CD4 (□) cells. Next, cells were washed several times with PBS and treated with trypsin to remove uninternalized viruses. Cells were washed and lysed using Triton X-100, and the amounts of internalized viral p24 were determined using a p24 enzymatic assay. Results shown represent the mean ± SD of triplicate samples. Experiments were repeated three times and gave reproducible results. Asterisks indicate significant differences from infection with virions produced by RM3-CD4 cells at P < .05. Levels of viral p24 in mock-infected cells were less than 20 pg/mL.
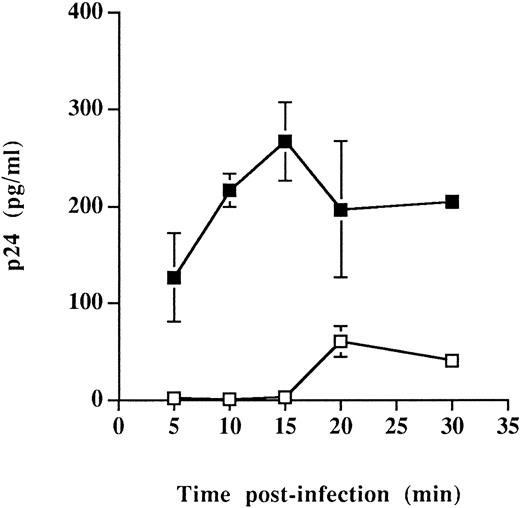
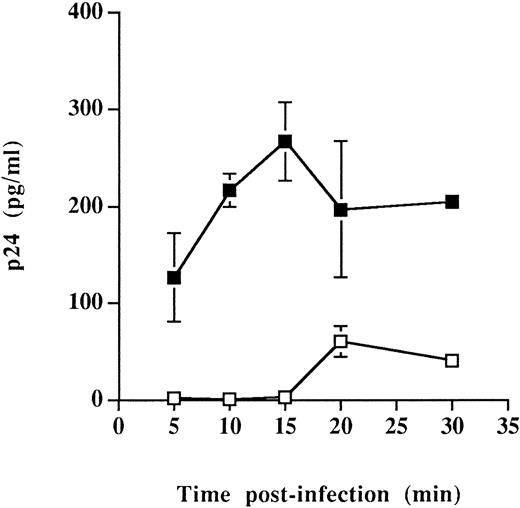
Similar types of infection studies were performed exclusively at 37°C and after shorter periods of monitoring time. These experiments were initiated based on cellular adhesion studies indicating that interactions between CD4 and MHC-II proteins were more efficient at 37°C than at 4°C (energy dependent)58 and on the assumption that the virus entry step was accelerated by the presence of host-derived MHC-II on HIV-1. The rate/efficiency of virus entry was investigated only in Sup-T1 cells because greater differences in the process of virus entry were seen in such cells (Fig 3). Entry of virus particles was found to be a more rapid event when infection was achieved using HIV-1 particles carrying host-derived HLA-DR (Fig 4). Indeed, high levels of the main viral core p24 protein were detected after incubation infection for 5 to 15 minutes at 37°C with progeny virus harvested from RAJI-CD4, whereas undetectable amounts of p24 were observed at similar time points in Sup-T1 inoculated with viral particles from RM3-CD4 cells. Altogether, these observations suggest that the presence of host-derived MHC-II glycoproteins on HIV-1 positively modulate the early events in the process of virus entry and may thus accelerate the kinetics of viral infection.
Faster kinetics of viral entry for virions bearing host-derived MHC-II. Sup-T1 cells were incubated at 37°C for the indicated periods of time with virus stocks harvested from either RAJI-CD4 (▪) or RM3-CD4 (□) cells. Next, cells were washed several times with PBS and treated with trypsin to remove uninternalized viruses. Cells were washed and lysed using Triton X-100, and the amounts of internalized viral p24 were determined using a p24 enzymatic assay. Data shown are the mean of triplicate samples. Error bars indicate sample standard deviations. Levels of viral p24 in mock-infected cells were less than 20 pg/mL.
Faster kinetics of viral entry for virions bearing host-derived MHC-II. Sup-T1 cells were incubated at 37°C for the indicated periods of time with virus stocks harvested from either RAJI-CD4 (▪) or RM3-CD4 (□) cells. Next, cells were washed several times with PBS and treated with trypsin to remove uninternalized viruses. Cells were washed and lysed using Triton X-100, and the amounts of internalized viral p24 were determined using a p24 enzymatic assay. Data shown are the mean of triplicate samples. Error bars indicate sample standard deviations. Levels of viral p24 in mock-infected cells were less than 20 pg/mL.
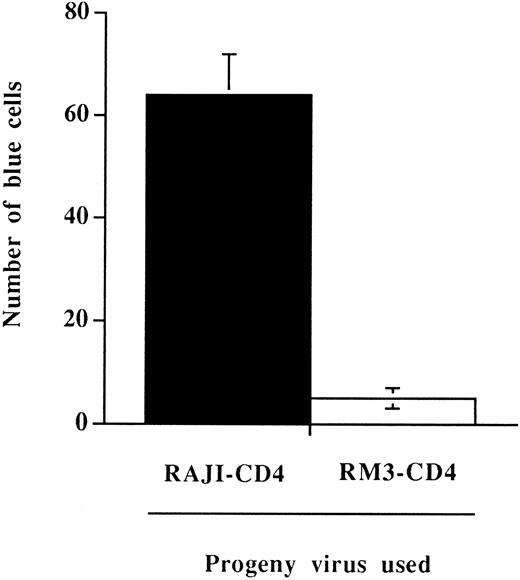
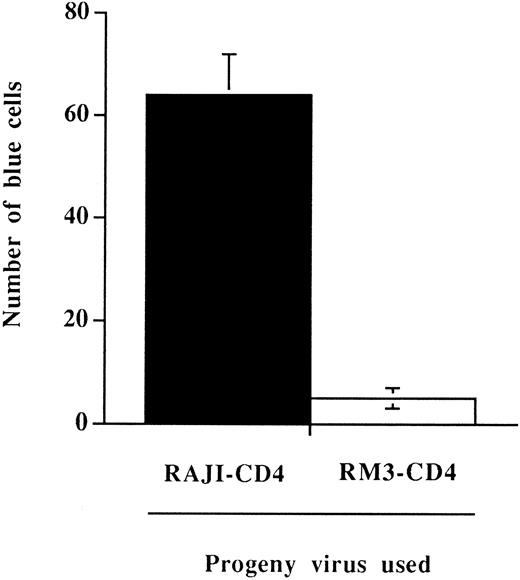
To directly assess this possibility, we have tested whether the more rapid process of virus entry could still be observed when measuring HIV-1 infectivity. This was achieved using the MAGI assay,34 a test that can quantitatively detect the presence of infectious viruses. The MAGI assay has been reported to be linear in its detection and as sensitive as end point dilution titration. Furthermore, this test is highly appropriate for our needs because it relies on a single round of viral infection with no reinfection events. The presence of host-derived MHC-II on HIV-1 was associated with a significant increase in the number of virally infected cells when the indicator cell line HeLa-CD4-LTR-β-gal was incubated for a brief period of time (15 minutes at 37°C) with normalized amounts of virus particles bearing or not bearing host-derived MHC-II (Fig 5). Results from this set of experiments indicate that host-derived MHC-II glycoproteins are part of infectious viral entities and are conferring an infectivity advantage to HIV-1 when susceptible cells are incubated for a limited period of time with virus particles.
Enhancement of viral infectivity in a defined incubation time period conferred by the presence on HIV-1 of host-derived MHC-II glycoproteins. HeLa-CD4-LTR-β-gal cells were incubated for 15 minutes at 37°C with 100 TCID50 of progeny virus produced by HIV-1IIIB–infected RAJI-CD4 (▪) or RM3-CD4 (□) cells. Cells were washed several times to remove unadsorbed viruses and incubated for 48 hours in a 37°C, 5% CO2 incubator. The number of blue-stained cells were counted using a light microscope. Results shown are the mean ± SD of triplicate determinations. The number of blue cells in mock-infected cells was always less than 5.
Enhancement of viral infectivity in a defined incubation time period conferred by the presence on HIV-1 of host-derived MHC-II glycoproteins. HeLa-CD4-LTR-β-gal cells were incubated for 15 minutes at 37°C with 100 TCID50 of progeny virus produced by HIV-1IIIB–infected RAJI-CD4 (▪) or RM3-CD4 (□) cells. Cells were washed several times to remove unadsorbed viruses and incubated for 48 hours in a 37°C, 5% CO2 incubator. The number of blue-stained cells were counted using a light microscope. Results shown are the mean ± SD of triplicate determinations. The number of blue cells in mock-infected cells was always less than 5.
DISCUSSION
Understanding how some specific steps of the replicative cycle of HIV-1 might be modulated by the acquisition of host-derived molecules is important to supplement our knowledge of the pathogenesis of acquired immune deficiency syndrome. In this regard, the effect(s) on the replicative cycle of HIV-1 that may be associated with virally embedded host cell membrane MHC-II needs to be investigated because MHC-II and the external viral envelope glycoprotein gp120 are both ligands for CD4, the primary cellular receptor for HIV. Studies presented here show that the incorporation of host-derived MHC-II glycoproteins on HIV-1 is accelerating the process of virus entry and infection into susceptible T-lymphoid cell lines.
As mentioned above, the membrane CD4 glycoprotein represents the high-affinity cellular receptor for HIV.17,18 The envelope protein of HIV has two major constituents, gp120 and gp41, which are noncovalently associated. The external portion of the envelope protein gp120 is the site of attachment to the CD4 molecule and the transmembrane domain gp41 catalyzes fusion between the virus and target cell membranes. Previous studies have shown that gp120 is easily shed from virus particles soon after budding from the cell membrane, leaving virus particles denuded of their external envelope glycoproteins gp120.32,33 McKeating et al59 have reported that the spontaneous shedding of gp120, due to the weak association with the transmembrane glycoprotein gp41, was linked to the loss of HIV-1 infectivity. Because the shedding of gp120 observed in vitro is also occurring in vivo,33 one may thus postulate that the infectious process will be greatly jeopardized if the number of gp120 molecules is too low to provide the threshold binding energy required to overcome the repulsive electrostatic forces between the negatively charged cellular and viral membranes.31
The exact mechanism by which acquisition of host MHC-II by HIV-1 is leading to an enhancement of the rate/efficiency of virus entry is still undefined. However, we can postulate that virally embedded host MHC-II glycoproteins are facilitating the penetration of the target cell either by a direct or indirect effect. In the first eventuality, besides the gp120-CD4 association, virally incorporated host MHC-II would also interact with CD4, its natural countereceptor, and would function as additional ligands for virus adsorption. Such interactions between virally acquired host molecules and their cell surface counterligands would be determinant for viruses expressing low levels of gp120, considering that multiple gp120 molecules need to be present for the infection to proceed.60 The adhesion of HIV to susceptible cells is mediated primarily by the gp120-CD4 interaction, and we believe that this initial contact will most likely facilitate the physical interaction between other molecules including host-derived virally acquired MHC-II and cell surface CD4. This postulate is based on cell-to-cell adhesion studies showing that binding of MHC-II to CD4 is not an initial adhesion event, but rather plays a role in late T-cell antigen-presenting cell interactions.58,61 The reported low-affinity binding of MHC-II to CD458,62,63 is another piece of evidence supporting the notion that interactions between virally embedded host MHC-II and cell surface CD4 would play a secondary role in the infection process. It has been previously postulated that the efficiency of virus entry could be responsible for changes in the pathogenic properties of different isolates of HIV-1.64 Therefore, secondary interactions could modulate the pathogenic potential of virions by allowing the formation of a multivalent complex that would enhance the stability of virus-cell binding. The second possibility that would explain the upregulation of HIV-1 entry is that host MHC-II on the virion surface would either increase the number of gp120 and/or stabilize the weak interaction between gp120 and gp41. Work is currently underway to identify with certainty the nature of the mechanism leading to the observed virally incorporated host MHC-II–mediated enhancement of HIV-1 entry.
The potentiation of the process of HIV-1 infection, which is mediated by the acquisition of host MHC-II, is most likely due to the presence of the HLA-DR determinant on progeny virions. This theory is based on our results suggesting that host HLA-DR are relatively more abundant on the virion surface than host-derived HLA-DP and HLA-DQ glycoproteins. However, we cannot totally exclude the possibility that virally embedded host HLA-DP and HLA-DQ might also be partly responsible for the enhancement of virus entry.
The biologic significance of the incorporation of host-derived molecules within budding HIV have been recently addressed by some investigators. Although a recent report has shown that incorporation of MHC-I molecules is not essential for HIV infectivity,11 several studies have demonstrated that incorporation of host proteins modulates the biology of HIV. For example, a synergy in virus neutralization was observed with a combination of an anti–LFA-1 monoclonal antibody and polyclonal anti–HIV-1 antibodies.65 It was proposed that host LFA-1 present on virions has the capacity to interact with its counterreceptor present on the target cell surface and that this additional virus-cell association would facilitate fusion mediated by virus envelope proteins. The acquisition of CD55 and CD59 by HIV-1 has been shown to confer protection against complement-mediated viral lysis.10,66 It has been previously suggested that the presence of a biologically active cell adhesion molecule CD44 on HIV-1 may affect viral tropism and infectivity.67 More recently, host-derived MHC-II glycoproteins on HIV-1 particles were shown to be immunologically functional in superantigen presentation to human T cells.68 In support of our findings, neutralization experiments conducted in vitro by Arthur et al7 showed that HIV infection could be inhibited with antisera directed against the HLA-DR determinant of MHC-II. The present report constitutes an additional evidence of the functional role played by host constituents acquired by HIV.
The physiological relevance of our observations for the pathogenesis of the disease is obvious considering that progeny virions in infected individuals will primarily originate from cells expressing cell surface MHC-II glycoproteins. Indeed, HIV-1 has been isolated from CD4-expressing T lymphocytes,69 monocytes/macrophages,70 and HIV-1 proviral DNA, and RNA have been detected in some members of the dendritic cell lineage, such as Langerhans cells, dendritic cells of the adenoid, and peripheral blood dendritic cells.71-73 The viral presence in cells of the dendritic cell lineage is of prime importance, because they express all polymorphic MHC-II gene products at high levels.74 This property is probably responsible for their higher potency in antigen presentation than peripheral blood monocytes/macrophages and lymphocytes.75 Another factor that could show some importance for the in vivo situation remains the relative abundance of virally incorporated host MHC-II. It has been previously estimated that between 375 and 600 HLA-DR molecules (α and β chains) are found associated with HIV-1IIIB budding from H9 cells.7 This observation suggests that virally embedded host HLA-DR outnumbered virus envelope glycoprotein gp120 by a factor of 1.7 to 2.8, considering that HIV possesses an average of 216 gp120 molecules per virion.76 This figure could be potentially much higher in favor of host cell membrane MHC-II based on our previous data indicating that the relative amounts of host-derived HLA-DR proteins may quantitatively differ depending of the producer cell line.52 For example, it is of interest to note that host-derived HLA-DR proteins were relatively more abundant on progeny virus originating from HIV-1–infected RAJI-CD4 than on virions produced by H9 cells.52 The in vivo significance of our findings is futher emphasized by the observation that host-derived MHC-II glycoproteins are acquired by clinical isolates of HIV-1 grown on phytohemagglutinin-stimulated primary peripheral blood mononuclear cells.52
Several crucial questions are arising from the present study. For example, could it be possible that the genetic background of the infected individual might affect the rate of progression of the clinical course? This is reminiscent of a previous study that has shown that the affinity between HLA-DR and CD4 might differ among various HLA-DR alleles.77 These results, when taken in conjunction with our observations, might imply that the incorporation of host HLA-DR alleles possessing the strongest affinities for CD4 may result in an increase of the infection process and, possibly, to a more rapid progression of the disease. Moreover, it is quite possible that some specific host HLA-DR alleles might be more preferentially and/or efficiently acquired by HIV-1, as we suggested previously.52 Finally, the incorporation of higher amounts of MHC-II within budding virions may also potentiate the process of virus entry and infection. Further investigations are needed to shed light on these matters.
The ability to design effective antiviral and immunological strategies for the treatment of HIV infection will greatly depend on our understanding of the components, being cellular or viral, expressed on the virion surface. The presence of cellular molecules incorporated within viral particles has important implications for vaccine development as indicated by the observation that anti–MHC-I78 and anti–HLA-DR79 antibodies protect macaques against infection with the simian immunodeficiency virus. The fundamental question is whether this could be exploited as an effective HIV vaccine? This strategy of immunization will be interesting if the immune response is not only directed against the foreign cellular protein acquired by the virus particle and if the elicited response shows no deleterious effect on the normal immune functions of the vaccinee.
In conclusion, this study has provided new information that will advance our knowledge of the functional role played by host proteins incorporated on HIV-1 particles. Further studies are necessary to determine if the putative interaction between virally incorporated MHC-II and T-cell receptor on the target cell could be responsible for some of the functional immunological defects seen in HIV-1–infected individuals.
ACKNOWLEDGMENT
We thank Maurice Dufour for technical assistance in flow cytometry studies. We are grateful to Dr R.-P. Sékaly for the following cell lines: RAJI, RM3, DAMP, pMNC, B7.21, and BT3.4. Several items were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program: HeLa-CD4-LTR-β-gal, Molt-4 clone 8, Sup-T1, WE17/10, and HIV-1IIIB. Sylvie Perron provided excellent technical assistance.
R.C. and J.-F.F. contributed equally to this work.
M.T. is supported by a Scholarship award from the Fonds de la Recherche en Santé du Québec (FRSQ). R.C. is the recipient of a PhD Fellowship from the FRSQ/Fonds pour la Formation de Chercheurs et l'Aide à la Recherche. J.-F.F. is supported by an NHRDP PhD Fellowship.
Address reprint requests to Michel Tremblay, PhD, Centre de Recherche en Infectiologie, Centre Hospitalier Universitaire de Québec, Pavillon CHUL, 2705 Blvd Laurier, Ste-Foy, Québec, Canada, G1V 4G2.